Classification and Visualization of Chemotherapy-Induced Cognitive Impairment in Volumetric Convolutional Neural Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
2.3. Feature Engineering
2.4. SE-Residual Neural Network and SE-DenseNet
2.5. Visualization through an Integrated Gradients Algorithm
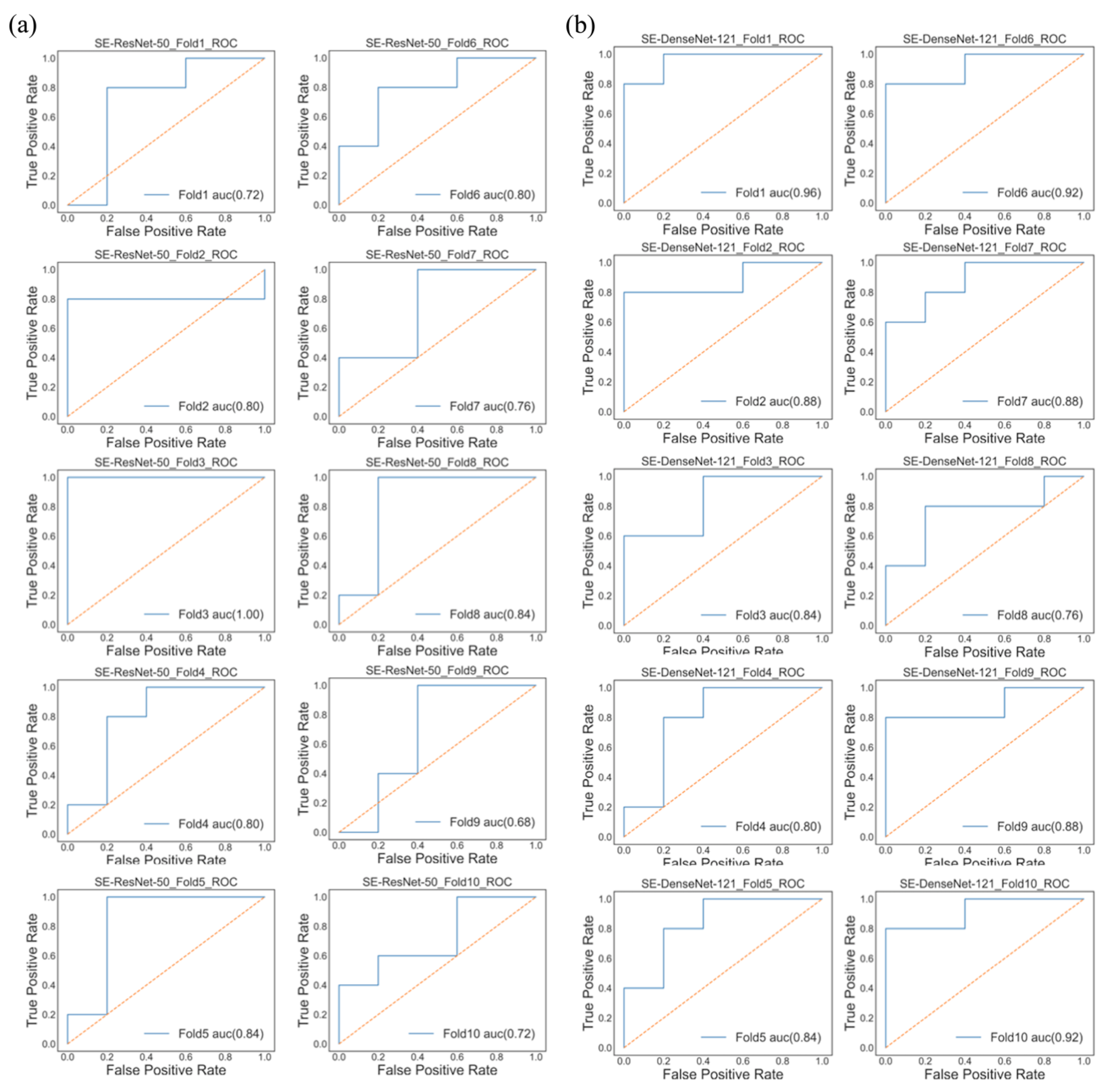
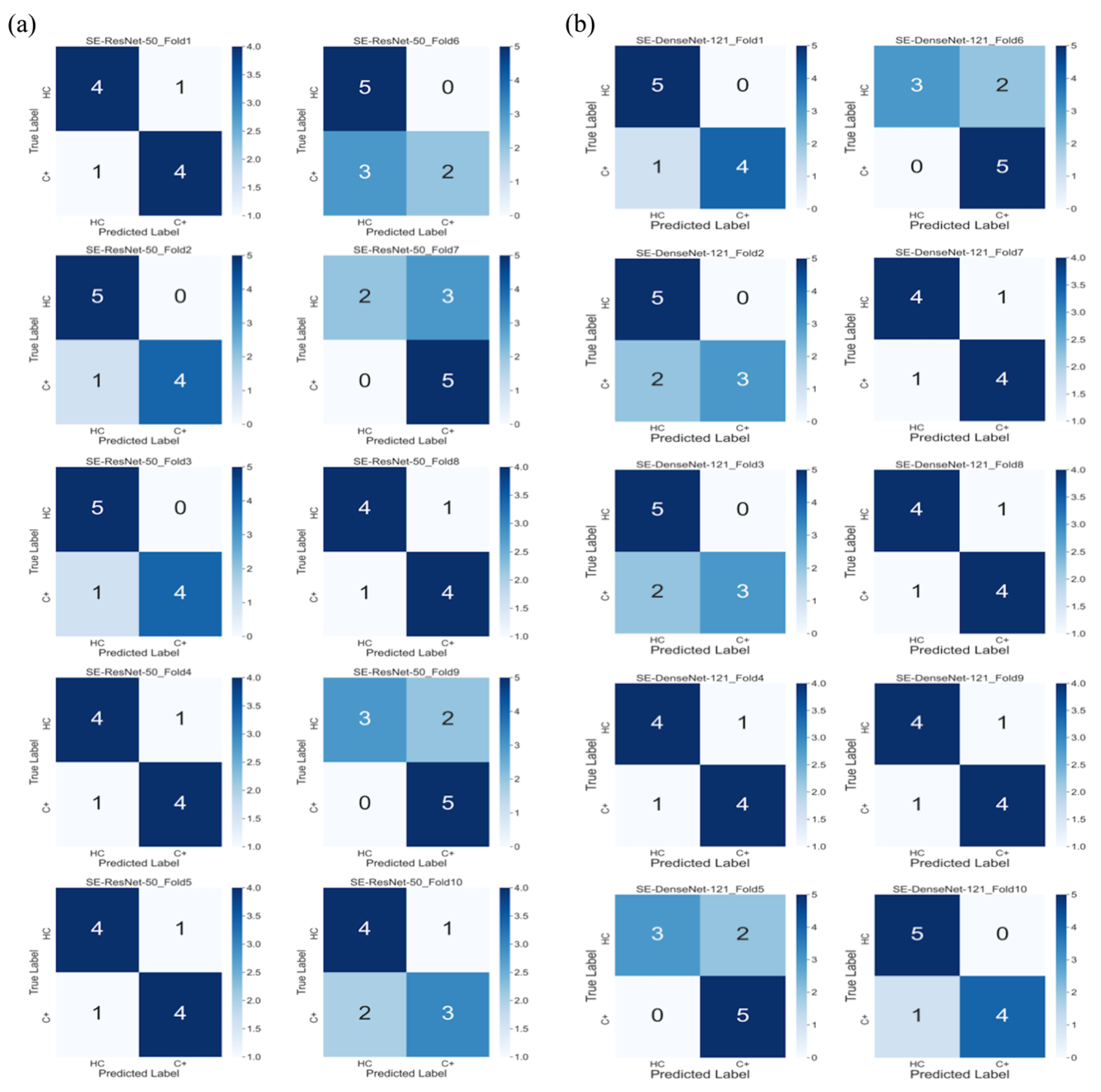
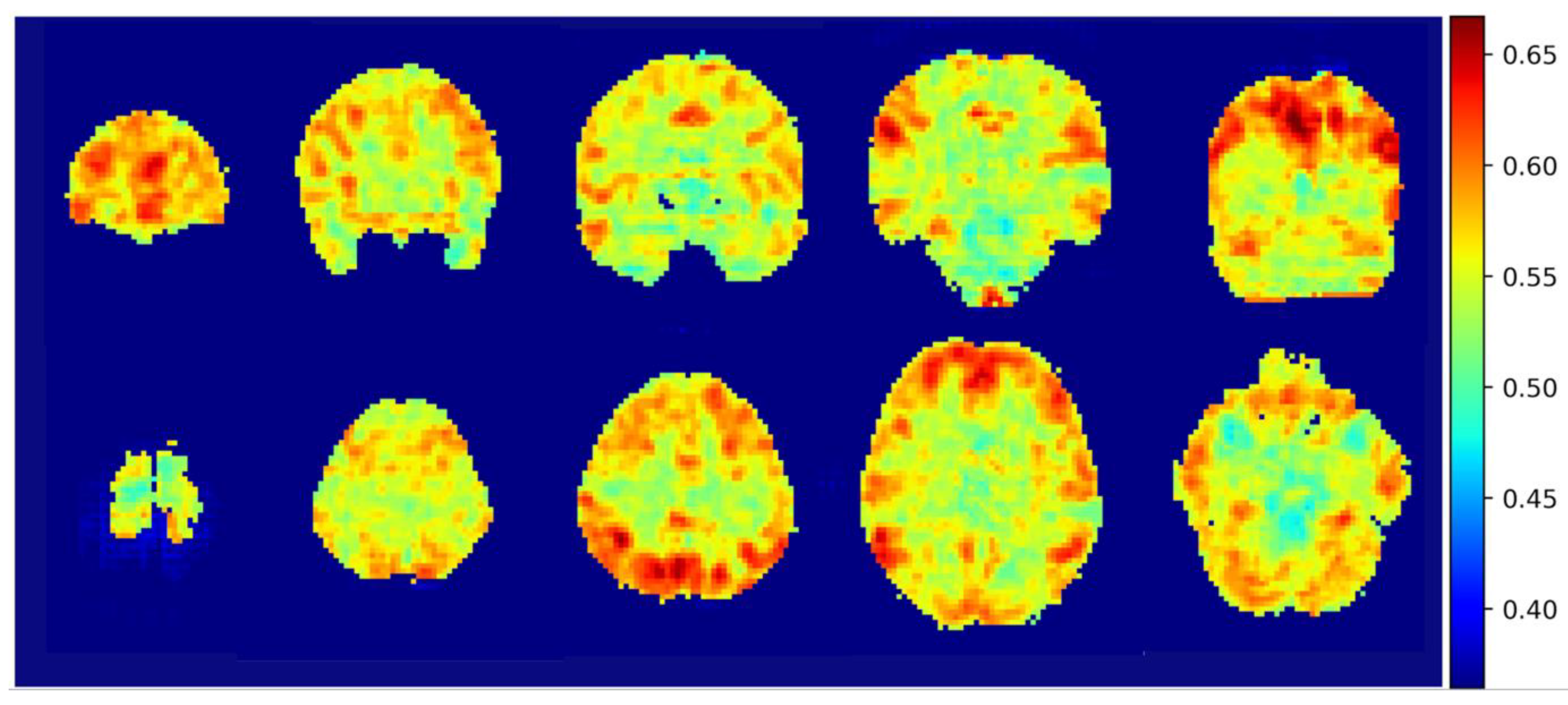
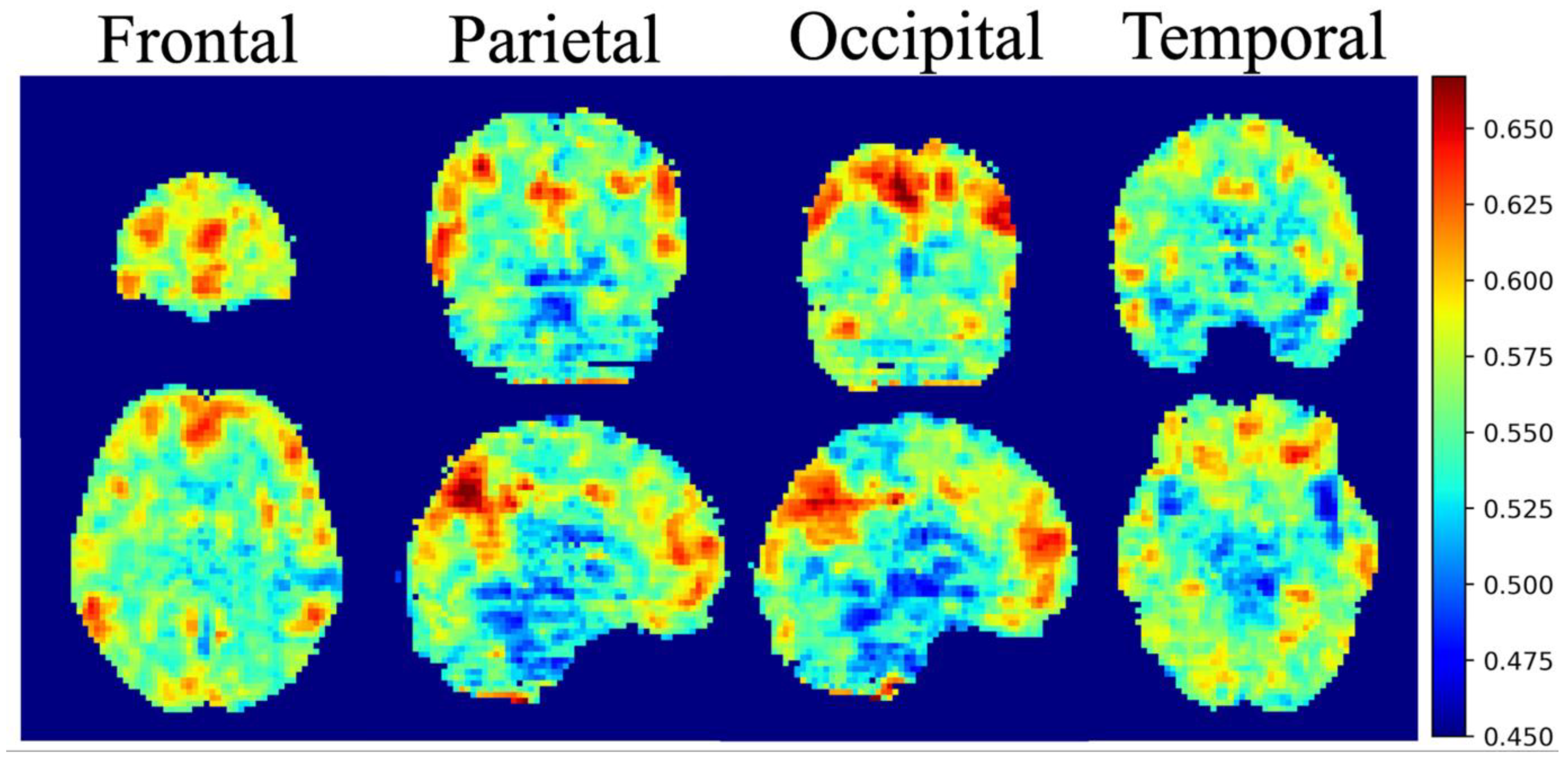
3. Results
3.1. Demographic Characteristics
3.2. Model Performance
3.3. Integrated Gradients Results
4. Discussion
4.1. Default Mode Network & Dorsal Attention Network
