Anti-Ageing Protein β-Klotho Rejuvenates Diabetic Stem Cells for Improved Gene-Activated Scaffold Based Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. SDF Plasmid Formulation and Preparation of Polyplex Vector
2.2. Expansion and Pre-Treatment of Normal and Diabetic ADSCs with β-Klotho
2.3. Development of SDF Gene-Activated Collagen-CS Scaffolds
2.4. Analysis of Soluble Factors
2.5. Quantitative Real-Time Polymerase Reaction (qRT-PCR) Analysis of Normal and Diabetic ADSCs on SDF-GAS
2.6. Immunofluorescent Imaging of Normal and Diabetic ADSCs on SDF-GAS and Quantification of Expressed Proteins
2.7. Statistical Analysis
3. Results
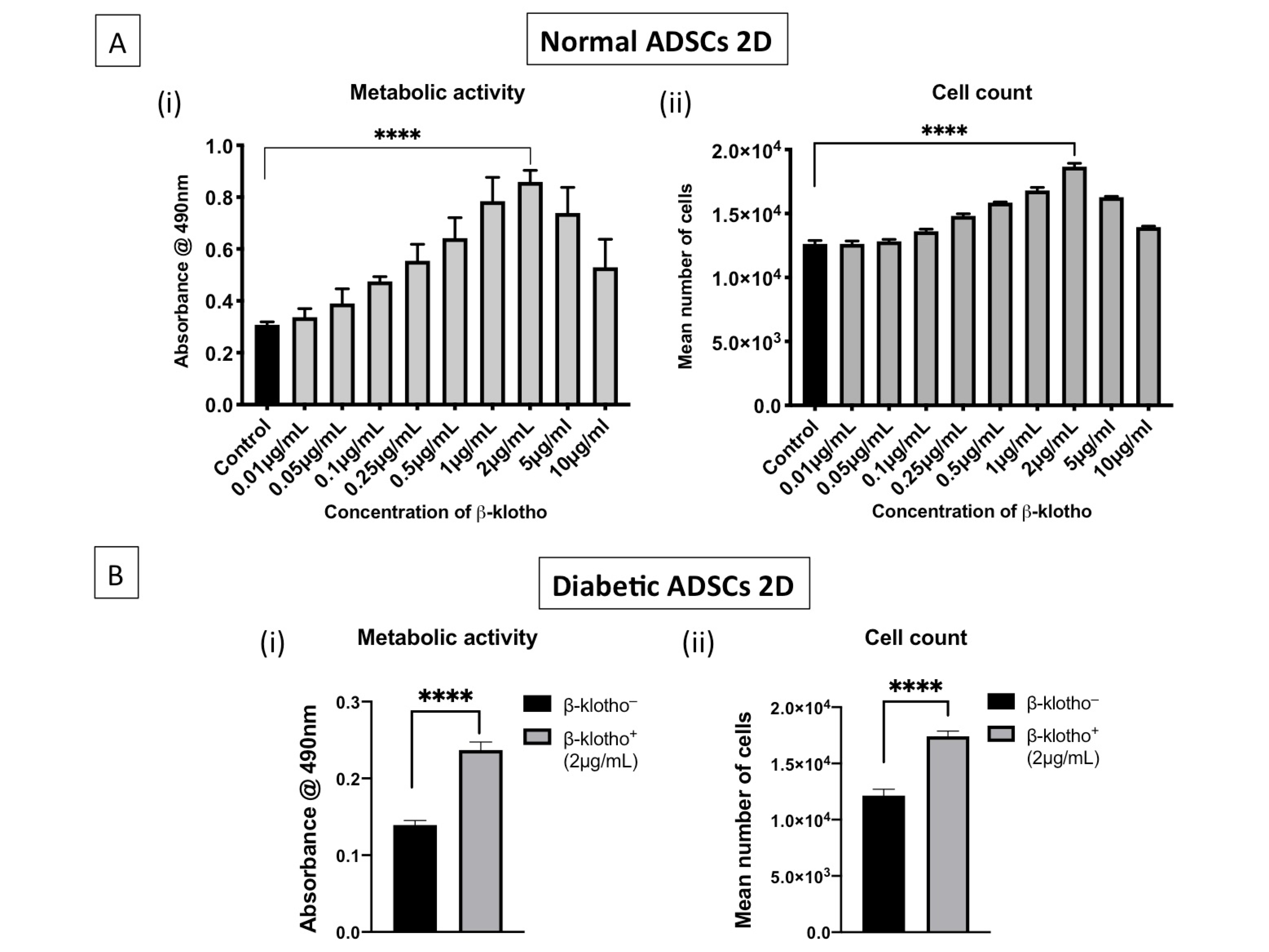
3.1. β-Klotho Pre-Treatment Increased the Proliferation and Viability of Normal and Diabetic ADSCs in 2D
3.2. β-Klotho Did Not Impede the Expression of SDF-1α and CXCR7
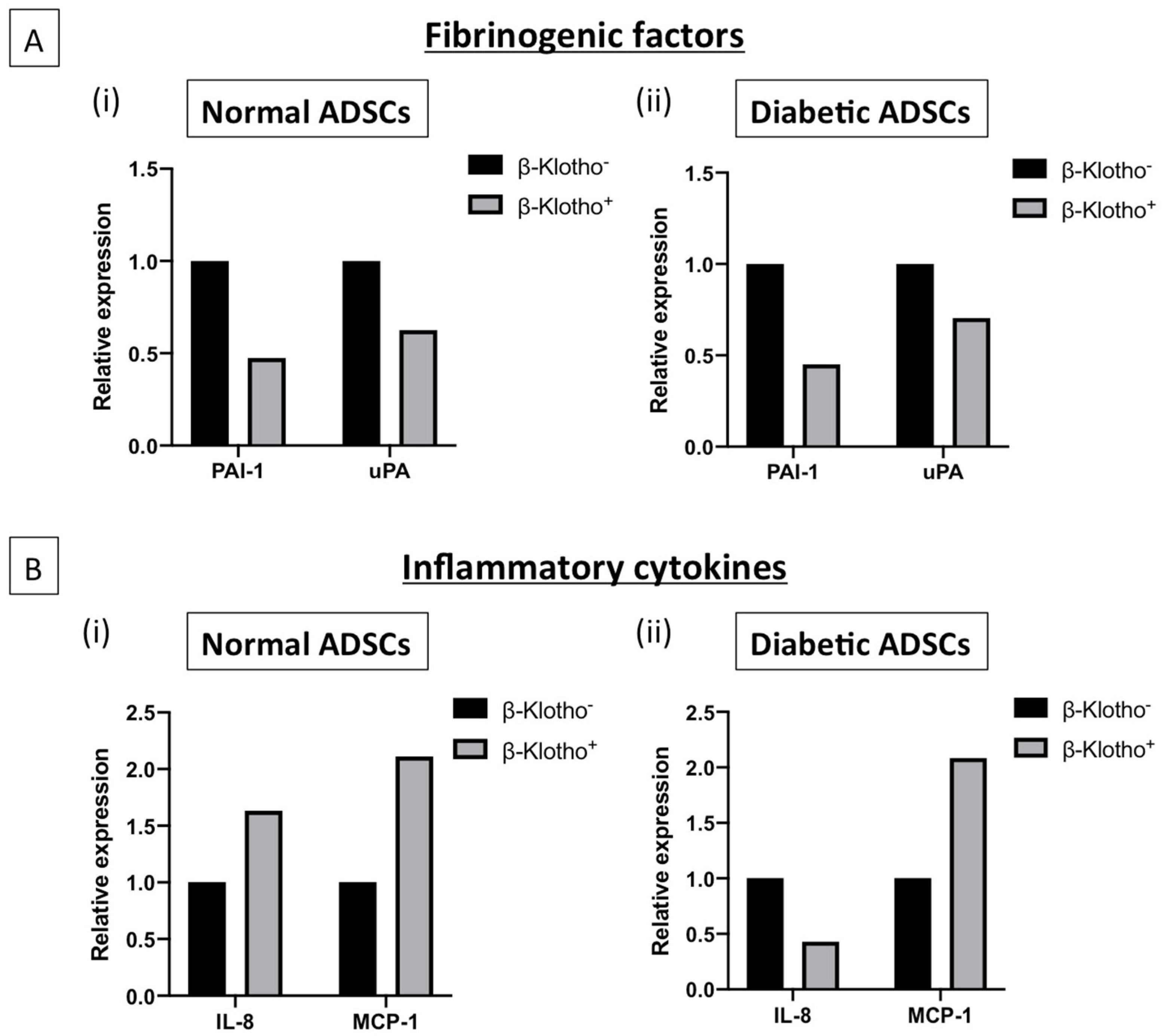
3.3. β-Klotho Pre-Treatment Reduced the Production of Fibrinogenic Factor Plasminogen Activator Inhibitor-1 and Attenuated Interleukin-8 Production in Diabetic ADSCs on SDF-GAS
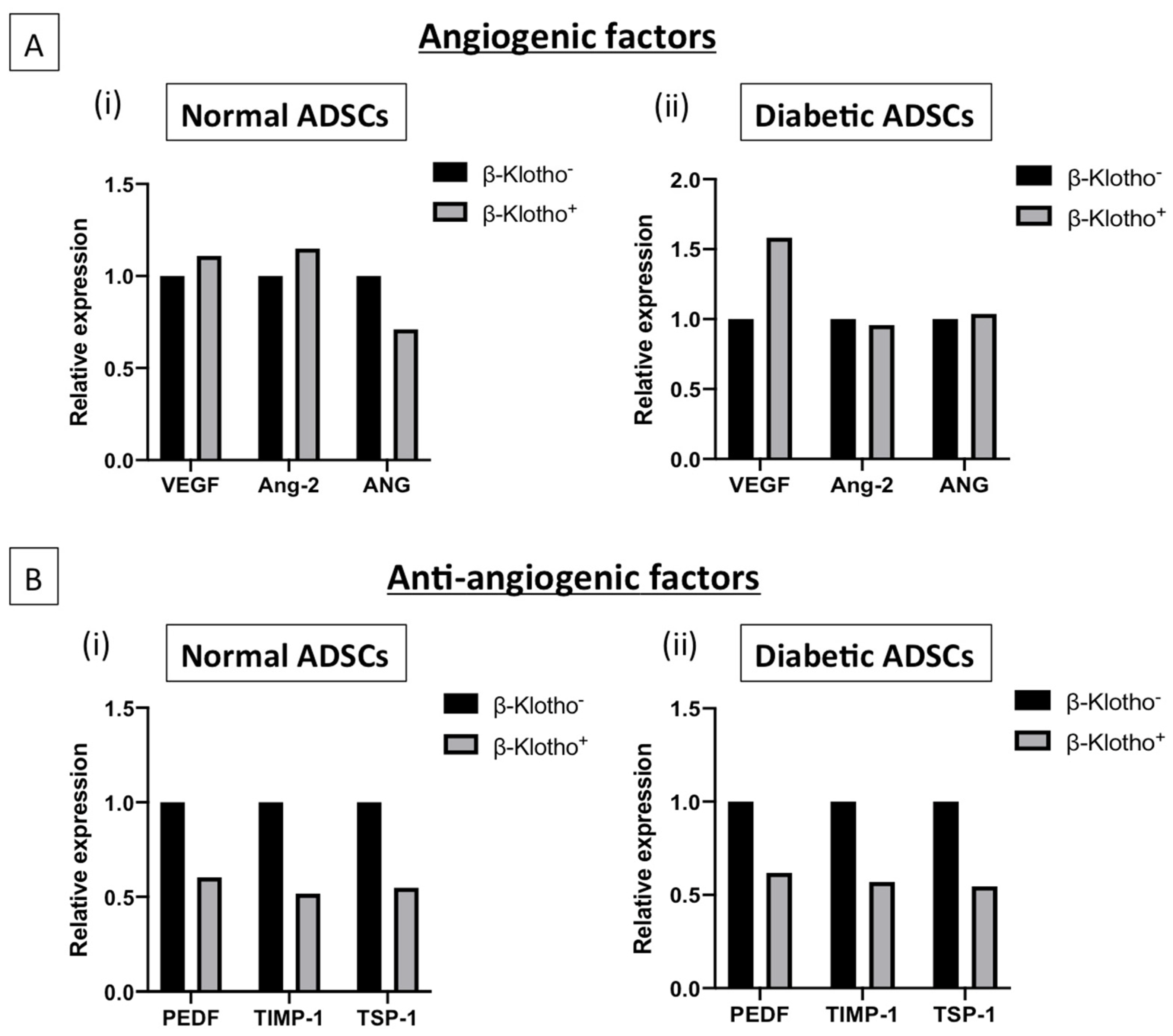
3.4. β-Klotho Pre-Treatment Facilitated Sustained Expression of Angiogenic Factors and Decreased the Expression of Anti-Angiogenic Factors in Diabetic ADSCs on SDF-GAS
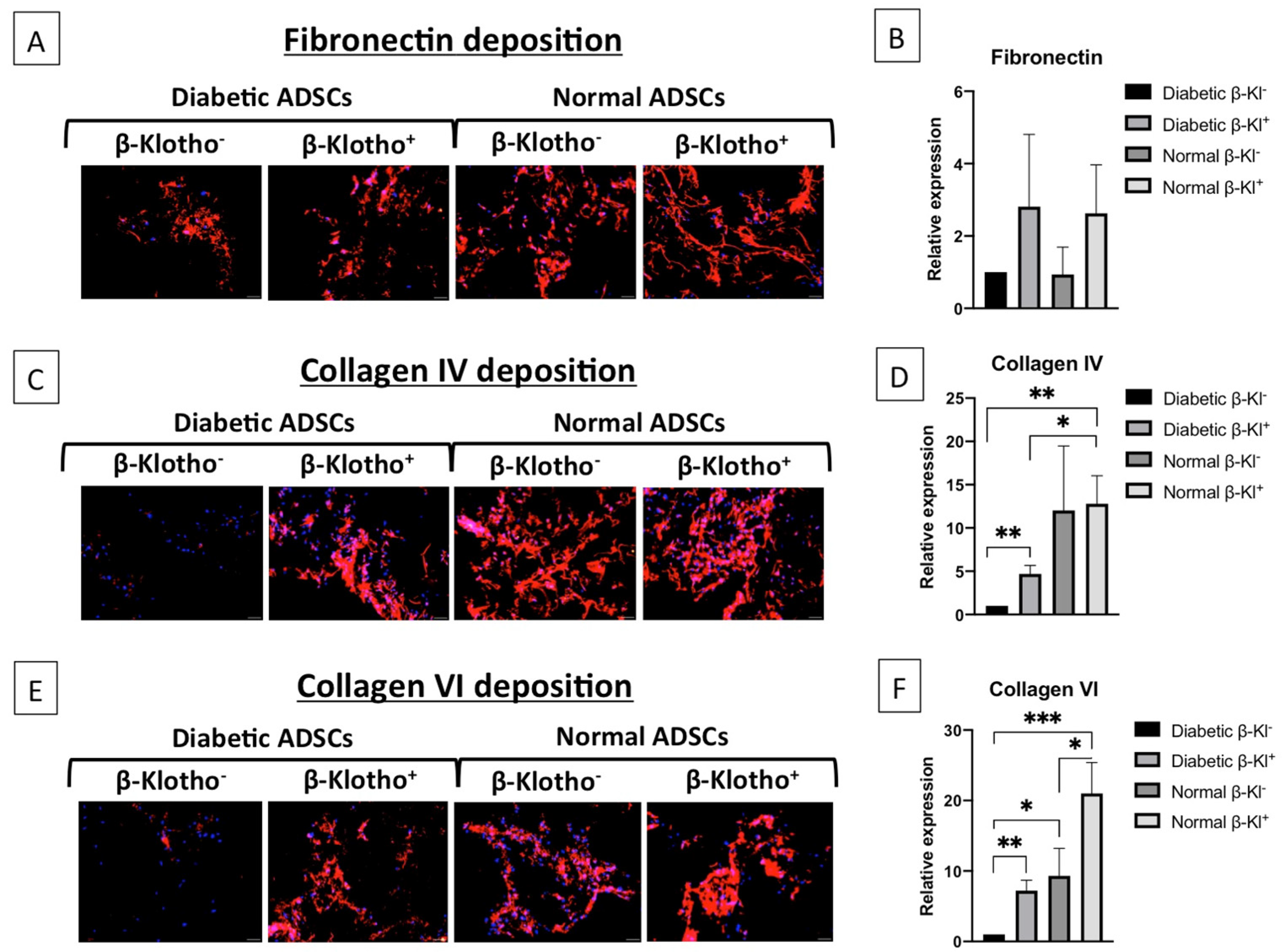
3.5. β-Klotho Pre-Treatment Improved the Expression of ECM Proteins in Diabetic ADSCs on SDF-GAS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed]
- Sargen, M.R.; Hoffstad, O.; Margolis, D.J. Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. J. Diabetes Complicat. 2013, 27, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Ramachandran, B.; Chakraborty, S.; Kannan, R.; Lonchin, S.; Muthuvijayan, V. Accelerated Healing of Diabetic Wounds Treated with L-Glutamic acid Loaded Hydrogels Through Enhanced Collagen Deposition and Angiogenesis: An In Vivo Study. Sci. Rep. 2017, 7, 10701. [Google Scholar] [CrossRef]
