Abstract
We explored the association between CYP2C19/3A4 mediated drug-gene-interaction (DGI), drug-drug-interaction (DDI) and drug-drug-gene-interaction (DDGI) and (es)citalopram dispensing course. A cohort study was conducted among adult Caucasians from the Lifelines cohort (167,729 participants) and linked dispensing data from the IADB.nl database as part of the PharmLines Initiative. Exposure groups were categorized into (es)citalopram starters with DGI, DDI and DDGI. The primary outcome was drug switching and/or dose adjustment, and the secondary was early discontinuation after the start of (es)citalopram. Logistic regression modeling was applied to estimate adjusted odd ratios with their confidence interval. We identified 316 (es)citalopram starters with complete CYP2C19/3A4 genetic information. The CYP2C19 IM/PM and CYP3A4 NM combination increased risks of switching and/or dose reduction (OR: 2.75, 95% CI: 1.03–7.29). The higher effect size was achieved by the CYP2C19 IM/PM and CYP3A4 IM combination (OR: 4.38, 95% CI: 1.22–15.69). CYP2C19/3A4 mediated DDIs and DDGIs showed trends towards increased risks of switching and/or dose reduction. In conclusion, a DGI involving predicted decreased CYP2C19 function increases the need for (es)citalopram switching and/or dose reduction which might be enhanced by co-presence of predicted decreased CYP3A4 function. For DDI and DDGI, no conclusions can be drawn from the results.
1. Introduction
Selective serotonin re-uptake inhibitors (SSRIs) such as citalopram and escitalopram ((es)citalopram) are among the first-line pharmacological options for depression in Europe and the US, and the use of SSRIs has increased considerably over the years [1,2]. However, reports showed that less than 50% of (es)citalopram users achieved disease symptom remission during their first treatment episode, and prognosis appeared unpredictable [3,4]. Such variable effectiveness may be explained by a large inter-individual pharmacokinetic variability among patients treated with (es)citalopram [5,6]. This variability is known to be caused partly by differences in metabolic activity of drug metabolizing Cytochrome P450 (CYP) enzymes [7].
(Es)citalopram is primarily metabolized by the combination of CYP2C19 and CYP3A4 enzymes, and to a lesser extent by CYP2D6 enzyme [8,9]. Genetic polymorphisms are known to affect the catalytic activity of these enzymes. Some studies have investigated the role of CYP2C19 and CYP2D6 polymorphisms on the exposure as well as the clinical impact of (es)citalopram [7,10]. Such interaction between the drug treatment and genetic variation is referred to as drug-gene interaction (DGI) [11]. To the best of our knowledge, no previous studies have explored the impact of the DGI related to CYP3A4 polymorphisms, or its combination with CYP2C19 polymorphisms, in (es)citalopram treatment. In addition, the concomitant administration of CYP2C19, CYP3A4, and/or CYP2D6 (CYP2C19/3A4/2D6) modulator drugs (inhibitor/inducer) produces a drug-drug-interaction (DDI) with (es)citalopram by affecting blood concentrations and hence modifying its effectiveness [12].
To make it even more complicated for treating physicians, (es)citalopram treatment may be affected by both genetics and drugs that modulate the activity of the metabolic pathways at the same time which potentially affect blood concentration even more unpredictably than DGI and DDI alone [13]. In other words, a drug-drug-gene-interaction (DDGI) is encountered when a DGI coincides with a DDI [14,15]. Generally, DDGIs show more pharmacokinetic diversity than DDIs and DGIs alone, since DDGIs concern several modes of interactions [15,16]. For example, a DDGI may involve the co-existence of a genetic polymorphism and a CYP-inhibitor for one CYP-enzyme or the co-presence of a genetic polymorphism in one or two metabolic pathways and a CYP modulator in another pathway [14,15].
Due to restricted study populations in trials and scarcity of health care databases with a possibility to link genetic and drug dispensing data, large-scale real-world pharmacogenetic studies are lacking on the impact of pharmacogenetic and drug interactions in general. Consequently, recent guidelines have only provided specific recommendations on the management of (es)citalopram-related DGIs and DDIs separately, but a knowledge gap remains regarding the pharmacotherapeutic management of DDGIs [17,18]. The PharmLines Initiative enables the unique linkage of genetic and drug data to perform an inception cohort study in a large population cohort which we used to explore the impact of DDIs, DGIs (specifically CYP2C19/3A4 polymorphisms), and DDGIs on short-term first-time (es)citalopram therapy [19]. To mirror treatment success, proxy outcomes such as drug switching, dose adjustment, and an early discontinuation after the first prescription of (es)citalopram are used [20,21].
2. Methods
2.1. Study Design, Setting and Data Sources
This retrospective cohort study was performed using data from the PharmLines Initiative which links the Lifelines cohort and the University of Groningen prescription IADB.nl database, two large databases in the Northern part of the Netherlands [19].
The Lifelines cohort is a three-generation prospective cohort covering 167,729 Dutch participants from the Northern provinces of the Netherlands [22,23]. It was established with the aim to study ‘complex interactions between environmental, phenotypic and genomic factors in the development of chronic diseases and healthy ageing’ [22,23]. The participants from the Lifelines cohort generally represent the characteristics of the adult population of the Northern part of the Netherlands [24]. More comprehensive information about the Lifelines cohort can be found in the publications of Stolk et al. and Scholtens et al. [22,23].
