Genomic Screening Identifies Individuals at High Risk for Hereditary Transthyretin Amyloidosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Population
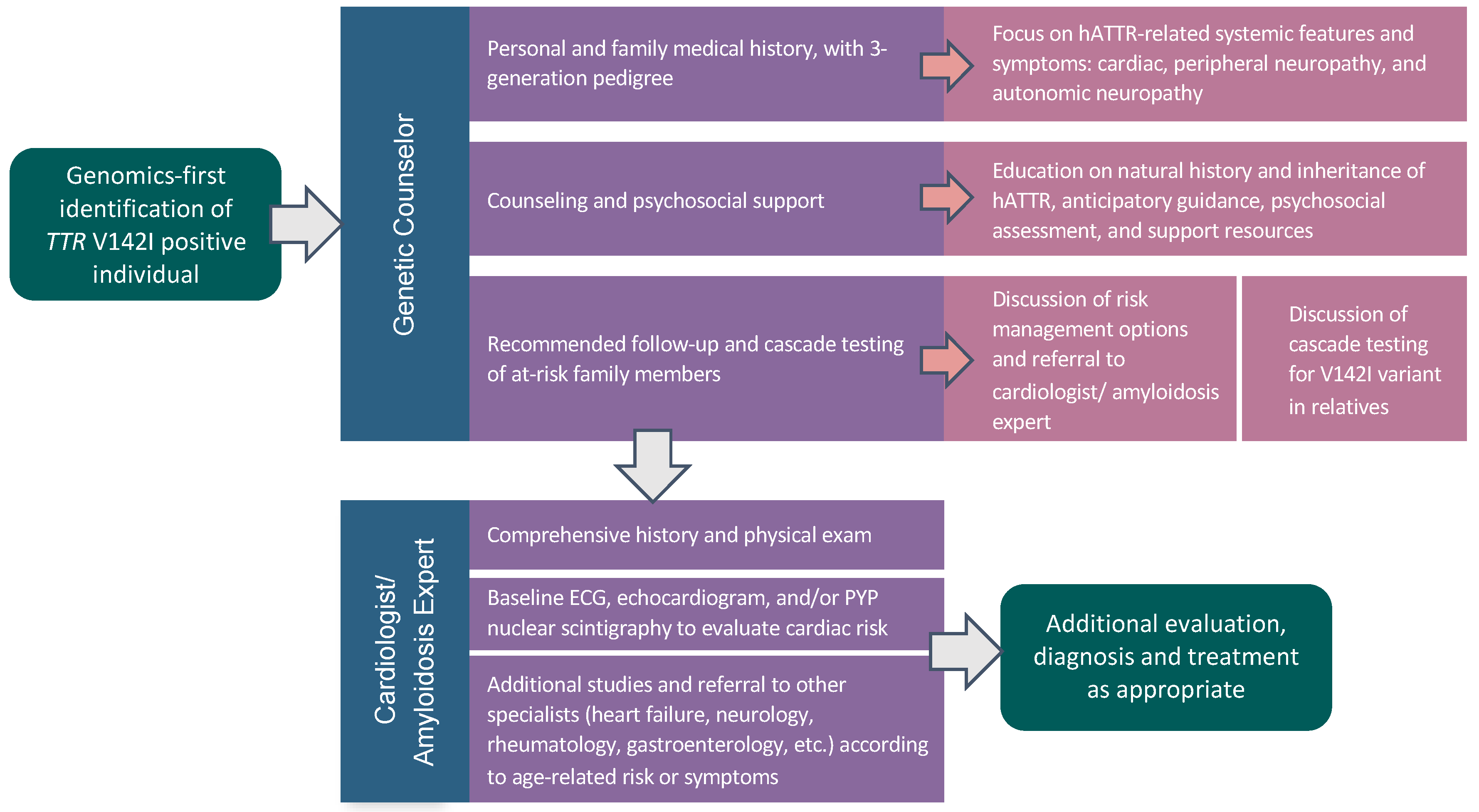
2.2. Pilot Genomic Screening Program
2.3. TTR V142I Results Disclosure
2.4. Characteristics of V142I-Positive Individuals at Result Disclosure
2.5. Post-Result Disclosure Follow-Up and Outcomes
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, M.F.; Giovanni, M.A. Bringing monogenic disease screening to the clinic. Nat. Med. 2020, 26, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, A.H.; Lester Kirchner, H.; Schwartz, M.L.B.; Kelly, M.A.; Schmidlen, T.; Jones, L.K.; Hallquist, M.L.G.; Rocha, H.; Betts, M.; Schwiter, R.; et al. Clinical outcomes of a genomic screening program for actionable genetic conditions. Genet. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Grzymski, J.J.; Elhanan, G.; Morales Rosado, J.A.; Smith, E.; Schlauch, K.A.; Read, R.; Rowan, C.; Slotnick, N.; Dabe, S.; Metcalf, W.J.; et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat. Med. 2020, 26, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.A.; Amendola, L.M.; Kuchta, K.; Dunnenberger, H.M.; Thompson, J.; Johnson, C.; Ilbawi, N.; Oshman, L.; Hulick, P.J. Primary Care Physician Experiences with Integrated Population-Scale Genetic Testing: A Mixed-Methods Assessment. J Pers. Med. 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Soper, E.R.; Odgis, J.A.; Cullina, S.; Bobo, D.; Moscati, A.; Rodriguez, J.E.; Team, C.G.; Regeneron Genetics, C.; Loos, R.J.F.; et al. Exome sequencing reveals a high prevalence of BRCA1 and BRCA2 founder variants in a diverse population-based biobank. Genome Med. 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Manickam, K.; Jones, L.K.; Wright, E.A.; Hartzel, D.N.; Gonzaga-Jauregui, C.; O’Dushlaine, C.; Leader, J.B.; Lester Kirchner, H.; Lindbuchler, D.a.M.; et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science 2016, 354. [Google Scholar] [CrossRef]
- Manickam, K.; Buchanan, A.H.; Schwartz, M.L.B.; Hallquist, M.L.G.; Williams, J.L.; Rahm, A.K.; Rocha, H.; Savatt, J.M.; Evans, A.E.; Butry, L.M.; et al. Exome sequencing-based screening for BRCA1/2 expected pathogenic variants among adult biobank participants. JAMA Netw. Open 2018, 1, e182140. [Google Scholar] [CrossRef]