- Rennert, R.; Sorkin, M.; Januszyk, M.; Duscher, D.; Kosaraju, R.; Chung, M.T.; Lennon, J.; Radiya-Dixit, A.; Raghvendra, S.; Maan, Z.N.; et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res. Ther. 2014, 5, 79. [Google Scholar] [CrossRef]
- Frame, J.D.; Still, J.; Lakhel-LeCoadou, A.; Carstens, M.H.; Lorenz, C.; Orlet, H.; Spence, R.; Berger, A.C.; Dantzer, E.; Burd, A. Use of Dermal Regeneration Template in Contracture Release Procedures: A Multicenter Evaluation. Plast. Reconstr. Surg. 2004, 113, 1330–1338. [Google Scholar] [CrossRef]
- Driver, V.R.; Lavery, L.A.; Reyzelman, A.M.; Dutra, T.G.; Dove, C.R.; Kotsis, S.V.; Kim, H.M.; Chung, K.C. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 2015, 23, 891–900. [Google Scholar] [CrossRef]
- Raftery, R.M.; Tierney, E.G.; Curtin, C.M.; Cryan, S.-A.; O’Brien, F.J. Development of a gene-activated scaffold platform for tissue engineering applications using chitosan-pDNA nanoparticles on collagen-based scaffolds. J. Control. Release 2015, 210, 84–94. [Google Scholar] [CrossRef]
- Duscher, D.; Barrera, J.F.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review. Gerontology 2016, 62, 216–225. [Google Scholar] [CrossRef]
- Laiva, A.L.; Raftery, R.M.; Keogh, M.B.; O’Brien, F.J. Pro-angiogenic impact of SDF-1α gene-activated collagen-based scaffolds in stem cell driven angiogenesis. Int. J. Pharm. 2018, 544, 372–379. [Google Scholar] [CrossRef]