The University of Groningen prescription database IADB.nl collected over 1.2 million prescriptions from 72 pharmacies. The information about gender, date of birth and four-digit postal codes (optional) from 730,000 recorded anonymous patients are available [25]. The prescription information of each participant is recorded such as dispensing date, Anatomical Therapeutic Chemical code (ATC code), quantity, duration, and DDD (defined daily dose) [25]. The participants recorded in the IADB.nl are found to be representative of the general population in the Netherlands as whole [25]. The IADB.nl is a reliable database and has been used in many pharmacoepidemiological studies [26,27,28]
The linking process of these two databases was facilitated by a trusted third party, the Statistic Netherlands. The linkage was performed at the individual level and relied on combined information of postal code, date of birth, and gender. Once the selection process was completed, identifiers from each database were cleared and then, a new unique identifier (pseudoID) was assigned. Using the pseudoID, genetic and prescription information of the participants from the Lifelines cohort and the IADB.nl, respectively, could be combined. Details on the linking process has been published elsewhere [19].
2.2. Study Population
Adult Lifelines participants (Caucasian, 18 years and older) with available genetic information (CYP2C19 and CYP3A4 genes) and who had their first citalopram (N06AB04) or escitalopram (N06AB10) prescription recorded in the PharmLines Iniative were eligible. Those who were not prescribed any (es)citalopram for at least 180 days before starting their drug dispensing were included. If there were several periods of (es)citalopram dispensing, only the first dispensing period was included in the analysis. Date of the first (es)citalopram prescription was regarded as an index date which indicates the start of follow-up.
2.3. Genotyping
Genotyping for single-nucleotide polymorphism (SNP) of CYP2C19 and CYP3A4 genes in the Lifelines cohort was performed using the Illumina CytoSNP-12v2 array [22]. The genotype data was imputed by using the Genome of the Netherlands reference panel [22]. The quality of genotyping data was checked using the following requirements i.e., (i) the p-value of Hardy-Weinberg equilibrium distribution was > 1 × 10−4, (ii) call rate of 95%, and (iii) minor allele frequency (MAF) was > 0.001 [22]. Additionally, principal component analysis was used to detect statistical outliers [22]. More detailed information on the genotyping process can be found in the publication of Scholtens et al. (2014) [22].
CYP2C19 and CYP3A4 genotypes were translated to haplotypes, which were used to predict corresponding phenotypes (Table 1, Table 2, Table 3 and Table 4). Relevant haplotypes were selected and genotypes were translated to predicted phenotypes based on available information from the Dutch Pharmacogenetics Working Group (DPWG). Corresponding predicted phenotypes include poor metabolizer (PM), intermediate metabolizer (IM), and normal metabolizer (NM) for CYP2C19 and CYP3A4, and ultra-rapid metabolizer (UM) for CYP2C19.

Table 1.
Pipeline translation table for CYP2C19 with haplotypes and their Single Nucleotide Polymorphisms (SNPs) information.

Table 2.
Pipeline translation table for CYP3A4 with haplotypes and their SNP information.

Table 3.
The translation of CYP2C19 and CYP3A4 haplotypes to their predicted metabolic activity.

Table 4.
The translation of CYP2C19 and CYP3A4 haplotype combinations to their predicted phenotypes.
2.4. Definition of Exposures
The exposure groups were defined as (es)citalopram users with a DGI, DDI, or DDGI. Participants who were predicted to be CYP2C19 UM, IM, or PM and/or CYP3A4 IM or PM and were prescribed (es)citalopram without co-prescription of CYP2C19/3A4/2D6 modulators (inhibitors/inducers) were classified as experiencing a DGI. For statistical power reasons, IM and PM groups were pooled into a combined IM/PM group, but we provided a sensitivity analysis for the separated IM and PM groups (Supplementary Materials S2).
Participants were classified to have a DDI when they were predicted as normal metabolizers (NM) of CYP2C19 and CYP3A4, and at the same time were co-prescribed a CYP2C19 and/or CYP3A4 and/or CYP2D6 modulator during the (es)citalopram treatment within a follow-up time frame of 90 days. A list of clinically relevant CYP2C19/3A4/2D6 modulators was based on Commentaren Medicatiebewaking (Health Base, NL) and the Flockhart tableTM (Supplementary S1) [32,33]. Only non-SSRI drugs were included as CYP2C19/3A4/2D6 modulators since our study population consists of first-time (es)citalopram users and it is uncommon to combine this with another SSRI drug in the early phase of drug treatments [34].
DDGI was defined as the occurrence of a DGI and DDI at the same time in which (es)citalopram patients with a CYP2C19/3A4 predicted deviating phenotype received a CYP2C19/3A4/2D6 modulator. The non-exposed reference group was defined as (es)citalopram users with a predicted normal CYP2C19/3A4 and who were not prescribed any CYP2C19/3A4/2D6 modulator during first-time (es)citalopram treatment.
2.5. Study Outcomes
Study outcomes were drug switching, dose adjustment, and early discontinuation. The incidence of these outcomes within the time frame of a 90 day follow-up after the index date were identified. This time frame was used since the acute phase treatment of SSRIs is considered to be between 6 and 12 weeks after the start of drug treatment. A previous report indicated that about 70% of antidepressant users stopped their therapy within 90 days [35]. However, since interactions commonly have an immediate effect, the presence of the outcomes within the time frame of a 45 day follow-up after the index date were also explored (Supplementary S3) [21]. Drug switching was defined as patients having an early discontinuation of (es)citalopram as well as the prescription of another antidepressant, regardless of the class, within 120 days after the index date. The follow-up time frame was expanded for dispensing of other antidepressants from 90 to 120 days after the index date in order to accommodate the possible time gap between the dispensing of (es)citalopram and the new antidepressant [36,37]. Meanwhile, dose adjustment was defined as having a dose reduction or a dose elevation for at least 25% of the first dose within 90 days after the index date. Early discontinuation was defined as discontinuing the prescription of (es)citalopram within 90 days after the index date, having no further re-prescription of (es)citalopram for at least 180 days after the stop date as well as no switching as described previously. In the preliminary analysis the effects of exposure on drug switching and dose reduction were in the same direction, therefore the outcomes were combined. Analysis on the separated outcomes are provided in the Supplementary S2.
