Impact of Tacrolimus Daily Dose Limitation in Renal Transplant Recipients Expressing CYP3A5: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Tacrolimus Dosage
2.3. CYP3A5 Genotyping
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
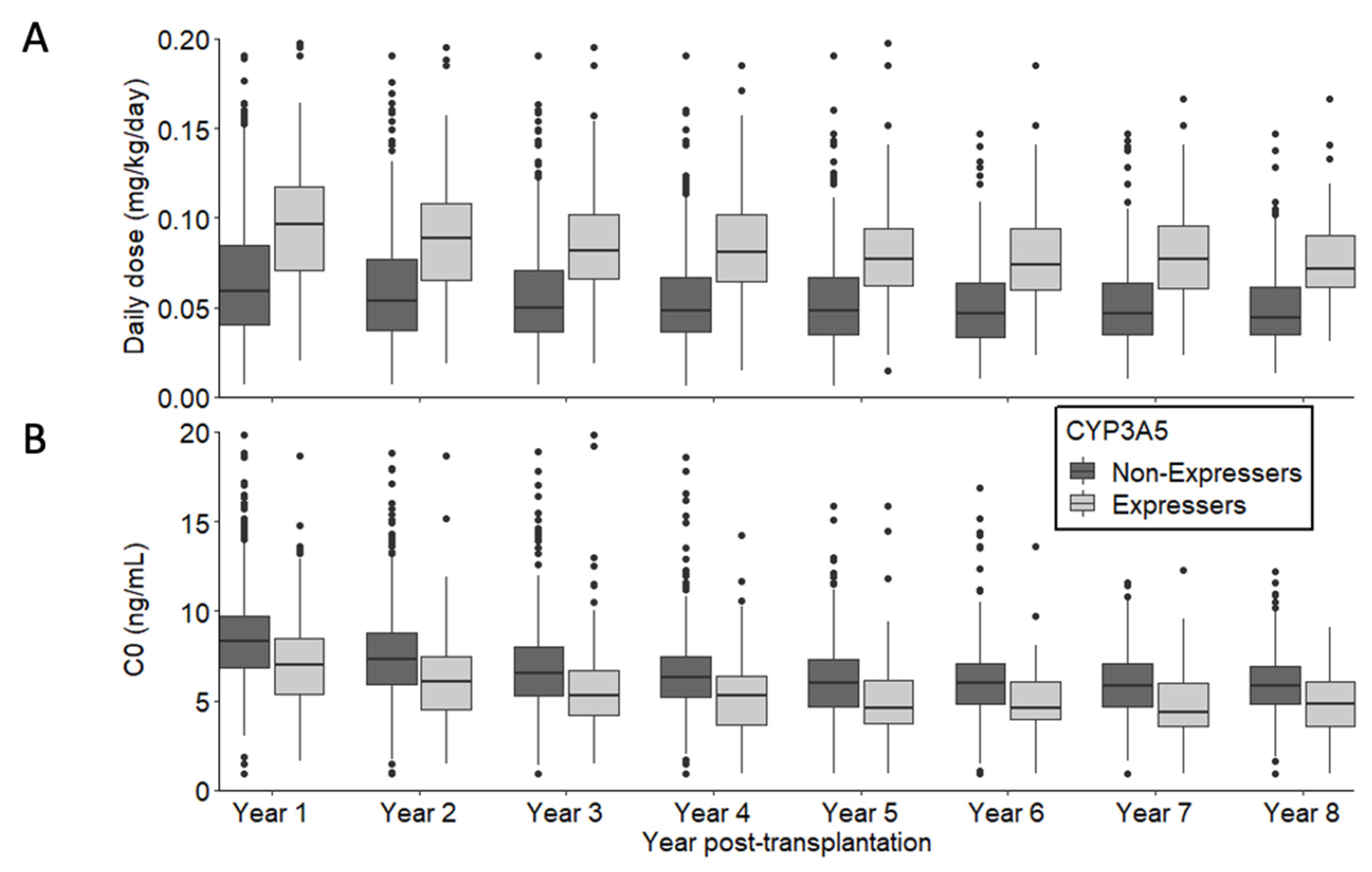
3.2. Tacrolimus Daily dose and Trough Blood Concentration
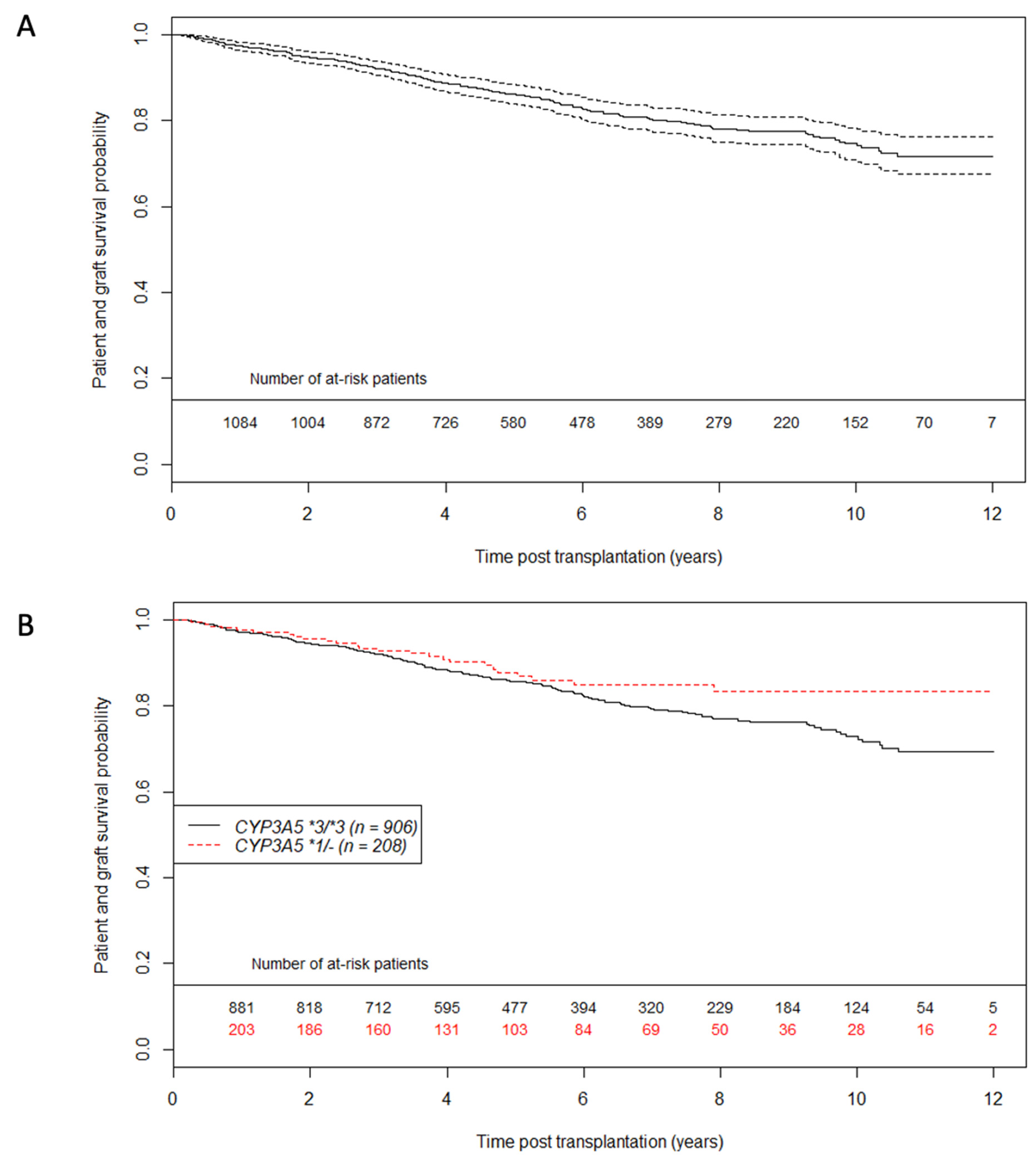
3.3. Primary Outcome: Patient—Graft Survival Analysis
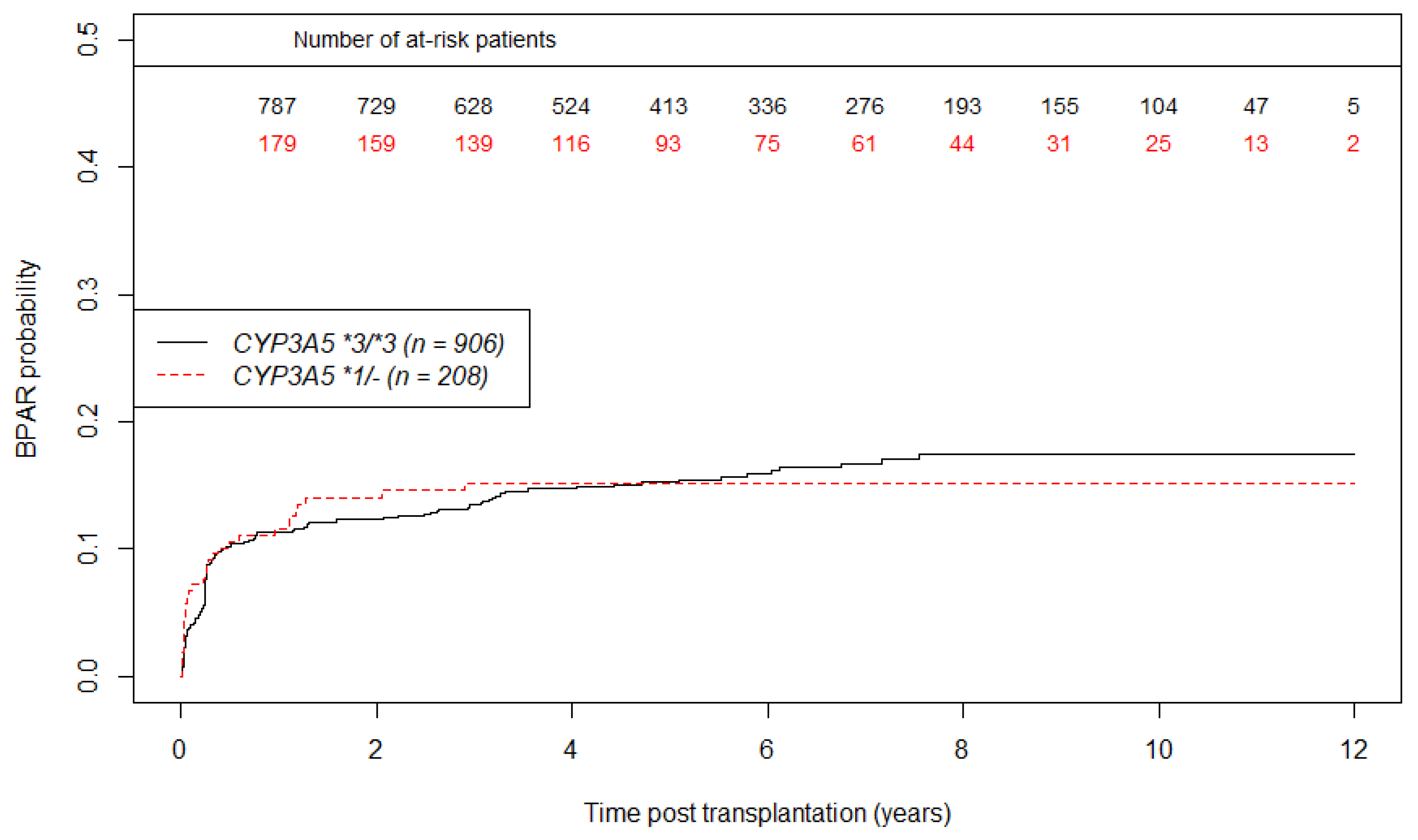
3.4. Secondary Outcomes: eGFR Evolution and BPAR Occurrence Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Stewart, D.E.; Cherikh, W.S.; Wainright, J.L.; Kucheryavaya, A.; Woodbury, M.; Snyder, J.J.; et al. OPTN/SRTR 2015 Annual Data Report: Kidney. Arab. Archaeol. Epigr. 2017, 17, 21–116. [Google Scholar] [CrossRef]
- Jouve, T.; Noble, J.; Rostaing, L.; Malvezzi, P. Tailoring tacrolimus therapy in kidney transplantation. Expert Rev. Clin. Pharmacol. 2018, 11, 581–588. [Google Scholar] [CrossRef]
- Dugast, E.; Soulillou, J.; Foucher, Y.; Papuchon, E.; Guerif, P.; Paul, C.; Riochet, D.; Chesneau, M.; Cesbron, A.; Renaudin, K.; et al. Failure of Calcineurin Inhibitor (Tacrolimus) Weaning Randomized Trial in Long-Term Stable Kidney Transplant Recipients. Arab. Archaeol. Epigr. 2016, 16, 3255–3261. [Google Scholar] [CrossRef]