- Buchanan, A.H.; Manickam, K.; Meyer, M.N.; Wagner, J.K.; Hallquist, M.L.G.; Williams, J.L.; Rahm, A.K.; Williams, M.S.; Chen, Z.-M.E.; Shah, C.K.; et al. Early cancer diagnoses through BRCA1/2 screening of unselected adult biobank participants. Genet. Med. 2018, 20, 554–558. [Google Scholar] [CrossRef]
- Rowczenio, D.M.; Noor, I.; Gillmore, J.D.; Lachmann, H.J.; Whelan, C.; Hawkins, P.N.; Obici, L.; Westermark, P.; Grateau, G.; Wechalekar, A.D. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum. Mutat. 2014, 35, E2403–E2412. [Google Scholar] [CrossRef]
- Sekijima, Y. Hereditary Transthyretin Amyloidosis. In GeneReviews; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 2001. [Google Scholar]
- Maurer, M.S.; Bokhari, S.; Damy, T.; Dorbala, S.; Drachman, B.M.; Fontana, M.; Grogan, M.; Kristen, A.V.; Lousada, I.; Nativi-Nicolau, J.; et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ. Heart Fail. 2019, 12, e006075. [Google Scholar] [CrossRef]
- Hawkins, P.N.; Ando, Y.; Dispenzeri, A.; Gonzalez-Duarte, A.; Adams, D.; Suhr, O.B. Evolving landscape in the management of transthyretin amyloidosis. Ann. Med. 2015, 47, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Kumar Dharmarajan, M.S.M. Transthyretin cardiac amyloidoses in older North Americans. J. Am. Geriatr. Soc. 2012, 60, 765. [Google Scholar] [CrossRef] [PubMed]
- Lahuerta Pueyo, C.; Aibar Arregui, M.Á.; Gracia Gutierrez, A.; Bueno Juana, E.; Menao Guillén, S. Estimating the prevalence of allelic variants in the transthyretin gene by analysing large-scale sequencing data. Eur. J. Hum. Genet. 2019, 27, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, D.R.; Alexander, A.A.; Tagoe, C.; Garvey, W.T.; Williams, S.M.; Tishkoff, S.; Modiano, D.; Sirima, S.B.; Kalidi, I.; Toure, A.; et al. The prevalence and distribution of the amyloidogenic transthyretin (TTR) V122I allele in Africa. Mol. Genet. Genomic. Med. 2016, 4, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hamidi Asl, K.; Yazaki, M.; Benson, M.D. A prospective evaluation of the transthyretin Ile122 allele frequency in an African-American population. Amyloid 2005, 12, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Damrauer, S.M.; Chaudhary, K.; Cho, J.H.; Liang, L.W.; Argulian, E.; Chan, L.; Dobbyn, A.; Guerraty, M.A.; Judy, R.; Kay, J.; et al. Association of the V122I Hereditary Transthyretin Amyloidosis Genetic Variant With Heart Failure Among Individuals of African or Hispanic/Latino Ancestry. JAMA 2019, 322, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, D.R.; Pastore, R.D.; Yaghoubian, R.; Kane, I.; Gallo, G.; Buck, F.S.; Buxbaum, J.N. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N. Engl. J. Med. 1997, 336, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.C.; Buxbaum, J.N.; Shah, A.M.; Falk, R.H.; Claggett, B.; Kitzman, D.W.; Mosley, T.H.; Butler, K.R.; Boerwinkle, E.; Solomon, S.D. The amyloidogenic V122I transthyretin variant in elderly black Americans. N. Engl. J. Med. 2015, 372, 21–29. [Google Scholar] [CrossRef]
- Carr, A.S.; Pelayo-Negro, A.L.; Jaunmuktane, Z.; Scalco, R.S.; Hutt, D.; Evans, M.R.B.; Heally, E.; Brandner, S.; Holton, J.; Blake, J.; et al. Transthyretin V122I amyloidosis with clinical and histological evidence of amyloid neuropathy and myopathy. Neuromuscul. Disord. 2015, 25, 511–515. [Google Scholar] [CrossRef]
- Maurer, M.S.; Hanna, M.; Grogan, M.; Dispenzieri, A.; Witteles, R.; Drachman, B.; Judge, D.P.; Lenihan, D.J.; Gottlieb, S.S.; Shah, S.J.; et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J. Am. Coll. Cardiol. 2016, 68, 161–172. [Google Scholar] [CrossRef]
- Yanagisawa, A.; Ueda, M.; Sueyoshi, T.; Okada, T.; Fujimoto, T.; Ogi, Y.; Kitagawa, K.; Tasaki, M.; Misumi, Y.; Oshima, T.; et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod. Pathol. 2015, 28, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Aus dem Siepen, F.; Hein, S.; Prestel, S.; Baumgärtner, C.; Schönland, S.; Hegenbart, U.; Röcken, C.; Katus, H.A.; Kristen, A.V. Carpal tunnel syndrome and spinal canal stenosis: Harbingers of transthyretin amyloid cardiomyopathy? Clin. Res. Cardiol. 2019, 108, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Sekijima, Y.; Yazaki, M.; Tojo, K.; Yoshinaga, T.; Doden, T.; Koyama, J.; Yanagisawa, S.; Ikeda, S.-I. Carpal tunnel syndrome: A common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid 2016, 23, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Sperry, B.W.; Reyes, B.A.; Ikram, A.; Donnelly, J.P.; Phelan, D.; Jaber, W.A.; Shapiro, D.; Evans, P.J.; Maschke, S.; Kilpatrick, S.E.; et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J. Am. Coll. Cardiol. 2018, 72, 2040–2050. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Office of the Commissioner. FDA Approves New Treatments for Heart Disease Caused by A Serious Rare Disease, Transthyretin Mediated Amyloidosis. FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatments-heart-disease-caused-serious-rare-disease-transthyretin-mediated#:~:text=On%20May%203%2C%20the%20U.S.,approved%20treatments%20for%20ATTR%2DCM (accessed on 20 December 2020).