2.6. Co-Variates
The following co-variates were recorded to compare groups: age, gender, dose of (es)citalopram at the index date, number of co-prescriptions, and pre-defined drugs as a proxy for certain co-existing comorbidities (Supplementary S1). (Es)citalopram users had to have at least two prescriptions of these proxy medications within six months before or after the index date to be assumed as having a chronic condition of the potential comorbidities [38]. The presence of NSAIDs co-prescription during (es)citalopram prescription was checked within the time frame of 90 days since the combination of NSAIDs and SSRIs was reported to increase the risk of gastrointestinal bleeding [39]. The potential comorbidities were clustered into one group, namely ‘potential comorbidities,’ in order to increase the power of the calculation. The distribution for each potential comorbidity was compared separately between outcomes and none of them were statistically significant different (p < 0.05). Lastly, the distribution of the number of CYP2C19/3A4/2D6 modulator prescriptions during the use of (es)citalopram was compared, since a previous study indicated that the higher the number of CYP2C19/3A4/2D6 modulator prescriptions, the more alteration in the clearance of (es)citalopram [12].
2.7. Statistical Analysis
The Chi-square (or Fisher′s exact test) and Mann-Whitney test were used to compare distribution of categorical and skewed distributed continuous variables between outcomes, respectively. Co-variates which differed significantly (p < 0.05) were entered into final multivariate logistic regression model to obtain adjusted odds ratio as measure of association (OR). We also provided adjusted p-values for false discovery rates due to multiple comparisons using the Benjamini-Hochberg method (q-values, with a q < 0.05 as the significance threshold). Since some participants did not have dosing information, a complete case analysis in cases of dosing comparison as well as dose adjustment analysis were performed. The baseline characteristics were compared between participants with complete information and participants without dosing information (Supplementary S2).
3. Results
Overall, 316 (es)citalopram users (median 45 years, 63% women) with CYP2C19 and CYP3A4 genetic information were available (Figure 1). Baseline characteristics of patients are displayed in Table 5. There were 32.6%, 7.3% and 4.4% of participants to have predicted CYP2C19 IM, PM, and UM, respectively, and there were 17.7% and 1.9% of our sample to have predicted CYP3A4 IM and PM, respectively. After combining both genetic information (regardless the presence of another exposure such as CYP modulators), we found that about 56% of the patients had at least one predicted deviating phenotype of CYP2C19 or CYP3A4. There were about 33%, 6%, 11%, and 4% of the participants having predicted CYP2C19 IM/PM + CYP3A4 NM, CYP2C19 IM/PM + CYP3A4 IM/PM, CYP2C19 NM + CYP3A4 IM/PM, and CYP2C19 UM + CYP3A4 NM/IM, respectively.
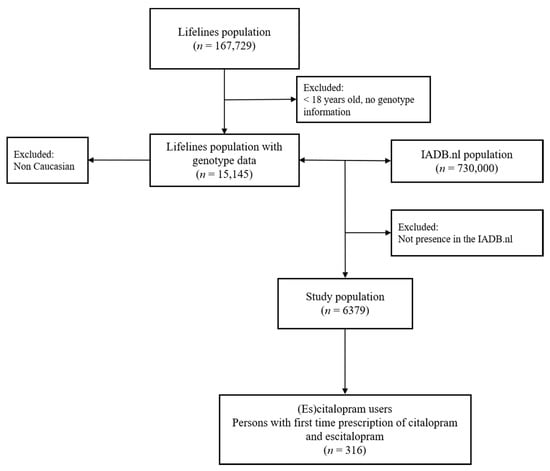
Figure 1.
Selection of (es)citalopram first time users.

Table 5.
Characteristics of patients starting (es)citalopram (n = 316).
Regardless of the number of prescribed CYP modulators, about 18% of the participants were exposed to CYP-modulators during (es)citalopram prescription and most of them were CYP2C19 inhibitors (13.9%). No combination of (es)citalopram with CYP2C19/3A4 inducer alone was identified. Two patients exposed to a combination of CYP modulators (one patient with a CYP2C19 and a CYP2D6 inhibitor, and one patient with a CYP2C19 inhibitor and a CYP3A4 inducer) were excluded since the number was too small to analyze. More than 60% of the participants had at least 20 mg citalopram or 10 mg escitalopram daily (≥ 1 Defined Daily Dose/DDD) at the start of their prescriptions. About 68% of the population had 1 to 2 potential comorbidities and about 78% of them used one to three different type of drugs during (es)citalopram prescription.
The more concomitant the CYP modulator used during (es)citalopram prescription, the more alteration in the (es)citalopram produced [12]. In our sample, we only found less than 10% of them using at least two concomitant CYP modulator at the same pathway. After looking on the combination of exposures (CYP2C19/3A4 genotypes and CYP modulators) among our study population, we found that 9%, 47%, and 8.5% of participants were exposed to DDIs, DGIs, and DDGIs, respectively. Frequency of each type of DDGIs is presented in Table 6.