- Tepper, O.M.; Carr, J.; Allen, R.J.; Chang, C.C.; Lin, C.D.; Tanaka, R.; Gupta, S.M.; Levine, J.P.; Saadeh, P.B.; Warren, S.M. Decreased Circulating Progenitor Cell Number and Failed Mechanisms of Stromal Cell-Derived Factor-1α Mediated Bone Marrow Mobilization Impair Diabetic Tissue Repair. Diabetes 2010, 59, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.; Freisinger, E.; Jones, R.K.; Slakey, U.P.; Dupin, C.L.; Newsome, E.R.; Alt, E.; Izadpanah, R. Persistent High Glucose Concentrations Alter the Regenerative Potential of Mesenchymal Stem Cells. Stem Cells Dev. 2010, 19, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative Senescence of Mesenchymal Stem Cells: A Continuous and Organized Process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Di Fagagna, F.D. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Saki, N.; Jalalifar, M.A.; Soleimani, M.; Hajizamani, S.; Rahim, F. Adverse Effect of High Glucose Concentration on Stem Cell Therapy. Int. J. Hematol. Stem Cell Res. 2013, 7, 34–40. [Google Scholar]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Hu, M.C.; Bian, A.; Neyra, J.A.; Zhan, M. Klotho, stem cells, and aging. Clin. Interv. Aging 2015, 10, 1233–1243. [Google Scholar] [CrossRef]
- Kuro-O, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Kuro-O, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Hwang, K.-H.; Park, K.S.; Kong, I.D.; Cha, S.-K. Biological Role of Anti-aging Protein Klotho. J. Lifestyle Med. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Van Heemst, D. Insulin, IGF-1 and longevity. Aging Dis. 2010, 1, 147–157. [Google Scholar] [PubMed]
- Holm, J.S.; Toyserkani, N.M.; Sorensen, J.A. Adipose-derived stem cells for treatment of chronic ulcers: Current status. Stem Cell Res. Ther. 2018, 9, 142. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel Freeze-Drying Methods to Produce a Range of Collagen–Glycosaminoglycan Scaffolds with Tailored Mean Pore Sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef]