- Abul-Husn, N.S.; Soper, E.R.; Braganza, G.T.; Rodriguez, J.E.; Zeid, N.; Cullina, S.; Bobo, D.; Moscati, A.; Merkelson, A.; Loos, R.J.F.; et al. Implementing genomic screening in diverse populations. medRxiv 2021. [Google Scholar] [CrossRef]
- Belbin, G.M.; Wenric, S.; Cullina, S.; Glicksberg, B.S.; Moscati, A.; Wojcik, G.L.; Shemirani, R.; Beckmann, N.D.; Cohain, A.; Sorokin, E.P.; et al. Towards a fine-scale population health monitoring system. bioRxiv 2019. [Google Scholar] [CrossRef]
- Tier 1 Genomics Applications and their Importance to Public Health | CDC. Available online: https://www.cdc.gov/genomics/implementation/toolkit/tier1.htm (accessed on 20 December 2020).
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef]
- Sema4: A Patient-Centered Health Intelligence Company. Available online: https://sema4.com/ (accessed on 20 December 2020).
- Gertz, M.A.; Mauermann, M.L.; Grogan, M.; Coelho, T. Advances in the treatment of hereditary transthyretin amyloidosis: A review. Brain Behav. 2019, 9, e01371. [Google Scholar] [CrossRef] [PubMed]
- Conceição, I.; González-Duarte, A.; Obici, L.; Schmidt, H.H.J.; Simoneau, D.; Ong, M.-L.; Amass, L. “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J. Peripher. Nerv. Syst. 2016, 21, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Popejoy, A.B.; Fullerton, S.M. Genomics is failing on diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.; Alexander, A.; Koziol, J.; Tagoe, C.; Fox, E.; Kitzman, D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am. Heart J. 2010, 159, 864–870. [Google Scholar] [CrossRef]
- Buxbaum, J.N.; Ruberg, F.L. Transthyretin V122I (pV142I)* cardiac amyloidosis: An age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet. Med. 2017, 19, 733–742. [Google Scholar] [CrossRef]
- Dungu, J.N.; Papadopoulou, S.A.; Wykes, K.; Mahmood, I.; Marshall, J.; Valencia, O.; Fontana, M.; Whelan, C.J.; Gillmore, J.D.; Hawkins, P.N.; et al. Afro-Caribbean heart failure in the United Kingdom: Cause, outcomes, and ATTR V122I cardiac amyloidosis. Circ. Heart Fail. 2016, 9. [Google Scholar] [CrossRef]
- Connors, L.H.; Prokaeva, T.; Lim, A.; Théberge, R.; Falk, R.H.; Doros, G.; Berg, A.; Costello, C.E.; O’Hara, C.; Seldin, D.C.; et al. Cardiac amyloidosis in African Americans: Comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am. Heart J. 2009, 158, 607–614. [Google Scholar] [CrossRef]
- Gopal, D.M.; Ruberg, F.L.; Siddiqi, O.K. Impact of genetic testing in transthyretin (ATTR) cardiac amyloidosis. Curr. Heart Fail. Rep. 2019, 16, 180–188. [Google Scholar] [CrossRef]
- Grandis, M.; Obici, L.; Luigetti, M.; Briani, C.; Benedicenti, F.; Bisogni, G.; Canepa, M.; Cappelli, F.; Danesino, C.; Fabrizi, G.M.; et al. Recommendations for pre-symptomatic genetic testing for hereditary transthyretin amyloidosis in the era of effective therapy: A multicenter Italian consensus. Orphanet. J. Rare Dis. 2020, 15, 348. [Google Scholar] [CrossRef]
- 23andMe. A New 23andMe Genetic Health Risk Report Brings to Light Underdiagnosed Condition—23andMe Blog. Available online: https://blog.23andme.com/health-traits/a-new-23andme-genetic-health-risk-report-brings-to-light-underdiagnosed-condition/ (accessed on 20 December 2020).