Table 6.
Frequency of DDGI (overlapping condition of DDI and DGI).
There were 25 (7.9%), 7 (2.2%), 80 (25%), and 47 (15%) of (es)citalopram users experiencing drug switching, dose reduction, dose elevation, and early discontinuation, respectively. Number of co-prescriptions seemed to influence the rate of switching (p = 0.02). Female gender and a higher dose at the index date are less prevalent in the subgroup that experienced dose elevation of (es)citalopram (p = 0.003 and 0.002, respectively) (Table 7).

Table 7.
Baseline comparisons.
In our dataset, participants with a predicted CYP2C19 IM phenotype had an increased risk of drug switching and/or dose reduction (aOR: 3.16, 95% CI: 1.41–7.09) but CYP2C19 PM did not show a comparable result (aOR: 0.54, 95% CI: 0.07–4.52) (Table 8). Meanwhile, both CYP2C19 IM and PM had a comparable trend on the risk of early discontinuation (aOR: 0.35, 95% CI: 0.15–0.79 and aOR: 0.41, 95% CI: 0.09–1.89, respectively) (Table 9).

Table 8.
Association between DDI, DGI, and DDGI with drug switching and/or dose reduction.

Table 9.
Association between DDI, DGI, and DDGI with early discontinuation.
Furthermore, there was an indication showing that co-presence of CYP3A4 IM/PM in individuals with CYP2C19 IM/PM increased the risk of switching and/or dose reduction of (es)citalopram to a larger extent than the combination of CYP2C19 IM/PM and CYP3A4 NM (aOR: 4.38, 95% CI: 1.22–15.69 and aOR: 2.75, 95% CI: 1.03–7.29, respectively). This effect might be facilitated by the combination of CYP2C19 IM and CYP3A4 IM since there was only one participant with CYP2C19 PM and no participants with CYP3A4 PM experiencing switching or dose reduction (Table 8). Meanwhile, CYP3A4 IM/PM in the co-presence of CYP2C19 NM did not seem to influence the risk of switching and/or dose reduction (aOR: 1.02, 95% CI: 0.19–5.24). No participants with the CYP2C19 UM and CYP3A4 NM/IM combination experienced drug switching and/or dose reduction and no significant association with early discontinuation as well as with dose elevation was observed (Table 8, Table 9 and Table 10).

Table 10.
Association between DDI, DGI, and DDGI with dose elevation.
DDIs seemed to increase the risk of drug switching and/or dose reduction (aOR: 2.82, 95% CI: 0.49–15.97), which was mainly facilitated by the co-presence of CYP2C19 inhibitors, but seemingly not to increase the risk of dose elevation and early discontinuation (Table 8, Table 9 and Table 10).
DDGIs also seemed to increase the risk of drug switching and/or dose reduction (aOR: 2.33, 95% CI: 0.42–12.78). However, there were only two participants with DDGIs experiencing drug switching or dose reduction, consisting of one participant with a DDGI affecting one pathway and the other one with a DDGI affecting two pathways (Supplementary S2). Consequently, a separated analysis of DDGIs based on the number of pathways affected produced comparable effect sizes (DDGI affecting one pathway: aOR: 2.52, 95% CI: 0.26–24.61; DDGI affecting two pathways: aOR: 2.17, 95% CI: 0.23–20.67).
Overall, there were no associations between the exposures and any outcomes tested reaching the statistical significance threshold of a false discovery rate-adjusted p-value (q > 0.05).
Analysis using a time frame of 45 days after the index date produced comparable results. CYP2C19 IM increased the risk of switching and the effect size was also larger in combination with CYP3A4 IM/PM (aOR: 6.41, 95% CI: 1.19–34.40) than with CYP3A4 NM (aOR: 2.66, 95% CI: 0.65–10.96). CYP2C19 IM seemingly increased the risk of dose reduction (aOR: 2.69, 95% CI: 0.43–16.96). Lastly, DDIs and DDGIs have a tendency to increase the risk of dose reduction and switching, respectively. Detailed data can be found in Supplementary S3.
4. Discussion
In this explorative inception cohort study, we presented for both CYP2C19 and CYP3A4 the associations of DGI, DDI, and DDGI and the risk of switching or dose adjustments and early discontinuation in the first treatment episode of (es)citalopram. In our relatively small samples, we found an indication that participants with DGI involving predicted CYP2C19 IM tended to experience switching and/or dose reduction, instead of early discontinuation, regardless of the CYP3A4 predicted phenotype. For participants with DGI involving predicted CYP3A4 IM/PM, no influence on switching and/or dose reduction was found. Yet, the effect of CYP2C19 IM might be enhanced by the presence of CYP3A4 IM. DDI and DDGI might be associated with an increased risk of switching or dose reduction, but the associations were not significant with wide confidence intervals.
We found that participants with CYP2C19 IM were more likely to experience switching than those with NM. This is consistent with the study reported by Mrazek et al. which showed that individuals with CYP2C19 reduced catalytic function were less tolerant to citalopram than those with increased catalytic function [40]. We also found that (es)citalopram users with CYP2C19 IM tended to experience dose reductions more than those with CYP2C19 NM. Decreasing the maximum daily dose of (es)citalopram in patients with CYP2C19 IM by 25% of the normal maximum dose is recommended by the DPWG [41]. As a note, we possibly managed to find some associations on CYP2C19 IM and the outcomes because we had a large enough number of (es)citalopram users with the genotype (about 33% of the cohort).