- Block, T.J.; Marinkovic, M.; Tran, O.N.; Gonzalez, A.O.; Marshall, A.; Dean, D.D.; Chen, X.-D. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res. Ther. 2017, 8, 239. [Google Scholar] [CrossRef]
- Hoch, A.I.; Binder, B.Y.; Genetos, D.C.; Leach, J.K. Differentiation-Dependent Secretion of Proangiogenic Factors by Mesenchymal Stem Cells. PLoS ONE 2012, 7, e35579. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Luo, W.; Ng, T.W.; Leung, P.C.; Zhang, C.; Leung, K.C.-F.; Jin, L. Nanoparticle-encapsulated baicalein markedly modulates pro-inflammatory response in gingival epithelial cells. Nanoscale 2017, 9, 12897–12907. [Google Scholar] [CrossRef]
- Chan, J.C.; Duszczyszyn, D.A.; Castellino, F.J.; Ploplis, V.A. Accelerated Skin Wound Healing in Plasminogen Activator Inhibitor-1-Deficient Mice. Am. J. Pathol. 2001, 159, 1681–1688. [Google Scholar] [CrossRef]
- Saksela, O. Cell-Associated Plasminogen Activation: Regulation And Physiological Functions. Annu. Rev. Cell Dev. Biol. 1988, 4, 93–126. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C.; et al. Pro-Inflammatory Chemokine CCL2 (MCP-1) Promotes Healing in Diabetic Wounds by Restoring the Macrophage Response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, K.; Tham, E.-L.; Wei, L.-L.; Ma, N.; Dodd, P.C.; Luo, Y.; Kirchhofer, D.; McVey, J.H.; Dorling, A. Inhibition of Angiopoietin-2 Production by Myofibrocytes Inhibits Neointimal Hyperplasia After Endoluminal Injury in Mice. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Qi, W.; Yang, C.; Dai, Z.; Che, D.; Feng, J.; Mao, Y.; Cheng, R.; Wang, Z.; He, X.; Zhou, T.; et al. High Levels of Pigment Epithelium–Derived Factor in Diabetes Impair Wound Healing Through Suppression of Wnt Signaling. Diabetes 2014, 64, 1407–1419. [Google Scholar] [CrossRef]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef]
- Streit, M.; Velasco, P.; Riccardi, L.; Spencer, L.; Brown, L.F.; Janes, L.; Lange-Asschenfeldt, B.; Yano, K.; Hawighorst, T.; Iruela-Arispe, L.; et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000, 19, 3272–3282. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Tepper, O.M.; Capla, J.M.; Galiano, R.D.; Ceradini, D.J.; Callaghan, M.J.; Kleinman, M.E.; Gurtner, G.C. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow–derived cells. Blood 2005, 105, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Braun, H.; Schmidt, B.M.; Raiss, M.; Baisantry, A.; Mircea-Constantin, D.; Wang, S.; Gross, M.-L.; Serrano, M.; Schmitt, R.; Melk, A. Cellular Senescence Limits Regenerative Capacity and Allograft Survival. J. Am. Soc. Nephrol. 2012, 23, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Small, S.M.; Peltekian, L.; Kessler, R.L.; Potthoff, M.J. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Mol. Metab. 2017, 6, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Sun, Z. Klotho Deficiency Accelerates Stem Cells Aging by Impairing Telomerase Activity. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2018, 74, 1396–1407. [Google Scholar] [CrossRef]
- Yannas, I.V.; Burke, J.F.; Gordon, P.L.; Huang, C.; Rubenstein, R.H. Design of an artificial skin. II. Control of chemical composition. J. Biomed. Mater. Res. 1980, 14, 107–132. [Google Scholar] [CrossRef]