- Conceição, I.; Damy, T.; Romero, M.; Galán, L.; Attarian, S.; Luigetti, M.; Sadeh, M.; Sarafov, S.; Tournev, I.; Ueda, M. Early diagnosis of ATTR amyloidosis through targeted follow-up of identified carriers of TTR gene mutations. Amyloid 2019, 26, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Lorenzini, M.; Longhi, S.; Milandri, A.; Gagliardi, C.; Bartolomei, I.; Salvi, F.; Maurer, M.S. Cardiac amyloidosis: The great pretender. Heart Fail. Rev. 2015, 20, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sirugo, G.; Williams, S.M.; Tishkoff, S.A. The missing diversity in human genetic studies. Cell 2019, 177, 26–31. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Participants (N = 32) |
|---|---|
| Age, median (range) | 57 (30–79) |
| Female, No. (%) | 26 (81) |
| Self-reported race/ethnicity, No. (%) | |
| African American/African | 17 (53) |
| Hispanic/Latinx | 15 (47) |
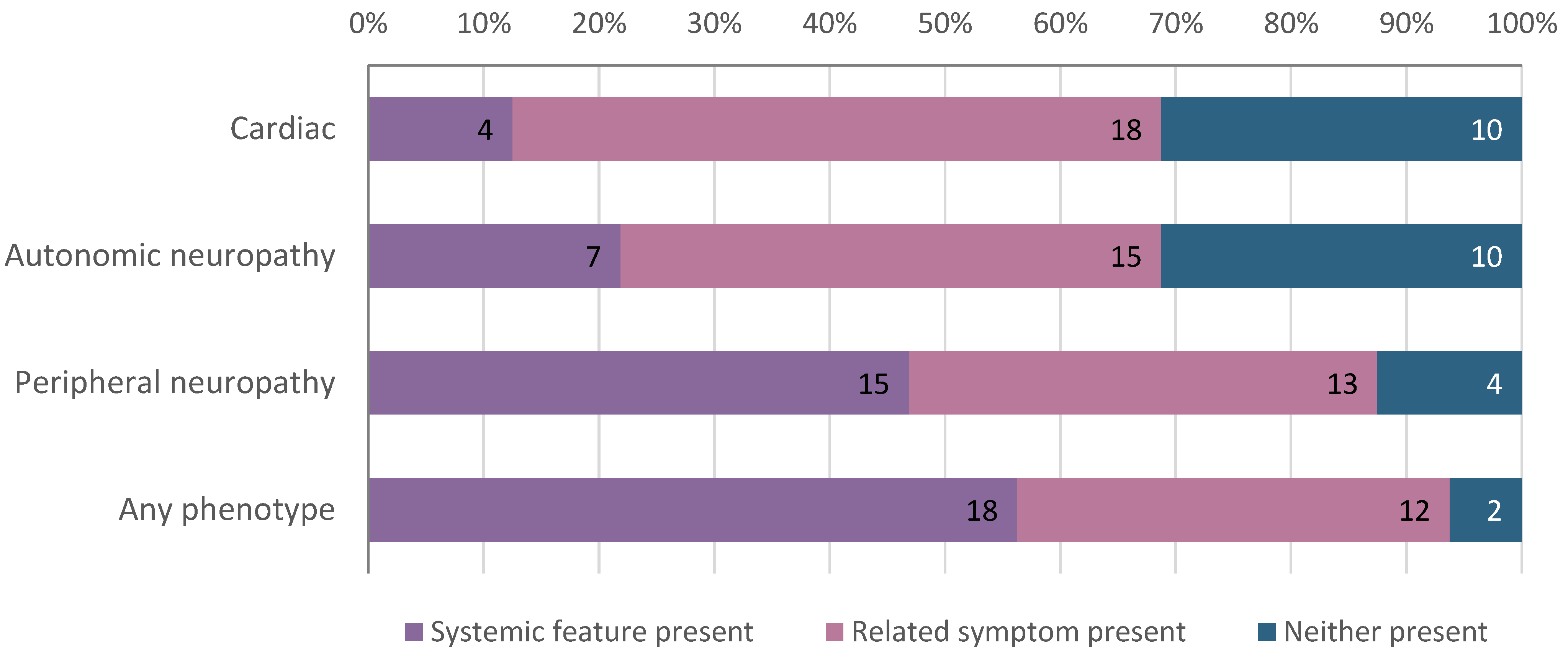
| Personal history of hATTR-related feature, No. (%) | 18 (56) |
| Family history of hATTR-related feature, No. (%) | 15 (47) |
| Follow-Up with Specialists (N = 32) | No. (%) | Weeks Post-Results Disclosure, Median (Range) |
|---|---|---|
| Cardiologist/Cardiovascular geneticist | 18 (56) | 4.9 (0.4–34.9) |
| Heart failure specialist | 2 (6) | 15.4 (1.9–28.9) |
| Neurologist | 2 (6) | 27.4 (16.0–38.7) |