Unfortunately, we did not find any significant association between patients with CYP2C19 PM and UM to the outcomes which was probably due to a limited sample size. Some clinical studies reported that patients with CYP2C19 PM were exposed to (es)citalopram blood concentration to a greater extent than CYP2C19 IM and that patients with CYP2C19 UM had a lower exposure to (es)citalopram compared to CY2C19 NMs [7]. Jukic et al. using about 2000 genotyped persons from the Oslo population found that escitalopram users with CYP2C19 UM and PM (33% of the study population) had a three times higher odds of switching to another antidepressant than those with CYP2C19 NM [20].
To the best of our knowledge, this is the first study to examine the impact of CYP3A4 alone and in combination with CYP2C19 on (es)citalopram treatment. Decreased function of CYP3A4 in the CYP2C19 NM participants did not seem to influence the outcomes, but might have increased the effect of CYP2C19 IM. A comparable trend of effects has been reported for CYP2D6. The effect of the CYP2D6 variant in individuals with CYP2C19 NM on the AUC of citalopram was limited. However, when there was a co-presence of CYP2C19 *1/*2 (IM), the influence of CYP2D6 *1/*4 (IM) became stronger [10].
In our dataset, there were about nine percent of (es)citalopram users exposed to potential DDIs. This might be because about 79% of our study population had at least one comorbidity and therefore, they used other drug(s) which might potentially interact with (es)citalopram. In the Lifelines population, the most prevalent potential CYP2C19 mediated DDI was citalopram and omeprazole [42]. Omeprazole was reported to increase s-citalopram plasma concentration by about 50% to 120% [43,44]. Therefore, it has been recommended that patients with omeprazole or esomeprazole should have a dose adjustment of (es)citalopram [45].
Although we did not find any significant associations between DDGI and the outcomes, this study is the first to explore the impact of complex DDGI on the (es)citalopram treatment at the population level. Generally, DDGI may come in two main scenarios [14,15]. Firstly, it may only affect one metabolic pathway of a drug, for example overlapping conditions between a CYP2C19 UM/IM/PM and a CYP2C19 inhibitor in (es)citalopram users. In this scenario, we might expect that the level of blood concentration of (es)citalopram in an individual with a CYP2C19 UM and a CYP2C19 inhibitor might be different from an individual with a CYP2C19 IM and a CYP2C19 inhibitor [15]. This is because the more the number of active allelic variants in the CYP450, the more difficult for their phenotypes to be converted by the co-presence of inhibitors [46]. The second main scenario is the alteration of two or even three metabolic pathways of a drug. The alteration can be a result of the presence of deviating genotypes in one/two metabolic pathway(s) and the co-presence of CYP modulator in one/two other pathway(s). In this scenario, each possible combination of co-inhibition produced by genetic variation and CYP modulators might result in variation of (es)citalopram concentration in the blood [15]. Therefore, the effect of DDGI can vary depending on the scenario of interactions, the metabolic contribution of the inhibited pathway(s), and the potency of CYP modulators [15]. In this study, since we had only two patients with DDGI experiencing switching (Supplementary S2), we could not explore more about the impact of the different scenarios on the outcomes. It is plausible because the number of patients with DDI and DGI were limited, we could expect that the number of patients exposed to DDGI is even less. Hence, further study with a larger dataset is needed to provide solid evidence about the impact of DDGI in clinical practice which can be used to support the lack of pharmacotherapeutic management of DDGI in the current guidelines.
Since genotyping is still not a part of routine clinical testing, prescribers often have no indication about the genotype of the patients at the time of prescription. Consequently, the presence of DGI and DDGI related to (es)citalopram, exposing 56% of our study population, is potentially missed by health practitioners. Therefore, in order to avoid DGI and DDGI complex interaction, pre-emptive genotyping, inclusion of genetic information in electronic health records as well as a sophisticated computerized drug interaction surveillance system are needed in clinical practice.
Several potential limitations need to be discussed. First, we did not have data on the blood concentration of (es)citalopram as the best indicator to show the effect of interactions. Consequently, we could not ascertain the effect of DDI/DGI/DDGI on the citalopram metabolism and validate the associations between the exposures and the outcomes. In addition, we did not have information about the genotype status of CYP2D6. Therefore, we could not assess the combined effects of CYP2C19/3A4/2D6 polymorphisms on (es)citalopram efficacy. CYP2D6 is the most polymorphic CYP enzyme and the prevalence of people with CYP2D6 IM and PM genotypes in the Caucasian population is 40% and 10%, respectively [47]. Therefore, there might be some persons with CYP2D6 polymorphisms among our participants. Despite its minor metabolic contribution on (es)citalopram disposition, CYP2D6 polymorphism might corroborate the alteration of citalopram clearance in the presence of CYP2C19 polymorphism [10]. It was reported in a small DGI study among healthy persons that one participant with CYP2C19 PM and CYP2D6 PM taking citalopram developed severe side effects and was withdrawn early before the study was completed [48]. Therefore, there was a possibility that the co-presence of combined CYP2C19/3A4/2D6 polymorphisms might produce a substantial effect on citalopram disposition.