- Naesens, M.; Kuypers, D.R.; Sarwal, M. Calcineurin Inhibitor Nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, P.; Navalesi, R. The metabolic effects of cyclosporin and tacrolimus. J. Endocrinol. Investig. 2000, 23, 482–490. [Google Scholar] [CrossRef]
- Ekberg, H.; Tedesco-Silva, H.; Demirbas, A.; Vítko, S.; Nashan, B.; Guerkan, A.; Margreiter, R.; Hugo, C.; Grinyó, J.M.; Frei, U.; et al. Reduced Exposure to Calcineurin Inhibitors in Renal Transplantation. N. Engl. J. Med. 2007, 357, 2562–2575. [Google Scholar] [CrossRef] [Green Version]
- Schiff, J.; Cole, E.; Cantarovich, M. Therapeutic Monitoring of Calcineurin Inhibitors for the Nephrologist. Clin. J. Am. Soc. Nephrol. 2007, 2, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yin, W.-J.; Li, D.-Y.; Ding, J.-J.; Zhou, L.-Y.; Wang, J.-L.; Ma, R.-R.; Liu, K.; Zhou, G.; Zuo, X.-C. Evaluating tacrolimus pharmacokinetic models in adult renal transplant recipients with different CYP3A5 genotypes. Eur. J. Clin. Pharmacol. 2018, 74, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Anglicheau, D.; Legendre, C.; Beaune, P.; Thervet, E. Cytochrome P450 3A polymorphisms and immunosuppressive drugs: An update. Pharmacogenomics 2007, 8, 835–849. [Google Scholar] [CrossRef]
