Clinical Outcomes Based on Measurable Residual Disease Status in Patients with Core-Binding Factor Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
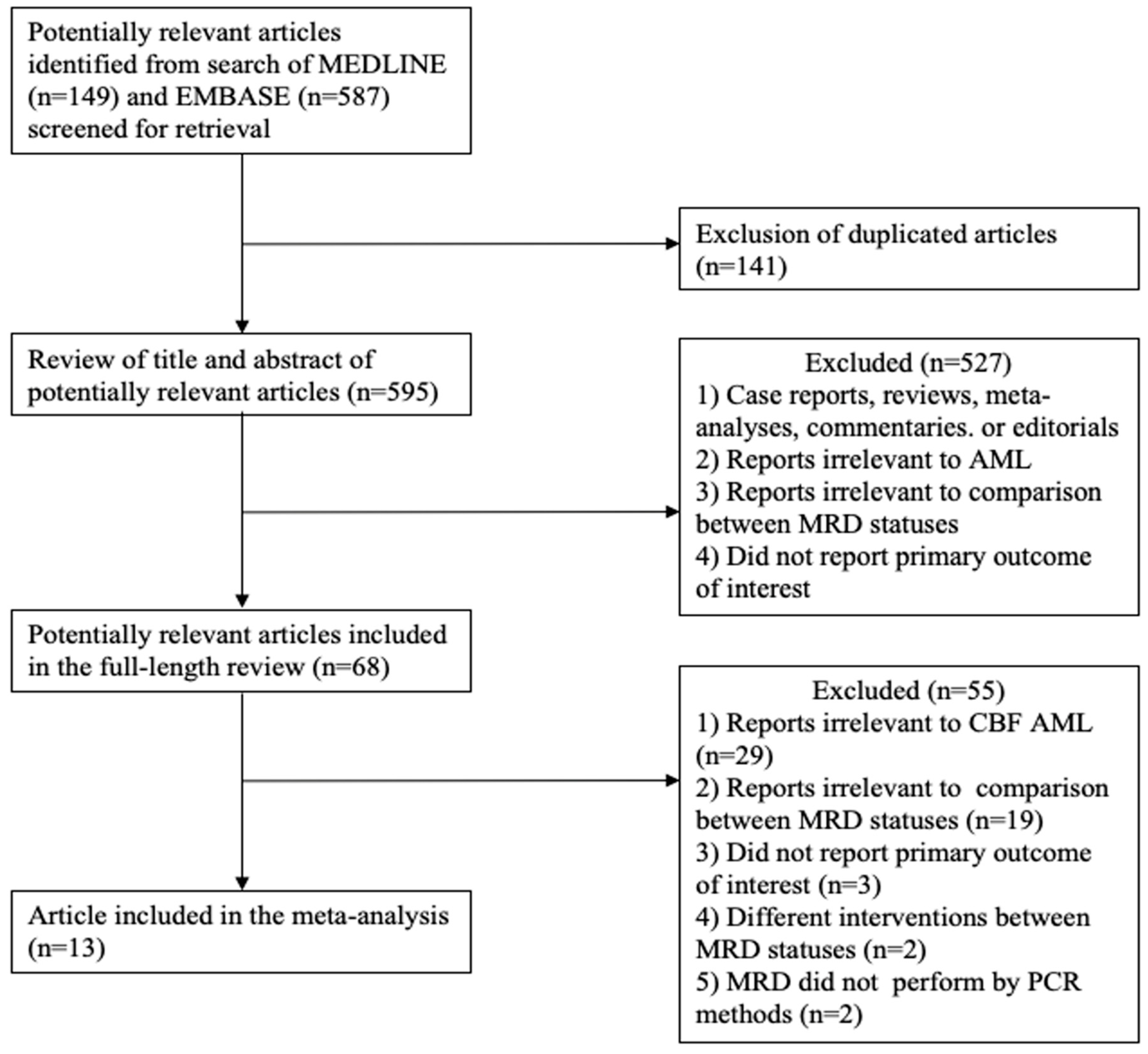
2.1. Data Sources and Searches
2.2. Selection Criteria and Data Extraction
2.3. Definition of Outcomes
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
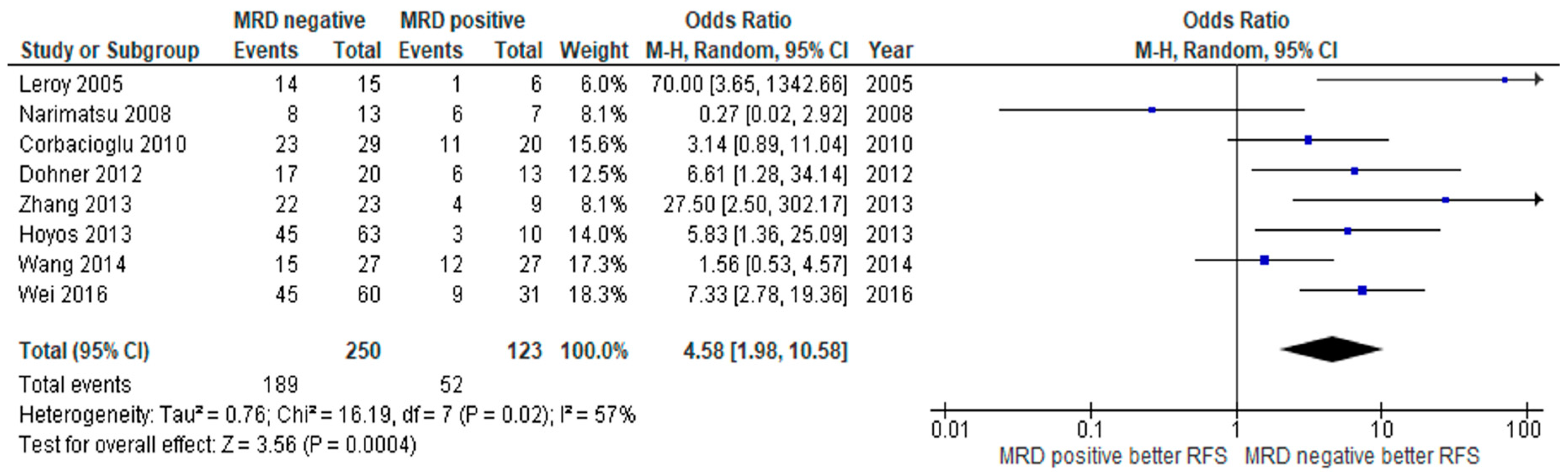
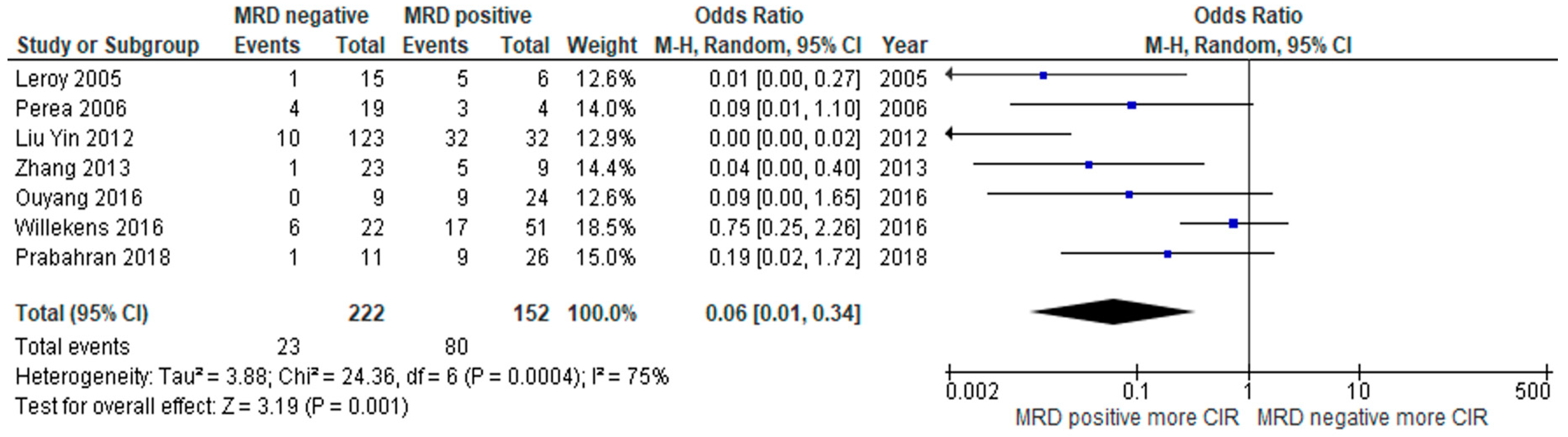
3.2. Clinical Outcome
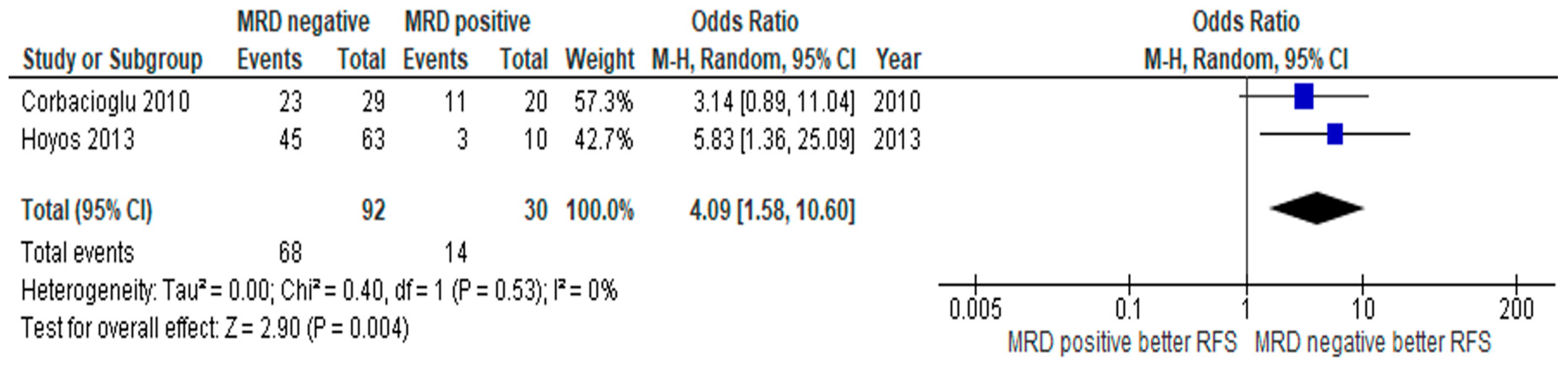
3.3. Subgroup Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tallman, M.S.; Gilliland, D.; Rowe, J.M. Drug therapy for acute myeloid leukemia. Blood 2005, 106, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Sinha, C.; Cunningham, L.C.; Liu, P.P. Core binding factor acute myeloid leukemia: New prognostic categories and therapeutic opportunities. Semin. Hematol. 2015, 52, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Mrózek, K.; Heinonen, K.; de La Chapelle, A.; Bloomfield, C.D. Clinical significance of cytogenetics in acute myeloid leukemia. Semin. Oncol. 1997, 24, 17. [Google Scholar] [PubMed]