| Interventions among Individuals Seen by a Specialist (N = 16) | No.* (%) | Weeks Post-Results Disclosure, Median (Range) |
| ECG | 10 of 12 (83) | 3.0 (0.4–20.0) |
| Echocardiogram | 12 of 16 (75) | 13.5 (2.1–41.0) |
| Tc-99m-PYP scintigraphy | 10 of 13 (77) | 8.3 (2.1–38.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soper, E.R.; Suckiel, S.A.; Braganza, G.T.; Kontorovich, A.R.; Kenny, E.E.; Abul-Husn, N.S. Genomic Screening Identifies Individuals at High Risk for Hereditary Transthyretin Amyloidosis. J. Pers. Med. 2021, 11, 49. https://doi.org/10.3390/jpm11010049
Soper ER, Suckiel SA, Braganza GT, Kontorovich AR, Kenny EE, Abul-Husn NS. Genomic Screening Identifies Individuals at High Risk for Hereditary Transthyretin Amyloidosis. Journal of Personalized Medicine. 2021; 11(1):49. https://doi.org/10.3390/jpm11010049
Chicago/Turabian StyleSoper, Emily R., Sabrina A. Suckiel, Giovanna T. Braganza, Amy R. Kontorovich, Eimear E. Kenny, and Noura S. Abul-Husn. 2021. "Genomic Screening Identifies Individuals at High Risk for Hereditary Transthyretin Amyloidosis" Journal of Personalized Medicine 11, no. 1: 49. https://doi.org/10.3390/jpm11010049
APA StyleSoper, E. R., Suckiel, S. A., Braganza, G. T., Kontorovich, A. R., Kenny, E. E., & Abul-Husn, N. S. (2021). Genomic Screening Identifies Individuals at High Risk for Hereditary Transthyretin Amyloidosis. Journal of Personalized Medicine, 11(1), 49. https://doi.org/10.3390/jpm11010049