- Terrazzino, S.; Quaglia, M.; Stratta, P.; Canonico, P.L.; Genazzani, A. The effect of CYP3A5 6986A>G and ABCB1 3435C>T on tacrolimus dose-adjusted trough levels and acute rejection rates in renal transplant patients: A systematic review and meta-analysis. Pharmacogenet. Genom. 2012, 22, 642–645. [Google Scholar] [CrossRef]
- Dai, Y.; Hebert, M.F.; Isoherranen, N.; Davis, C.L.; Marsh, C.; Shen, D.D.; Thummel, K.E. Effect of Cyp3a5polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab. Dispos. 2006, 34, 836–847. [Google Scholar] [CrossRef] [Green Version]
- Rojas, L.; Neumann, I.; Herrero, M.J.; Boso, V.; Reig, J.; Poveda, J.L.; Megías, J.; Bea, S.; Aliño, S.F. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: A systematic review and meta-analysis of observational studies. Pharmacogenomics J. 2014, 15, 38–48. [Google Scholar] [CrossRef]
- Flahault, A.; Anglicheau, D.; Loriot, M.-A.; Thervet, E.; Pallet, N. Clinical impact of the CYP3A5 6986A>G allelic variant on kidney transplantation outcomes. Pharmacogenomics 2017, 18, 165–173. [Google Scholar] [CrossRef]
- Kuypers, D.R.J.; de Jonge, H.; Naesens, M.; Lerut, E.; Verbeke, K.; Vanrenterghem, Y. CYP3A5 and CYP3A4 but not MDR1 Single-nucleotide Polymorphisms Determine Long-term Tacrolimus Disposition and Drug-related Nephrotoxicity in Renal Recipients. Clin. Pharmacol. Ther. 2007, 82, 711–725. [Google Scholar] [CrossRef]