- Heimbach, D.; Luterman, A.; Burke, J.; Cram, A.; Herndon, D. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann. Surg. 1988, 208, 313–320. [Google Scholar] [CrossRef]
- Stiefel, D.; Schiestl, C.; Meuli, M. Integra Artificial Skin® for burn scar revision in adolescents and children. Burns 2010, 36, 114–120. [Google Scholar] [CrossRef]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1α gene-activated collagen scaffold drives functional differentiation of human Schwann cells for wound healing applications. Biotechnol. Bioeng. 2020. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Massee, M.; Chinn, K.; Lim, J.J.; Godwin, L.; Young, C.S.; Koob, T.J. Type I and II Diabetic Adipose-Derived Stem Cells Respond In Vitro to Dehydrated Human Amnion/Chorion Membrane Allograft Treatment by Increasing Proliferation, Migration, and Altering Cytokine Secretion. Adv. Wound Care 2016, 5, 43–54. [Google Scholar] [CrossRef]
- Engelhardt, E.; Toksoy, A.; Goebeler, M.; Debus, S.; Bröcker, E.-B.; Gillitzer, R. Chemokines IL-8, GROα, MCP-1, IP-10, and Mig Are Sequentially and Differentially Expressed During Phase-Specific Infiltration of Leukocyte Subsets in Human Wound Healing. Am. J. Pathol. 1998, 153, 1849–1860. [Google Scholar] [CrossRef]
- Bodnar, R.J. Chemokine Regulation of Angiogenesis During Wound Healing. Adv. Wound Care 2015, 4, 641–650. [Google Scholar] [CrossRef]
- Mirshahi, F.; Pourtau, J.; Li, H.; Muraine, M.; Trochon, V.; Legrand, E.; Vannier, J.; Soria, J.; Vasse, M.; Soria, C. SDF-1 activity on microvascular endothelial cells: Consequences on angiogenesis in in vitro and in vivo models. Thromb. Res. 2000, 99, 587–594. [Google Scholar] [CrossRef]
- Holash, J.; Maisonpierre, P.C.; Compton, D.; Boland, P.; Alexander, C.R.; Zagzag, D.; Yancopoulos, G.D.; Wiegand, S.J. Vessel Cooption, Regression, and Growth in Tumors Mediated by Angiopoietins and VEGF. Science 1999, 284, 1994–1998. [Google Scholar] [CrossRef]