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Healthcare and Welfare, Taiwan. Available online: https://www.mohw.gov.tw/mp-2.html (accessed on 2 June 2020).
- Argyriou, A.A.; Assimakopoulos, K.; Iconomou, G.; Giannakopoulou, F.; Kalofonos, H.P. Either Called “Chemobrain” or “Chemofog”, the Long-Term Chemotherapy-Induced Cognitive Decline in Cancer Survivors Is Real. J. Pain Symptom. Manag. 2011, 41, 126–139. [Google Scholar] [CrossRef]
- Hermelink, K. Chemotherapy and Cognitive Function in Breast Cancer Patients: The So-Called Chemo Brain. J. Natl. Cancer Inst. Monogr. 2015, 2015, 67–69. [Google Scholar] [CrossRef] [Green Version]
- LeCun, Y.; Boser, B.; Denker, J.S.; Henderson, D.; Howard, R.E.; Hubbard, W.; Jackel, L.D. Backpropagation applied to handwritten zip code recognition. Neural Comput. 1989, 1, 541–551. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. Imagenet classification with deep convolutional neural networks. Commun. ACM 2017, 60, 84–90. [Google Scholar] [CrossRef]
- Deng, J.; Dong, W.; Socher, R.; Li, L.-J.; Li, K.; Fei-Fei, L. Imagenet: A large-scale hierarchical image database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009. [Google Scholar]
- Chollet, F. Deep Learning with Python; Manning Publications Co.: Greenwich, CT, USA, 2017. [Google Scholar]
- Guidotti, R.; Monreale, A.; Ruggieri, S.; Turini, F.; Giannotti, F.; Pedreschi, D. A survey of methods for explaining black box models. ACM Comput. Surv. (CSUR) 2018, 51, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Abadi, M.; Barham, P.; Chen, J.; Chen, Z.; Davis, A.; Dean, J.; Devin, M.; Ghemawat, S.; Irving, G.; Isard, M. Tensorflow: A system for large-scale machine learning. In Proceedings of the 12th {USENIX} Symposium on Operating Systems Design and Implementation ({OSDI} 16), Savannah, GA, USA, 2–4 November 2016. [Google Scholar]
- Chollet, F. Keras. GitHub. 2015. Available online: https://github.com/fchollet/keras (accessed on 2 June 2020).