Furthermore, though, our study population from the PharmLines database is rather large (6379 participants), the statistical power of the study is relatively low to detect significant associations between multiple exposures as DGI, DDI, DDGI and outcomes. Therefore, the results of this study should be interpreted as hypothesis-generating rather than confirmative to explore potential effects of DGI, DDI, and DDGI on the prognosis of (es)citalopram treatment. Much larger studies are required to further confirm our findings. Lastly, about 30% of our dataset had no information about the dose of (es)citalopram. The missingness may probably not be related to other variables since it may be because pharmacists or pharmacy technicians forgot to include the dose information before sending the prescription data to the IADB.nl. In the baseline comparisons, we found that patients without dosing information were significantly older than those with complete information (Supplementary S2). Hence, we might underestimate the effect of age on the dose adjustments of (es)citalopram. Among those with complete information, age seemed not to influence the dose elevation or reduction of (es)citalopram (Table 7).
5. Conclusions
In conclusion, the predicted CYP2C19 IM phenotype increased the need of drug switching and/or dose reduction, and the co-presence of CYP3A4 IM enhanced these effects. Therefore, when patients receive (es)citalopram, it is important to not only consider the genetic information for CYP2C19 but also the genetic status of CYP3A4 as well.
Despite the fact that DDI and DDGI showed trends towards increased risks of switching and/or dose reduction, no conclusions can be drawn from the results because there were great uncertainties surrounding the estimates. Therefore, further real-world studies with larger samples are needed to confirm the results.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4426/10/4/256/s1, Table S1: List of comorbidities; Table S2.1: Separated analysis of drug switching and dose reduction; Table S2.2: Differentiation of DDGI affecting one and two pathways; Table S2.3: Sensitivity Analysis for specific combination of CYP2C19 & CYP3A4 Phenotypes; Table S2.4: Demographics of participants, discriminated between complete and missing dose information; Table S3.1: Frequency of DDGI for time frame of 45 days; Table S3.2: Baseline comparisons for time frame of 45 days; Table S3.3: Association between exposures and outcomes for time frame of 45 days.
Author Contributions
Conceptualization: M.A.B., P.L., J.H.J.B., R.H.S., E.H. and B.W.; Data curation, M.A.B., P.L. and J.H.J.B.; Formal analysis, M.A.B.; Investigation, M.A.B., P.L. and J.H.J.B.; Methodology, M.A.B., E.H. and B.W.; Project administration, J.H.J.B.; Resources, P.L. and J.H.J.B.; Software, J.H.J.B.; Supervision, R.H.S., E.H. and B.W.; Writing—original draft, M.A.B. and B.W.; Writing—review & editing, P.L., J.H.J.B., R.H.S. and E.H. All authors have read and agreed to the published version of the manuscript.
Funding
The Lifelines Biobank initiative has been made possible by funds from FES (Fonds Economische Structuurversterking), SNN (Samenwerkingsverband Noord Nederland) and REP (Ruimtelijk Economisch Programma). The IADB.nl is funded by the University of Groningen. Muh. Akbar Bahar obtained a DIKTI scholarship from the Ministry of Research, Technology and Higher Education of Indonesia. The funding organizations had no role and influence in the study design and results.
Acknowledgments
The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centres delivering data to Lifelines, and all the study participants, and the participating IADB.nl pharmacies for kindly providing their data for research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abbing-Karahagopian, V.; Huerta, C.; Souverein, P.C.; De Abajo, F.; Leufkens, H.G.M.; Slattery, J.; Alvarez, Y.; Miret, M.; Gil, M.; Oliva, B.; et al. Antidepressant prescribing in five European countries: Application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur. J. Clin. Pharmacol. 2014, 70, 849–857. [Google Scholar] [CrossRef]
- Kaplan, C.; Zhang, Y. Assessing the comparative-effectiveness of antidepressants commonly prescribed for depression in the US Medicare population. J. Ment. Health Policy Econ. 2012, 15, 171–178. [Google Scholar]
- Li, G.; Shen, Y.; Luo, J.; Li, H. Efficacy of escitalopram monotherapy in the treatment of major depressive disorder: A pooled analysis of 4 Chinese clinical trials. Medicine 2017, 96, e8142. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowitz, B.; McGrath, P.J.; et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: Implications for clinical practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [CrossRef]
- Fredricson Overo, K. Kinetics of citalopram in man; plasma levels in patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 1982, 6, 311–318. [Google Scholar] [CrossRef]
- Jin, Y.; Pollock, B.G.; Frank, E.; Cassano, G.B.; Rucci, P.; Müller, D.J.; Kennedy, J.L.; Forgione, R.N.; Kirshner, M.; Kepple, G.; et al. Effect of age, weight, and CYP2C19 genotype on escitalopram exposure. J. Clin. Pharmacol. 2010, 50, 62–72. [Google Scholar] [CrossRef]