- Kämpfer, H.; Pfeilschifter, J.; Frank, S. Expressional Regulation of Angiopoietin-1 and -2 and the Tie-1 and -2 Receptor Tyrosine Kinases during Cutaneous Wound Healing: A Comparative Study of Normal and Impaired Repair. Lab. Investig. 2001, 81, 361–373. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenes. Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef]
- Poschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef]
- Theocharidis, G.; Drymoussi, Z.; Kao, A.P.; Barber, A.H.; Lee, D.A.; Braun, K.M.; Connelly, J.T. Type VI Collagen Regulates Dermal Matrix Assembly and Fibroblast Motility. J. Investig. Dermatol. 2016, 136, 74–83. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Chai, M.; Tao, R.; Lei, Y.; Jia, Y.; Shu, J.; Ren, J.; Li, G.; Wei, W.; et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose‑derived stem cells into fibroblasts. Int. J. Mol. Med. 2018, 43, 890–900. [Google Scholar] [CrossRef]
- Bi, H.; Li, H.; Zhang, C.; Mao, Y.; Nie, F.; Xing, Y.; Sha, W.; Wang, X.; Irwin, D.M.; Tan, H.-R. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res. Ther. 2019, 10, 302. [Google Scholar] [CrossRef]

| Soluble Factor | Function | |
|---|---|---|
| Fibrinogenic factors | Plasminogen Activator Inhibitor-1 (PAI-1) | Impedes fibrinolysis [29] |
| Urokinase Plasminogen Activator (uPA) | Promotes fibrinolysis (antagonist to PAI-1) [30] | |
| Inflammatory Cytokines | Interleukin-8 (IL-8) | Inflammatory response, neutrophil recruitment to the wound site [31] |
| Monocyte Chemoattractant Protein-1 (MCP-1) | Inflammatory response, monocyte recruitment to the wound site [32] | |