- Glowacki, F.; Lionet, A.; Buob, D.; Labalette, M.; Allorge, D.; Provôt, F.; Hazzan, M.; Noël, C.; Broly, F.; Cauffiez, C. CYP3A5 and ABCB1 polymorphisms in donor and recipient: Impact on Tacrolimus dose requirements and clinical outcome after renal transplantation. Nephrol. Dial. Transplant. 2011, 26, 3046–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaranta, S.; Chevalier, D.; Allorge, D.; Lo-Guidice, J.M.; Migot-Nabias, F.; Kenani, A.; Imbenotte, M.; Broly, F.; Lacarelle, B.; Lhermitte, M. Ethnic differences in the distribution ofCYP3A5gene polymorphisms. Xenobiotica 2006, 36, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Arab. Archaeol. Epigr. 2016, 17, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B Methodol. 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Kuypers, D.R. Intrapatient Variability of Tacrolimus Exposure in Solid Organ Transplantation: A Novel Marker for Clinical Outcome. Clin. Pharmacol. Ther. 2019, 107, 347–358. [Google Scholar] [CrossRef]
- Laird, N.M.; Ware, J.H. Random-Effects Models for Longitudinal Data. Biometrics 1982, 38, 963. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. Last Modified in 2020. Available online: https://www.r-project.org/ (accessed on 5 January 2021).
