Whole Body 3.0 T Magnetic Resonance Imaging in Lymphomas: Comparison of Different Sequence Combinations for Staging Hodgkin’s and Diffuse Large B Cell Lymphomas
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
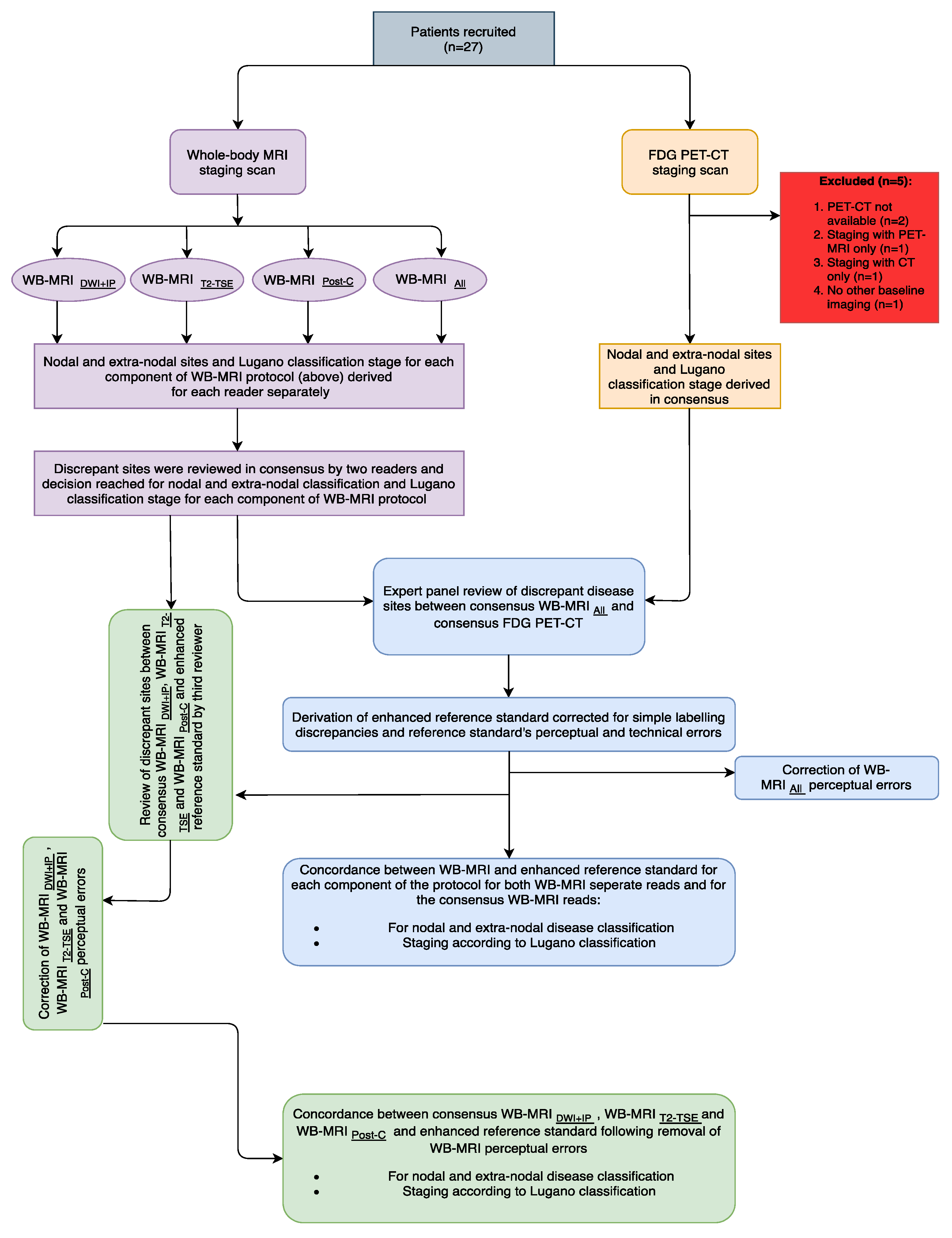
2.2. Study Summary
2.3. Multi-Parametric Whole Body MRI Protocol
2.4. 18F-FDG PET-CT Protocol
2.5. Whole Body MRI Interpretation
- (1)
- “WB-MRI DWI+IP”: Whole-body pre-contrast in-phase mDixon + whole-body DWI (b1000)
- (2)
- “WB-MRI T2-TSE”: Whole-body T2-TSE only,
- (3)
- “WB-MRI Post-C”: Whole-body post-contrast water-only mDixon, DCE liver/spleen and CE lung mDixon,
- (4)
- “WB-MRI All”: all components from 1–3 above.