- Chang, M.; Tybring, G.; Dahl, M.L.; Lindh, J.D. Impact of cytochrome P450 2C19 polymorphisms on citalopram/escitalopram exposure: A systematic review and meta-analysis. Clin. Pharmacokinet. 2014, 53, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Rochat, B.; Amey, M.; Gillet, M.; Meyer, U.A.; Baumann, P. Identification of three cytochrome P450 isozymes involved in N-demethylation of citalopram enantiomers in human liver microsomes. Pharmacogenetics 1997, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- von Moltke, L.L.; Greenblatt, D.J.; Giancarlo, G.M.; Granda, B.W.; Harmatz, J.S.; Shader, R.I. Escitalopram (S-citalopram) and its metabolites in vitro: Cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab. Dispos. 2001, 29, 1102–1109. [Google Scholar] [PubMed]
- Fudio, S.; Borobia, A.M.; Piñana, E.; Ramírez, E.; Tabarés, B.; Guerra, P.; Carcas, A.; Frías, J. Evaluation of the influence of sex and CYP2C19 and CYP2D6 polymorphisms in the disposition of citalopram. Eur. J. Pharmacol. 2010, 626, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Westervelt, P.; Cho, K.; Bright, D.R.; Kisor, D.F. Drug-gene interactions: Inherent variability in drug maintenance dose requirements. Pharm. Ther. 2014, 39, 630–637. [Google Scholar]
- Wenzel-Seifert, K.; Brandl, R.; Hiemke, C.; Haen, E. Influence of concomitant medications on the total clearance and the risk for supra-therapeutic plasma concentrations of Citalopram. A population-based cohort study. Pharmacopsychiatry 2014, 47, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Thirumaran, R.K.; Heck, J.W.; Hocum, B.T. CYP450 genotyping and cumulative drug–gene interactions: An update for precision medicine. Future Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Verbeurgt, P.; Mamiya, T.; Oesterheld, J. How common are drug and gene interactions? Prevalence in a sample of 1143 patients with CYP2C9, CYP2C19 and CYP2D6 genotyping. Pharmacogenomics 2014, 15, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.A.; Setiawan, D.; Hak, E.; Wilffert, B. Pharmacogenetics of drug–drug interaction and drug–drug–gene interaction: A systematic review on CYP2C9, CYP2C19 and CYP2D6. Pharmacogenomics 2017, 18, 701–739. [Google Scholar] [CrossRef] [PubMed]
- Malki, M.A.; Pearson, E.R. Drug–drug–gene interactions and adverse drug reactions. Pharm. J. 2019, 20, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef]
- Swen, J.J.; Nijenhuis, M.; de Boer, A.; Grandia, L.; Maitland-van der Zee, A.H.; Mulder, H.; Rongen, G.A.P.J.M.; Van Schaik, R.H.N.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From bench to byte—An update of guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef]
- Sediq, R.; van der Schans, J.; Dotinga, A.; Alingh, R.A.; Wilffert, B.; Bos, J.H.; Schuiling-Veninga, C.C.; Hak, E. Concordance assessment of self-reported medication use in the netherlands three-generation lifelines Cohort study with the pharmacy database iaDB. nl: The Pharmlines initiative. Clin. Epidemiol. 2018, 10, 981. [Google Scholar] [CrossRef]
- Jukić, M.M.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2087 patients. Am. J. Psychiatry 2018, 175, 463–470. [Google Scholar] [CrossRef]
- Bijl, M.J.; Visser, L.E.; Hofman, A.; Vulto, A.G.; Van Gelder, T.; Stricker, B.H.C.; Van Schaik, R.H. Influence of the CYP2D6* 4 polymorphism on dose, switching and discontinuation of antidepressants. Br. J. Clin. Pharmacol. 2008, 65, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.; Dotinga, A.; Vonk, J.M.; Van Dijk, F.; van Zon, S.K.; Wijmenga, C.; Wolffenbuttel, B.H.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2014, 44, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Stolk, R.P.; Rosmalen, J.G.; Postma, D.S.; de Boer, R.A.; Navis, G.; Slaets, J.P.; Ormel, J.; Wolffenbuttel, B.H. Universal risk factors for multifactorial diseases. Eur. J. Epidemiol. 2008, 23, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Klijs, B.; Scholtens, S.; Mandemakers, J.J.; Snieder, H.; Stolk, R.P.; Smidt, N. Representativeness of the LifeLines cohort study. PLoS ONE 2015, 10, e0137203. [Google Scholar] [CrossRef]
- Visser, S.T.; Schuiling-Veninga, C.C.; Bos, J.H.; de Jong-van den Berg Lolkje, T.W.; Postma, M.J. The population-based prescription database IADB. nl: Its development, usefulness in outcomes research and challenges. Expert Rev. Pharm. Outcomes Res. 2013, 13, 285–292. [Google Scholar]
- Bahar, M.; Hak, E.; Bos, J.H.; Borgsteede, S.D.; Wilffert, B. The burden and management of cytochrome P450 2D6 (CYP2D6)-mediated drug–drug interaction (DDI): Co-medication of metoprolol and paroxetine or fluoxetine in the elderly. Pharmacoepidemiol. Drug Saf. 2017, 26, 752–765. [Google Scholar] [CrossRef]
- Bahar, M.A.; Wang, Y.; Bos, J.H.; Wilffert, B.; Hak, E. Discontinuation and dose adjustment of metoprolol after metoprolol-paroxetine/fluoxetine co-prescription in Dutch elderly. Pharmacoepidemiol. Drug Saf. 2018, 27, 621–629. [Google Scholar] [CrossRef]