- Komine, N.; Satoh, S.; Saito, M.; Numakura, K.; Inoue, T.; Tsuruta, H.; Narita, S.; Komatsuda, A.; Nanjo, H.; Kagaya, H.; et al. Influence of CYP3A5 genetic differences in tacrolimus on quantitative interstitial fibrosis and long-term graft function in kidney transplant recipients. Int. Immunopharmacol. 2018, 58, 57–63. [Google Scholar] [CrossRef]
- Taber, D.J.; Su, Z.; Fleming, J.; McGillicuddy, J.W.; Posadas-Salas, M.A.; Treiber, F.A.; Dubay, D.; Srinivas, T.R.; Mauldin, P.D.; Moran, W.P.; et al. Tacrolimus Trough Concentration Variability and Disparities in African American Kidney Transplantation. Transplantation 2017, 101, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Andrews, L.; Van Gelder, T.; Shi, Y.; Van Schaik, R.; Wang, L.; Hesselink, D. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: Recent developments and ethnic considerations. Expert Opin. Drug Metab. Toxicol. 2016, 12, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, M.; Van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Mesnard, L.; Muthukumar, T.; Burbach, M.; Li, C.; Shang, H.; Dadhania, D.; Lee, J.R.; Sharma, V.K.; Xiang, J.; Suberbielle, C.; et al. Exome Sequencing and Prediction of Long-Term Kidney Allograft Function. PLoS Comput. Biol. 2016, 12, e1005088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groopman, E.E.; Marasa, M.; Cameron-Christie, S.; Petrovski, S.; Aggarwal, V.S.; Rasouly, H.M.; Li, Y.; Zhang, J.; Nestor, J.; Krithivasan, P.; et al. Diagnostic Utility of Exome Sequencing for Kidney Disease. N. Engl. J. Med. 2019, 380, 142–151. [Google Scholar] [CrossRef] [PubMed]


| CYP3A5 *3/*3 N = 906 | CYP3A5 *1/- N = 208 | p-Value | Available Data | |
|---|---|---|---|---|
| Year of transplantation | 0.20 | 1114 | ||
| 232 (25.6%) | 40 (19.2%) | ||
| 239 (26.4%) | 54 (26.0%) | ||
| 284 (31.3%) | 72 (34.6%) | ||
| 151 (16.7%) | 42 (20.2%) | ||
| Recipient age (years) | 52.4 (40.1;60.3) | 49.9 (37.9;59.6) | 0.18 | 1114 |
| Recipient male | 561 (61.9%) | 127 (61.1%) | 0.88 | 1114 |
| Recipient BMI (kg/m²) | 24.4 (21.4;27.6) | 24.6 (22.0;27.4) | 0.76 | 1112 |
| Positive anti-HLA class I antibodies | 169 (18.7%) | 40 (19.2%) | 0.93 | 1114 |
| Positive anti-HLA class II antibodies | 180 (20.1%) | 47 (22.7%) | 0.47 | 1101 |
| Retransplantation | 152 (16.8%) | 35 (16.8%) | 1.00 | 1114 |
| Time spent in dialysis (years) | 2.1 (1.1;3.6) | 2.5 (1.3;4.6) | 0.02 | 1111 |
| Renal replacement therapy modality | 0.14 | 1114 | ||
| 116 (12.8%) | 18 (8.7%) | ||
| 689 (76.0%) | 171 (82.2%) | ||
| 101 (11.1%) | 19 (9.1%) | ||