2.6. 18F-FDG PET-CT Interpretation
Expert Panel Review and Derivation of Enhanced Reference Standard
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
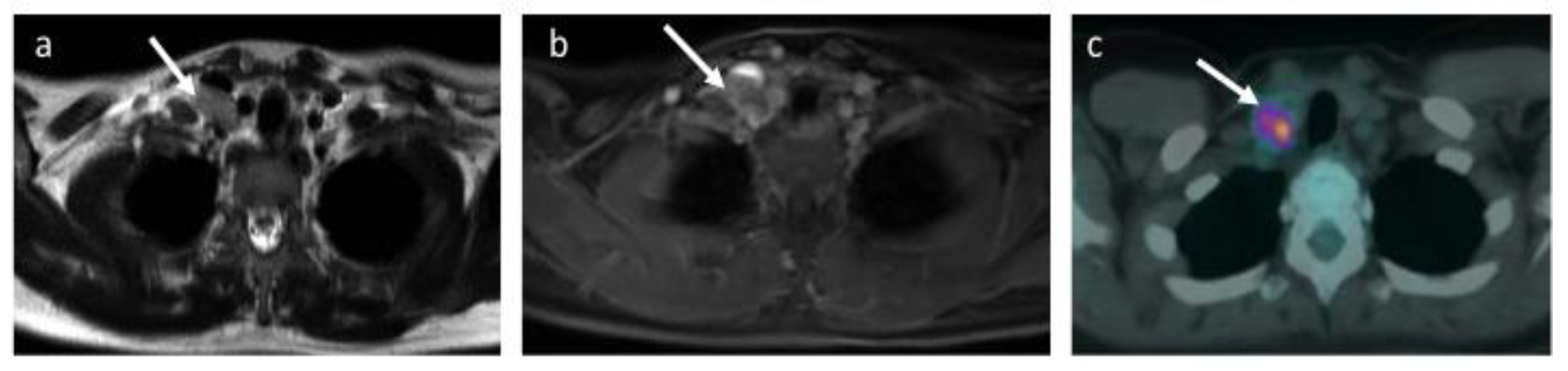
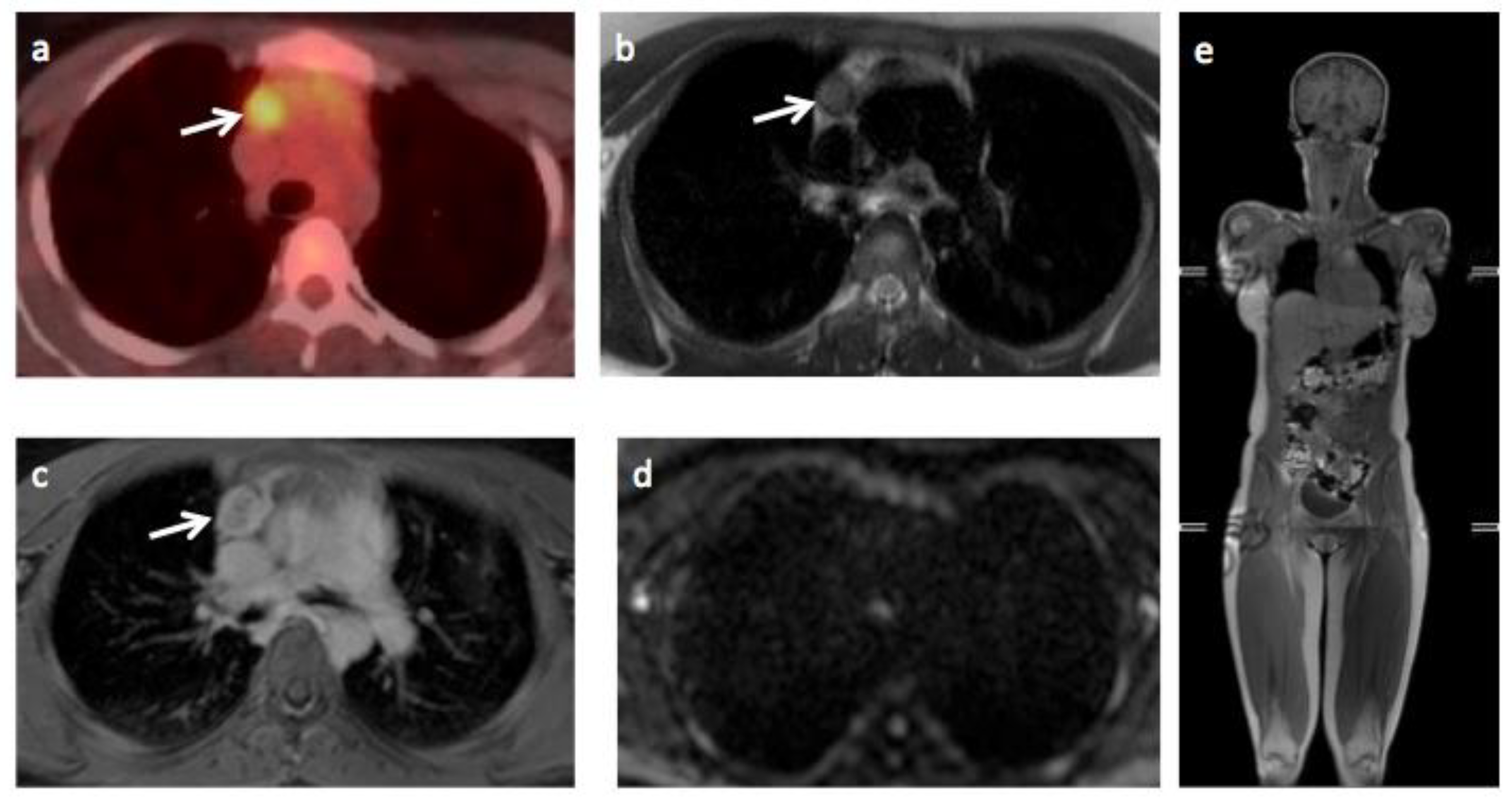
3.2. Expert Panel Review and Enhanced Reference Standard
3.3. Comparison of Whole-Body MRI and Enhanced Reference Standard
3.4. Comparison of Whole-Body MRI and Enhanced Reference Standard following Correction for Perceptual Errors
3.5. Overall Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saito, E.; Matsuoka, J. International comparison of Hodgkin and non-Hodgkin lymphoma incidence. Jpn. J. Clin. Oncol. 2020, 50, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; Italian Lymphoma Foundation; et al. European Mantle Cell Lymphoma Consortium Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Cronin, C.G.; Swords, R.; Truong, M.T.; Viswanathan, C.; Rohren, E.; Giles, F.J.; O’Dwyer, M.; Bruzzi, J.F. Clinical Utility of PET/CT in Lymphoma. Am. J. Roentgenol. 2010, 194, W91–W103. [Google Scholar] [CrossRef] [PubMed]
- Ghielmini, M.; Vitolo, U.; Kimby, E.; Montoto, S.; Walewski, J.; Pfreundschuh, M.; Federico, M.; Hoskin, P.; McNamara, C.; Caligaris-Cappio, F.; et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: Diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann. Oncol. 2013, 24, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Karanikas, G.; Kletter, K.; Prosch, H.; Kiesewetter, B.; Skrabs, C.; Porpaczy, E.; Weber, M.; Pinker-Domenig, K.; Berzaczy, D.; et al. Evaluation of Diffusion-Weighted MRI for Pretherapeutic Assessment and Staging of Lymphoma: Results of a Prospective Study in 140 