- Daud, A.N.; Bergman, J.E.; Oktora, M.P.; Kerstjens-Frederikse, W.S.; Groen, H.; Bos, J.H.; Hak, E.; Wilffert, B. Maternal use of drug substrates of placental transporters and the effect of transporter-mediated drug interactions on the risk of congenital anomalies. PLoS ONE 2017, 12, e0173530. [Google Scholar] [CrossRef]
- Gaedigk, A.; Ingelman-Sundberg, M.; Miller, N.A.; Leeder, J.S.; Whirl-Carrillo, M.; Klein, T.E.; PharmVar Steering Committee. The Pharmacogene Variation (PharmVar) Consortium: Incorporation of the human cytochrome P450 (CYP) allele nomenclature database. Clin. Pharmacol. Ther. 2018, 103, 399–401. [Google Scholar] [CrossRef]
- García-Martín, E.; Martínez, C.; Pizarro, R.M.; García-Gamito, F.J.; Gullsten, H.; Raunio, H.; Agúndez, J.A. CYP3A4 variant alleles in white individuals with low CYP3A4 enzyme activity. Clin. Pharmacol. Ther. 2002, 71, 196–204. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, Y.Z.; Kan, Q.C.; Zhang, L.R.; Li, Z.S.; Lu, H.; Wang, Z.Y.; Chu, Q.J.; Zhang, J. CYP3A4* 1G genetic polymorphism influences CYP3A activity and response to fentanyl in Chinese gynecologic patients. Eur. J. Clin. Pharmacol. 2010, 66, 61. [Google Scholar] [CrossRef] [PubMed]
- Borgsteede, S. Commentaren Medicatiebewaking; Health Base: Houten, The Netherlands, 2015. [Google Scholar]
- Flockhart, D. Drug Interactions: Cytochrome P450 Drug Interaction Table; Indiana University School of Medicine: Indianapolis, IN, USA, 2012. [Google Scholar]
- Palaniyappan, L.; Insole, L.; Ferrier, N. Combining antidepressants: A review of evidence. Adv. Psychiatr. Treat. 2009, 15, 90–99. [Google Scholar] [CrossRef]
- Olfson, M.; Marcus, S.C.; Tedeschi, M.; Wan, G.J. Continuity of antidepressant treatment for adults with depression in the United States. Am. J. Psychiatry 2006, 163, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Saragoussi, D.; Chollet, J.; Bineau, S.; Chalem, Y.; Milea, D. Antidepressant switching patterns in the treatment of major depressive disorder: A General Practice Research Database (GPRD) study. Int. J. Clin. Pract. 2012, 66, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.A.; Dusetzina, S.B.; Dominik, R.C.; Gaynes, B.N. Prescription refill records as a screening tool to identify antidepressant non-adherence. Pharmacoepidemiol. Drug Saf. 2010, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Van Boven, J.F.; Van Raaij, J.J.; Van Der Galiën, R.; Postma, M.J.; Van Der Molen, T.; Dekhuijzen, P.R.; Vegter, S. Impact of multiple-dose versus single-dose inhaler devices on COPD patients’ persistence with long-acting β 2-agonists: A dispensing database analysis. NPJ Prim. Care Respir. Med. 2014, 24, 14069. [Google Scholar] [CrossRef][Green Version]
- De Jong, J.C.; Van Den Berg, P.B.; Tobi, H.; De Jong, L.T.; Van Den Berg. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br. J. Clin. Pharmacol. 2003, 55, 591–595. [Google Scholar] [CrossRef]
- Mrazek, D.A.; Biernacka, J.M.; O’Kane, D.J.; Black, J.L.; Cunningham, J.M.; Drews, M.S.; Snyder, K.A.; Stevens, S.R.; Rush, A.J.; Weinshilboum, R.M. CYP2C19 variation and citalopram response. Pharmacogenet. Genom. 2011, 21, 1–9. [Google Scholar] [CrossRef]
- Dutch Pharmacogenetics Working Group. Dutch Pharmacogenetics Working Group Guidelines; Dutch Pharmacogenetics Working Group: The Hague, The Netherlands, 2018. [Google Scholar]
- Bahar, M.; Bos, J.H.; Borgsteede, S.D.; Dotinga, A.; Alingh, R.A.; Wilffert, B.; Hak, E. Prevalence and Accuracy of Information on CYP2D6, CYP2C19, and CYP2C9 Related Substrate and Inhibitor Co-Prescriptions in the General Population: A Cross-Sectional Descriptive Study as Part of the PharmLines Initiative. Front. Pharmacol. 2020, 11, 624. [Google Scholar] [CrossRef]
- Malling, D.; Poulsen, M.; Søgaard, B. The effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram in healthy subjects. Br. J. Clin. Pharmacol. 2005, 60, 287–290. [Google Scholar] [CrossRef]
- Rocha, A.; Coelho, E.B.; Sampaio, S.A.; Lanchote, V.L. Omeprazole preferentially inhibits the metabolism of (+)-(S)-citalopram in healthy volunteers. Br. J. Clin. Pharmacol. 2010, 70, 43–51. [Google Scholar] [CrossRef]
- Gjestad, C.; Westin, A.A.; Skogvoll, E.; Spigset, O. Effect of proton pump inhibitors on the serum concentrations of the selective serotonin reuptake inhibitors citalopram, escitalopram, and sertraline. Ther. Drug Monit. 2015, 37, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Storelli, F.; Matthey, A.; Lenglet, S.; Thomas, A.; Desmeules, J.; Daali, Y. Impact of CYP2D6 functional allelic variations on phenoconversion and drug–drug interactions. Clin. Pharmacol. Ther. 2018, 104, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Wijnen, P.A.H.M.; Op den Buijsch, R.A.M.; Drent, M.; Kuipers, P.M.J.C.; Neef, C.; Bast, A.; Bekers, O.; Koek, G.H. The prevalence and clinical relevance of cytochrome P450 polymorphisms. Aliment. Pharmacol. Ther. 2007, 26, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Herrlin, K.; Yasui-Furukori, N.; Tybring, G.; Widén, J.; Gustafsson, L.L.; Bertilsson, L. Metabolism of citalopram enantiomers in CYP2C19/CYP2D6 phenotyped panels of healthy Swedes. Br. J. Clin. Pharmacol. 2003, 56, 415–421. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).