| Recipient blood type | 0.36 | 1114 | ||
| 415 (45.8%) | 82 (39.4%) | ||
| 36 (4.0%) | 9 (4.3%) | ||
| 86 (9.5%) | 25 (12.0%) | ||
| 369 (40.7%) | 92 (44.2%) | ||
| Donor age (years) | 52.0 (41.0;62.0) | 51.0 (40.8;61.0) | 0.52 | 1114 |
| Donor male | 537 (59.3%) | 122 (58.7%) | 0.93 | 1114 |
| Donor BMI (kg/m²) | 25.6 (22.9;28.6) | 25.0 (22.5;28.6) | 0.46 | 1114 |
| Donor blood type | 0.24 | 1114 | ||
| 396 (43.7%) | 75 (36.1%) | ||
| 26 (2.9%) | 7 (3.4%) | ||
| 78 (8.6%) | 22 (10.6%) | ||
| 406 (44.8%) | 104 (50.0%) | ||
| Donor vital status | 0.73 | 1114 | ||
| 77 (8.5%) | 16 (7.7%) | ||
| 383 (42.3%) | 95 (45.7%) | ||
| 418 (46.1%) | 89 (42.8%) | ||
| 28 (3.1%) | 8 (3.8%) | ||
| HLA-A-B-DR incompatibilities > 4 | 221 (24.4%) | 65 (31.2%) | 0.05 | 1113 |
| Cold ischemia time (hours) | 16.0 (12.0;21.0) | 16.0 (12.0;20.0) | 0.77 | 1098 |
| Machine perfusion conservation | 175 (19.4%) | 37 (18.0%) | 0.72 | 1106 |
| HR | CI95% | p-Value | |
|---|---|---|---|
| CYP3A5 *1/- (versus CYP3A5 *3/*3) | 0.70 | (0.46; 1.07) | 0.10 |
| Recipient age > 60 years old (yes versus no) | 2.13 | (1.46; 3.12) | <0.01 |
| Donor age > 60 years old (yes versus no) | 1.62 | (1.10; 2.37) | 0.01 |
| Male recipient (yes versus no) | 1.38 | (1.02; 1.89) | 0.04 |
| Retransplantation (yes versus no) | 1.52 | (1.02; 2.26) | 0.04 |
| Renal replacement therapy modality | |||
| Ref. | ||
| 1.10 | (0.69; 1.75) | 0.68 |
| 0.38 | (0.15; 0.97) | 0.04 |
| Time spent in dialysis (per 1 year) | 1.04 | (1.01; 1.07) | < 0.01 |
| Donor vital status | |||
| Ref. | ||
| 1.53 | (0.60; 3.88) | 0.37 |
| 1.79 | (0.71; 4.53) | 0.22 |
| 3.44 | (1.10; 10.74) | 0.03 |
| Cold ischemia time (per 10 h) | 1.09 | (0.86; 1.38) | 0.49 |
| Occurrence of BPAR (yes versus no) | 2.69 | (1.95; 3.71) | <0.01 |
| Association with 1-year Egfr (Baseline Effect) | Association with eGFR Evolution from 1 year Post Transplantation (Slope Effect) | |||||
|---|---|---|---|---|---|---|
| Coefficients | CI95% | p-Value | Coefficients | CI95% | p-Value | |
| Referential value | 99.95 | (89.49; 110.41) | <0.01 | −10.40 | (−15.88; −4.93) | <0.01 |
| CYP3A5 *1/- (ref: CYP3A5 *3/*3) | −0.87 | (−4.56; 2.82) | 0.64 | 2.57 | (0.38; 4.75) | 0.02 |
| Recipient age (years) | −0.10 | (−0.24; 0.03) | 0.15 | 0.08 | (0.02; 0.15) | 0.01 |
| Male recipient (yes versus non) | 1.26 | (−1.77; 4.28) | 0.42 | 1.84 | (0.05; 3.63) | 0.04 |
| Recipient BMI (kg/m²) | −0.42 | (−0.64; −0.20) | <0.01 | |||
| Renal replacement therapy modality (ref: peritoneal dialysis) | ||||||
| 5.18 | (0.7; 9.65) | 0.02 | −4.09 | (−6.72; −1.47) | <0.01 |