- Song, X.W.; Dong, Z.Y.; Long, X.Y.; Li, S.F.; Zuo, X.N.; Zhu, C.Z.; He, Y.; Yan, C.G.; Zang, Y.F. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 2011, 6, e25031. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Bengio, Y.; Simard, P.; Frasconi, P. Learning long-term dependencies with gradient descent is difficult. IEEE Trans. Neural Netw. 1994, 5, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Glorot, X.; Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. In Proceedings of the Thirteenth International Conference on Artificial Intelligence and Statistics, Sardinia, Italy, 13–15 May 2010. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Identity mappings in deep residual networks. In Proceedings of the European Conference on Computer Vision, Amsterdam, The Netherlands, 8–16 October 2016; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Huang, G.; Liu, Z.; Weinberger, K.Q.; van der Maaten, L. Densely connected convolutional networks. arXiv 2016, arXiv:1608.06993. [Google Scholar]
- Hu, J.; Shen, L.; Sun, G. Squeeze-and-excitation networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018. [Google Scholar]
- Sundararajan, M.; Taly, A.; Yan, Q. Axiomatic attribution for deep networks. arXiv 2017, arXiv:1703.01365. [Google Scholar]
- Chen, V.C.H.; Lin, K.Y.; Tsai, Y.H.; Weng, J.C. Connectome analysis of brain functional network alterations in breast cancer survivors with and without chemotherapy. PLoS ONE 2020, 15, e0232548. [Google Scholar] [CrossRef]
- Van den Heuvel, M.P.; Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 2013, 17, 683–696. [Google Scholar] [CrossRef]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tononi, G.; Sporns, O.; Edelman, G.M. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. USA 1994, 91, 5033–5037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shine, J.M.; Breakspear, M. Understanding the Brain, By Default. Trends Neurosci. 2018, 41, 244–247. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Andrews-Hanna, J.R. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 2012, 18, 251–270. [Google Scholar] [CrossRef]
- Palacios, E.M.; Sala-Llonch, R.; Junque, C.; Roig, T.; Tormos, J.M.; Bargallo, N.; Vendrell, P. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol. 2013, 70, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Petrella, J.R.; Sheldon, F.C.; Prince, S.E.; Calhoun, V.D.; Doraiswamy, P.M. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology 2011, 76, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Pu, W.; Wang, J.; Liu, H.; Wu, G.; Liu, C.; Mwansisya, T.E.; Tao, H.; Chen, X.; Huang, X. Inefficient DMN suppression in schizophrenia patients with impaired cognitive function but not patients with preserved cognitive function. Sci. Rep. 2016, 6, 21657. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yan, Y.; Wang, X.; Tao, L.; Chen, Q.; Bian, Y.; He, X.; Liu, Y.; Ding, W.; Yu, Y.; et al. Executive Function Alternations of Breast Cancer Patients After Chemotherapy: Evidence From Resting-state Functional MRI. Acad. Radiol. 2016, 23, 1264–1270. [Google Scholar] [CrossRef] [Green Version]
- Kesler, S.R. Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol. Aging 2014, 35, S11–S19. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Chen, X.; Yan, Y.; He, X.; Hu, S.; Kong, J.; Wu, M.; Wei, Y.; Zhou, Y.; Wang, L.; et al. Functional connectivity change of brain default mode network in breast cancer patients after chemotherapy. Neuroradiology 2016, 58, 921–928. [Google Scholar] [CrossRef]