Patients. Clin. Cancer Res. 2014, 20, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Koike, S.; Kashima, S.; Awai, K. Geographic Distribution of CT, MRI and PET Devices in Japan: A Longitudinal Analysis Based on National Census Data. PLoS ONE 2015, 10, e0126036. [Google Scholar] [CrossRef] [PubMed]
- Punwani, S.; Taylor, S.A.; Bainbridge, A.; Prakash, V.; Bandula, S.; De Vita, E.; Olsen, O.E.; Hain, S.F.; Stevens, N.; Daw, S.; et al. Pediatric and Adolescent Lymphoma:Comparison of Whole-Body STIR Half-Fourier RARE MR Imaging with an Enhanced PET/CT Reference for Initial Staging. Radiology 2010, 255, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Lecouvet, F.E.; Tunariu, N.; Koh, D.-M.; De Keyzer, F.; Collins, D.J.; Sala, E.; Schlemmer, H.P.; Petralia, G.; Vargas, H.A.; et al. METastasis Reporting and Data System for Prostate Cancer: Practical Guidelines for Acquisition, Interpretation, and Reporting of Whole-body Magnetic Resonance Imaging-based Evaluations of Multiorgan Involvement in Advanced Prostate Cancer. Eur. Urol. 2017, 71, 81–92. [Google Scholar] [CrossRef]
- Latifoltojar, A.; Hall-Craggs, M.; Bainbridge, A.; Rabin, N.; Popat, R.; Rismani, A.; D’Sa, S.; Dikaios, N.; Sokolska, M.; Antonelli, M.; et al. Whole-body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. Eur. Radiol. 2017, 27, 5325–5336. [Google Scholar] [CrossRef]
- Latifoltojar, A.; Humphries, P.D.; Menezes, L.J.; Haroon, A.; Daw, S.; Shankar, A.; Punwani, S. Whole-body magnetic resonance imaging in paediatric Hodgkin lymphoma—Evaluation of quantitative magnetic resonance metrics for nodal staging. Pediatric Radiol. 2019, 49, 1285–1298. [Google Scholar] [CrossRef]