- Solh, M.; Yohe, S.; Weisdorf, D.; Ustun, C. Core-binding factor acute myeloid leukemia: Heterogeneity, monitoring, and therapy. Am. J. Hematol. 2014, 89, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.-C.; Lo-Coco, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- Ravandi, F.; Walter, R.B.; Freeman, S.D. Evaluating measurable residual disease in acute myeloid leukemia. Blood Adv. 2018, 2, 1356–1366. [Google Scholar] [CrossRef]
- Mosna, F.; Capelli, D.; Gottardi, M. Minimal residual disease in acute myeloid leukemia: Still a work in progress? J. Clin. Med. 2017, 6, 57. [Google Scholar] [CrossRef]
- del Principe, M.I.; Lo-Coco, F.; Maurillo, L.; Sconocchia, G.; Cefalo, M.; Consalvo, M.I.; Sarlo, C.; Conti, C.; De Santis, G.; De Bellis, E.; et al. Minimal residual disease in acute myeloid leukemia of adults: Determination, prognostic impact and clinical applications. Mediterr. J. Hematol. Infect. Dis. 2016, 8, 2016052. [Google Scholar] [CrossRef]
- Berry, D.A.; Zhou, S.; Higley, H.; Mukundan, L.; Fu, S.; Reaman, G.H.; Wood, B.L.; Kelloff, G.J.; Jessup, J.M.; Radich, J.P. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia. JAMA Oncol. 2017, 3, e170580. [Google Scholar] [CrossRef]