- Cheng, H.; Li, W.; Gong, L.; Xuan, H.; Huang, Z.; Zhao, H.; Wang, L.S.; Wang, K. Altered resting-state hippocampal functional networks associated with chemotherapy-induced prospective memory impairment in breast cancer survivors. Sci. Rep. 2017, 7, 45135. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.M.; Koovakkattu, D.; Kesler, S.R. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Kesler, S.R.; Wefel, J.S.; Hosseini, S.M.; Cheung, M.; Watson, C.L.; Hoeft, F. Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc. Natl. Acad. Sci. USA 2013, 110, 11600–11605. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.Y.; Chen, V.C.; Yeh, D.C.; Huang, S.L.; Zhang, X.R.; Chai, J.W.; Huang, Y.H.; Chou, M.C.; Weng, J.C. Association of functional dorsal attention network alterations with breast cancer and chemotherapy. Sci. Rep. 2019, 9, 104. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist 2014, 20, 150–159. [Google Scholar] [CrossRef]
- Anticevic, A.; Cole, M.W.; Murray, J.D.; Corlett, P.R.; Wang, X.J.; Krystal, J.H. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012, 16, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; He, X.; Tao, L.; Cheng, H.; Li, J.; Zhang, J.; Qiu, B.; Yu, Y.; Wang, K. The attention network changes in breast cancer patients receiving neoadjuvant chemotherapy: Evidence from an arterial spin labeling perfusion study. Sci. Rep. 2017, 7, 42684. [Google Scholar] [CrossRef] [Green Version]
- Dumas, J.A.; Makarewicz, J.; Schaubhut, G.J.; Devins, R.; Albert, K.; Dittus, K.; Newhouse, P.A. Chemotherapy altered brain functional connectivity in women with breast cancer: A pilot study. Brain Imaging Behav. 2013, 7, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Scherling, C.; Collins, B.; Mackenzie, J.; Bielajew, C.; Smith, A. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: An FMRI study. Front. Hum. Neurosci. 2011, 5, 122. [Google Scholar] [CrossRef] [Green Version]
- Cimprich, B.; Reuter-Lorenz, P.; Nelson, J.; Clark, P.M.; Therrien, B.; Normolle, D.; Berman, M.G.; Hayes, D.F.; Noll, D.C.; Peltier, S.; et al. Prechemotherapy alterations in brain function in women with breast cancer. J. Clin. Exp. Neuropsychol. 2010, 32, 324–331. [Google Scholar] [CrossRef]
| C+ (N = 55) | HC (N = 65) | p-Value | |
|---|---|---|---|
| Age (years, mean ± SD) | 50.00 ± 8.09 | 44.71 ± 7.76 | <0.001 |
| Age range (years) | 32–65 | 31–67 | |
| Years of education | 11.58 ± 3.8 | 13.56 ± 3.06 | 0.002 |
| (mean ± SD) |
| Model/Performance | Accuracy | Precision | Recall | AUC | F1-Score |
|---|---|---|---|---|---|
| SE-ResNet-50 | 0.8 (0.07) | 0.78 (0.13) | 0.7 (0.18) | 0.72 (0.09) | 0.73 (0.1) |
| SE-DenseNet-121 | 0.8 (0.04) | 0.86 (0.12) | 0.8 (0.13) | 0.87 (0.06) | 0.81 (0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.-Y.; Chen, V.C.-H.; Tsai, Y.-H.; McIntyre, R.S.; Weng, J.-C. Classification and Visualization of Chemotherapy-Induced Cognitive Impairment in Volumetric Convolutional Neural Networks. J. Pers. Med. 2021, 11, 1025. https://doi.org/10.3390/jpm11101025
Lin K-Y, Chen VC-H, Tsai Y-H, McIntyre RS, Weng J-C. Classification and Visualization of Chemotherapy-Induced Cognitive Impairment in Volumetric Convolutional Neural Networks. Journal of Personalized Medicine. 2021; 11(10):1025. https://doi.org/10.3390/jpm11101025
Chicago/Turabian StyleLin, Kai-Yi, Vincent Chin-Hung Chen, Yuan-Hsiung Tsai, Roger S. McIntyre, and Jun-Cheng Weng. 2021. "Classification and Visualization of Chemotherapy-Induced Cognitive Impairment in Volumetric Convolutional Neural Networks" Journal of Personalized Medicine 11, no. 10: 1025. https://doi.org/10.3390/jpm11101025
APA StyleLin, K.-Y., Chen, V. C.-H., Tsai, Y.-H., McIntyre, R. S., & Weng, J.-C. (2021). Classification and Visualization of Chemotherapy-Induced Cognitive Impairment in Volumetric Convolutional Neural Networks. Journal of Personalized Medicine, 11(10), 1025. https://doi.org/10.3390/jpm11101025