- Lecouvet, F.E.; Van Nieuwenhove, S.; Jamar, F.; Lhommel, R.; Guermazi, A.; Pasoglou, V.P. Whole-Body MR Imaging: The Novel, "Intrinsically Hybrid," Approach to Metastases, Myeloma, Lymphoma, in Bones and Beyond. PET Clin. 2018, 13, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Kishi, S.; Tsuchida, T.; Yamauchi, T.; Ikegaya, S.; Urasaki, Y.; Rt, Y.F.; Ueda, T.; Okazawa, H.; Kimura, H. Evaluation of staging and early response to chemotherapy with whole-body diffusion-weighted MRI in malignant lymphoma patients: A comparison with FDG-PET/CT. J. Magn. Reson. Imaging 2015, 41, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Regacini, R.; Puchnick, A.; Shigueoka, D.C.; Iared, W.; Lederman, H.M. Whole-body diffusion-weighted magnetic resonance imaging versus FDG-PET/CT for initial lymphoma staging: Systematic review on diagnostic test accuracy studies. Sao Paulo Med. J. 2015, 133, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chan, T.; Zhang, J.; Leung, A.Y.H.; Kwong, Y.L.; Khong, P.-L. Whole-Body Diffusion-Weighted Imaging: The Added Value to Whole-Body MRI at Initial Diagnosis of Lymphoma. Am. J. Roentgenol. 2011, 197, W384–W391. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Vermoolen, M.A.; Akkerman, E.A.; Kersten, M.J.; Fijnheer, R.; Ludwig, I.; Beek, F.J.A.; Van Leeuwen, M.S.; Bierings, M.B.; Bruin, M.C.A.; et al. Whole-body MRI, including diffusion-weighted imaging, for staging lymphoma: Comparison with CT in a prospective multicenter study. J. Magn. Reson. Imaging 2014, 40, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bray, T.J.; Singh, S.; Latifoltojar, A.; Rajesparan, K.; Rahman, F.; Narayanan, P.; Naaseri, S.; Lopes, A.; Bainbridge, A.; Punwani, S.; et al. Diagnostic utility of whole body Dixon MRI in multiple myeloma: A multi-reader study. PLoS ONE 2017, 12, e0180562. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Johnston, E.W.; Latifoltojar, A.; Sidhu, H.S.; Ramachandran, N.; Sokolska, M.; Bainbridge, A.; Moore, C.; Ahmed, H.U.; Dikaios, N. Multiparametric whole-body 3.0-T MRI in newly diagnosed intermediate- and high-risk prostate cancer: Diagnostic accuracy and interobserver agreement for nodal and metastatic staging. Eur. Radiol. 2019, 29, 3159–3169. [Google Scholar] [CrossRef]
- Ganeshalingam, S.; Koh, D.-M. Nodal staging. Cancer Imaging 2009, 9, 104–111. [Google Scholar] [CrossRef]
- Latifoltojar, A.; Punwani, S.; Lopes, A.; Humphries, P.D.; Klusmann, M.; Menezes, L.J.; Daw, S.; Shankar, A.; Neriman, D.; Fitzke, H.; et al. Whole-body MRI for staging and interim response monitoring in paediatric and adolescent Hodgkin’s lymphoma: A comparison with multi-modality reference standard including 18F-FDG-PET-CT. Eur. Radiol. 2019, 29, 202–212. [Google Scholar] [CrossRef]
- Johnson, S.A.; Kumar, A.; Matasar, M.J.; Schöder, H.; Rademaker, J. Imaging for Staging and Response Assessment in Lymphoma. Radiology 2015, 276, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.D.; Gleeson, T.; Coate, L.E.; Cronin, C.; Carney, D.; Eustace, S.J. A Comparison of Whole-Body MRI and CT for the Staging of Lymphoma. Am. J. Roentgenol. 2005, 185, 711–716. [Google Scholar] [CrossRef] [PubMed]
- NICE Guideline; Myeloma: Diagnosis and Management (February 2016). Available online: https://www.nice.org.uk/guidance/ng35/evidence/full-guideline-pdf-2306487277 (accessed on 5 December 2020).