| −3.54 | (−9.7; 2.62) | 0.26 | 2.66 | (−0.94; 6.26) | 0.15 |
| Time spent in dialysis (years) | 0.35 | (−0.01; 0.71) | 0.06 | −0.24 | (−0.45; −0.03) | 0.03 |
| Anti-HLA class II antibodies (yes versus no) | 6.48 | (2.71; 10.25) | <0.01 | −5.08 | (−7.32; −2.84) | <0.01 |
| Donor age (years) | −0.57 | (−0.67; −0.48) | <0.01 | |||
| Donor BMI (kg/m²) | −0.21 | (−0.47; 0.06) | 0.13 | 0.21 | (0.05; 0.37) | 0.01 |
| Donor vital status (ref: living donor) | ||||||
| −3.20 | (−6.78; 0.37) | 0.08 | |||
| −4.34 | (−7.97; −0.72) | 0.02 | |||
| −11.76 | (−17.69; −5.83) | <0.01 | |||
| HR | CI95% | p-Value | |
|---|---|---|---|
| CYP3A5 *1/- (versus CYP3A5 *3/*3) | 1.01 | (0.68; 1.49) | 0.97 |
| Male donor (yes versus no) | 0.64 | (0.47; 0.86) | <0.01 |
| HLA-A-B-DR incompatibilities > 4 (yes versus no) | 1.23 | (0.87; 1.74) | 0.24 |
| Positive anti-HLA class II antibodies (yes versus no) | 1.41 | (1.00; 2.01) | 0.05 |
| Cold ischemia time (per 10 hours) | 1.46 | (1.19; 1.80) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenain, R.; Maanaoui, M.; Hamroun, A.; Larrue, R.; Van Der Hauwaert, C.; Gibier, J.-B.; Gnemmi, V.; Gomis, S.; Labalette, M.; Broly, F.; et al. Impact of Tacrolimus Daily Dose Limitation in Renal Transplant Recipients Expressing CYP3A5: A Retrospective Study. J. Pers. Med. 2021, 11, 1002. https://doi.org/10.3390/jpm11101002
Lenain R, Maanaoui M, Hamroun A, Larrue R, Van Der Hauwaert C, Gibier J-B, Gnemmi V, Gomis S, Labalette M, Broly F, et al. Impact of Tacrolimus Daily Dose Limitation in Renal Transplant Recipients Expressing CYP3A5: A Retrospective Study. Journal of Personalized Medicine. 2021; 11(10):1002. https://doi.org/10.3390/jpm11101002
Chicago/Turabian StyleLenain, Rémi, Mehdi Maanaoui, Aghilès Hamroun, Romain Larrue, Cynthia Van Der Hauwaert, Jean-Baptiste Gibier, Viviane Gnemmi, Sébastien Gomis, Myriam Labalette, Franck Broly, and et al. 2021. "Impact of Tacrolimus Daily Dose Limitation in Renal Transplant Recipients Expressing CYP3A5: A Retrospective Study" Journal of Personalized Medicine 11, no. 10: 1002. https://doi.org/10.3390/jpm11101002
APA StyleLenain, R., Maanaoui, M., Hamroun, A., Larrue, R., Van Der Hauwaert, C., Gibier, J.-B., Gnemmi, V., Gomis, S., Labalette, M., Broly, F., Hennart, B., Pottier, N., Hazzan, M., Cauffiez, C., & Glowacki, F. (2021). Impact of Tacrolimus Daily Dose Limitation in Renal Transplant Recipients Expressing CYP3A5: A Retrospective Study. Journal of Personalized Medicine, 11(10), 1002. https://doi.org/10.3390/jpm11101002