| Angiogenic factors | Vascular Endothelial Growth Factor (VEGF) | Early stimulation of angiogenesis [33] |
| Angiogenin (ANG) | Stimulation of angiogenesis (late stage) | |
| Angiopoietin-2 (Ang-2) | Obstructs neo-vascularization, blood vessel maturation [34] | |
| Anti-angiogenic factors | Pigment Epithelium Derived Factor (PEDF) | Promotes vessel regression [35] |
| Tissue Inhibitor for Metalloproteinase-1 (TIMP-1) | Inhibits Matrix Metalloproteinases (MMPs) that break down the ECM to facilitate angiogenesis [36] | |
| Thrombospondin-1 (TSP-1) | Delays angiogenesis, poor vascularization [37] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suku, M.; Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Anti-Ageing Protein β-Klotho Rejuvenates Diabetic Stem Cells for Improved Gene-Activated Scaffold Based Wound Healing. J. Pers. Med. 2021, 11, 4. https://doi.org/10.3390/jpm11010004
Suku M, Laiva AL, O’Brien FJ, Keogh MB. Anti-Ageing Protein β-Klotho Rejuvenates Diabetic Stem Cells for Improved Gene-Activated Scaffold Based Wound Healing. Journal of Personalized Medicine. 2021; 11(1):4. https://doi.org/10.3390/jpm11010004
Chicago/Turabian StyleSuku, Meenakshi, Ashang Luwang Laiva, Fergal J. O’Brien, and Michael B. Keogh. 2021. "Anti-Ageing Protein β-Klotho Rejuvenates Diabetic Stem Cells for Improved Gene-Activated Scaffold Based Wound Healing" Journal of Personalized Medicine 11, no. 1: 4. https://doi.org/10.3390/jpm11010004
APA StyleSuku, M., Laiva, A. L., O’Brien, F. J., & Keogh, M. B. (2021). Anti-Ageing Protein β-Klotho Rejuvenates Diabetic Stem Cells for Improved Gene-Activated Scaffold Based Wound Healing. Journal of Personalized Medicine, 11(1), 4. https://doi.org/10.3390/jpm11010004