- Taylor, S.A.; Mallett, S.; Ball, S.; Beare, S.; Bhatnagar, G.; Bhowmik, A.; Boavida, P.; Bridgewater, J.; Clarke, C.S.; Duggan, M.; et al. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed non-small-cell lung cancer: The prospective Streamline L trial. Lancet Respir. Med. 2019, 7, 523–532. [Google Scholar] [CrossRef]
- Obara, P.; Loening, A.M.; Taviani, V.; Iagaru, A.; Hargreaves, B.A.; Vasanawala, S.S. Relative value of three whole-body MR approaches for PET-MR, including gadofosveset-enhanced MR, in comparison to PET-CT. Clin. Imaging 2018, 48, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Punwani, S.; Taylor, S.A.; Saad, Z.Z.; Bainbridge, A.; Groves, A.; Daw, S.; Shankar, A.; Halligan, S.; Humphries, P.D. Diffusion-weighted MRI of lymphoma: Prognostic utility and implications for PET/MRI? Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Punwani, S.; Prakash, V.; Bainbridge, A.; Taylor, S.A.; Bandula, S.; Olsen, O.E.; Hain, S.F.; Shankar, A.; Daw, S.; Humphries, P. Quantitative diffusion weighted MRI: A functional biomarker of nodal disease in Hodgkin lymphoma? Cancer Biomarkers 2011, 7, 249–259. [Google Scholar] [CrossRef]
- Lin, C.; Itti, E.; Luciani, A.; Zegai, B.; Lin, S.-J.; Kuhnowski, F.; Pigneur, F.; Gaillard, I.; Paone, G.; Meignan, M.; et al. Whole-Body Diffusion-Weighted Imaging with Apparent Diffusion Coefficient Mapping for Treatment Response Assessment in Patients With Diffuse Large B-Cell Lymphoma. Investig. Radiol. 2011, 46, 341–349. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Karanikas, G.; Kletter, K.; Prosch, H.; Kiesewetter, B.; Skrabs, C.; Porpaczy, E.; Weber, M.; Knogler, T.; Sillaber, C.; et al. Evaluation of Diffusion-Weighted Magnetic Resonance Imaging for Follow-up and Treatment Response Assessment of Lymphoma: Results of an 18F-FDG-PET/CT–Controlled Prospective Study in 64 Patients. Clin. Cancer Res. 2015, 21, 2506–2513. [Google Scholar] [CrossRef]


| T2-TSE | mDixon (Pre and Post-Contrast #) | DWI (b0, 100, 300, 1000) | DCE (Liver and Spleen) | Post-Contrast Lung | |
|---|---|---|---|---|---|
| Imaging Plane | Transverse | Coronal | Transverse | Transverse | Transverse |
| TE (ms) | 80 | 1.02/1.8 | 71 | 1.02/1.8 | 1.02/1.8 |
| TR (ms) | 1228 | 3.0 | 6371 | 3.0 | 3.0 |
| FOV (mm*mm) | 500*300 | 502*300 | 500*306 | 512*512 | 512*512 |
| Voxel Size (mm*mm) | 1*1 | 2.1*2.1 | 4*4.2 | 1.2*1.2 | 1.2*1.2 |
| Number of Slices | 40 | 120 | 40 | 80 | 68 |
| Slice Thickness (mm) | 5 | 5 | 5 | 5 | 6 |
| Acquisition Matrix | 500*286 | 144*238 | 124*72 | 256*254 | 336*332 |
| ETL | 91 | 2 | 39 | 2 | 2 |
| Acceleration Factor (SENSE) | 2 | 2 | 2.5 | 2.8 | 2.8 |
| Pixel Bandwidth (Hz) | 537 | 1992 | 3369 | 1890 | 42055 |
| Acquisition Time per Station (s) | 47 | 17 | 152 | 17 | 17 |
| Number of Stations | 6 | 4 | 6 | 1 | 2 |
| Patient Characteristic (N = 22) | Number or Median (Range) |
|---|---|
| Age | 32 (22–87) |
| Sex | Male/Female: 12/10 |
| Overall stage | |
| I | 7 |
| II | 8 |
| III | 2 |
| IV | 5 |
| Subtype | |
| HL | 14 |
| DLBCL | 8 |
| Chemotherapy regimen | |
| ABVD | 10 |
| RCHOP | 8 |
| ABVD-BEACOPP | 3 |
| Rituximab | 1 |
| Analyses (Reader 1) | Agreement Rate | TPR | FPR | Kappa (95% CI) |
|---|---|---|---|---|
| WB-MRI DWI+IP | ||||
| Nodal Sites | 92% (360/390) | 60% (31/52) | <1% (9/338) | 0.63 (0.51–0.75) |
| Extra-nodal sites | 97% (237/243) | 44% (4/9) | <1% (1/234) | 0.56 (0.25–0.87) |
| WB-MRI T2-TSE | ||||
| Nodal Sites | 94% (367/390) | 66% (34/52) | <1% (5/338) | 0.71 (0.60–0.82) |
| Extra-nodal sites | 98% (239/243) | 67% (6/9) | <1% (1/234) | 0.74 (0.50–0.98) |
| WB-MRI Post-C | ||||
| Nodal Sites | 96% (373/390) | 77% (40/52) | <1% (5/338) | 0.80 (0.71–0.89) |
| Extra-nodal sites | 96% (233/243) | 67% (6/9) | <1% (7/234) | 0.52 (0.27–0.78) |
| WB-MRI All | ||||
| Nodal Sites | 95% (371/390) | 67% (35/52) | <1% (2/338) | 0.76 (0.66-0.86) |
| Extra-nodal sites | >99% (241/243) | 89% (8/9) | <1% (1/234) | 0.88 (0.73–1.00) |
| Analyses (Reader 2) | Agreement Rate | TPR | FPR | Kappa (95% CI) |
|---|---|---|---|---|
| WB-MRI DWI+IP | ||||
| Nodal Sites | 95% (369/390) | 73% (38/52) | <1% (7/338) | 0.75 (0.65–0.85) |
| Extra-nodal sites | 97% (237/243) | 44% (4/9) | <1% (1/234) | 0.56 (0.25–0.87) |
| WB-MRI T2-TSE | ||||
| Nodal Sites | 96% (374/390) | 75% (39/52) | <1% (3/338) | 0.81 (0.71–0.90) |
| Extra-nodal sites | >99% (241/243) | 89% (8/9) | <1% (1/234) | 0.88 (0.73–1.00) |
| WB-MRI Post-C | ||||
| Nodal Sites | 96% (374/390) | 85% (44/52) | <1% (8/338) | 0.82 (0.74–0.91) |
| Extra-nodal sites | 99% (240/243) | 78% (7/9) | <1% (7/234) | 0.59 (0.35–0.83) |
| WB-MRI All | ||||
| Nodal Sites | 97% (378/390) | 83% (43/52) | <1% (3/338) | 0.86 (0.78 to 0.94) |
| Extra-nodal sites | >99% (242/243) | 89% (8/9) | 0% (0/234) | 0.94 (0.82 to 1.00) |
| Analyses (Consensus) | Agreement Rate | TPR | FPR | Kappa (95% CI) |
|---|---|---|---|---|
| WB-MRI DWI+IP | ||||
| Nodal Sites | 95% (372/390) | 75% (39/52) | <1% (5/338) | 0.79 (0.69–0.88) |
| Extra-nodal sites | 97% (240/243) | 67% (6/9) | 0% (0/234) | 0.79 (0.57–1.00) |
| WB-MRI T2-TSE | ||||
| Nodal Sites | 97% (377/390) | 83% (43/52) | <1% (4/338) | 0.85 (0.77–0.93) |
| Extra-nodal sites | >99% (242/243) | 89% (8/9) | 0% (0/234) | 0.93 (0.82–1.00) |
| WB-MRI Post-C | ||||
| Nodal Sites | 96% (373/390) | 77% (40/52) | <1% (5/338) | 0.80 (0.71–0.89) |
| Extra-nodal sites | >99% (242/243) | 89% (8/9) | 0% (0/234) | 0.94 (0.82–1.00) |
| WB-MRI All | ||||
| Nodal Sites | 98% (383/390) | 87% (45/52) | 0% (0/338) | 0.92 (0.86–0.98) |
| Extra-nodal sites | 100% (243/243) | 100% (9/9) | 0% (0/234) | 1.00 (1.00–1.00) |
| Analyses (Consensus/Post Correction) | Agreement Rate | TPR | FPR | Kappa (95% CI) |
|---|---|---|---|---|
| WB-MRI DWI+IP | ||||
| Nodal Sites | 97% (380/390) | 81% (42/52) | 0% (0/338) | 0.88 (0.81–0.95) |
| Extra-nodal sites | >99% (241/243) | 78% (7/9) | 0% (0/234) | 0.87 (0.69–1.00) |
| WB-MRI T2-TSE | ||||
| Nodal Sites | 98% (383/390) | 87% (45/52) | 0% (0/338) | 0.92 (0.86–0.98) |
| Extra-nodal sites | >99% (242/243) | 89% (8/9) | 0% (0/234) | 0.93 (0.82–1.00) |
| WB-MRI Post-C | ||||
| Nodal Sites | 98% (383/390) | 87% (45/52) | 0% (0/338) | 0.92 (0.86–0.98) |
| Extra-nodal sites | 100% (243/243) | 100% (9/9) | 0% (0/234) | 0.94 (0.84–0.97) |
| WB-MRI All | ||||
| Nodal Sites | 98% (383/390) | 87% (45/52) | 0% (0/338) | 0.92 (0.86–0.98) |
| Extra-nodal sites | 100% (243/243) | 100% (9/9) | 0% (0/234) | 1.00 (1.00–1.00) |
| WB-MRI | ||||
|---|---|---|---|---|
| DWI+IP | Post-C | T2-TSE | All | |
| Reader 1 | 0.55 (0.30–0.80) | 0.69 (0.46–0.93) | 0.69 (0.46–0.91) | 0.88 (0.72–0.97) |
| Reader 2 | 0.62 (0.37–0.87) | 0.76 (0.53–0.98) | 0.88 (0.71–1.00) | 0.88 (0.71–1.00) |
| Consensus | 0.75 (0.52–0.97) | 0.94 (0.82–1.00) | 0.94 (0.82–1.00) | 1.00 (1.00–1.00) |
| Post-correction | 0.81 (0.61–1.00) | 1.00 (1.00–1.00) | 0.94 (0.82–1.00) | 1.00 (1.00–1.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latifoltojar, A.; Duncan, M.K.J.; Klusmann, M.; Sidhu, H.; Bainbridge, A.; Neriman, D.; Fraioli, F.; Lambert, J.; Ardeshna, K.M.; Punwani, S. Whole Body 3.0 T Magnetic Resonance Imaging in Lymphomas: Comparison of Different Sequence Combinations for Staging Hodgkin’s and Diffuse Large B Cell Lymphomas. J. Pers. Med. 2020, 10, 284. https://doi.org/10.3390/jpm10040284
Latifoltojar A, Duncan MKJ, Klusmann M, Sidhu H, Bainbridge A, Neriman D, Fraioli F, Lambert J, Ardeshna KM, Punwani S. Whole Body 3.0 T Magnetic Resonance Imaging in Lymphomas: Comparison of Different Sequence Combinations for Staging Hodgkin’s and Diffuse Large B Cell Lymphomas. Journal of Personalized Medicine. 2020; 10(4):284. https://doi.org/10.3390/jpm10040284
Chicago/Turabian StyleLatifoltojar, Arash, Mark K. J. Duncan, Maria Klusmann, Harbir Sidhu, Alan Bainbridge, Deena Neriman, Francesco Fraioli, Jonathan Lambert, Kirit M. Ardeshna, and Shonit Punwani. 2020. "Whole Body 3.0 T Magnetic Resonance Imaging in Lymphomas: Comparison of Different Sequence Combinations for Staging Hodgkin’s and Diffuse Large B Cell Lymphomas" Journal of Personalized Medicine 10, no. 4: 284. https://doi.org/10.3390/jpm10040284
APA StyleLatifoltojar, A., Duncan, M. K. J., Klusmann, M., Sidhu, H., Bainbridge, A., Neriman, D., Fraioli, F., Lambert, J., Ardeshna, K. M., & Punwani, S. (2020). Whole Body 3.0 T Magnetic Resonance Imaging in Lymphomas: Comparison of Different Sequence Combinations for Staging Hodgkin’s and Diffuse Large B Cell Lymphomas. Journal of Personalized Medicine, 10(4), 284. https://doi.org/10.3390/jpm10040284