- Buccisano, F.; Maurillo, L.; Del Principe, M.I.; Del Poeta, G.; Sconocchia, G.; Lo-Coco, F.; Arcese, W.; Amadori, S.; Venditti, A. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood 2012, 119, 332–341. [Google Scholar] [CrossRef]
- Ossenkoppele, G.J.; Schuurhuis, G.J. MRD in AML: Does it already guide therapy decision-making? Hematol 2016, 2016, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; PRISMA-P Group; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: West Sussexc, UK, 2009. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Leroy, H.; De Botton, S.; Grardel-Duflos, N.; Darre, S.; Leleu, X.; Roumier, C.; Morschhauser, F.; Lai, J.-L.; Bauters, F.; Fenaux, P.; et al. Prognostic value of real-time quantitative PCR (RQ-PCR) in AML with t(8;21). Leuk 2005, 19, 367–372. [Google Scholar] [CrossRef]
- Perea, G.; Lasa, A.; Aventín, A.; Domingo, A.M.; Villamor, N.; de Llano, M.P.Q.; Llorente, A.; Junca, J.; Palacios, C.D.F.; Fernández, C.; et al. Prognostic value of minimal residual disease (MRD) in acute myeloid leukemia (AML) with favorable cytogenetics [t(8;21) and inv(16)]. Leuk 2006, 20, 87–94. [Google Scholar] [CrossRef]
- Narimatsu, H.; Iino, M.; Ichihashi, T.; Yokozawa, T.; Hayakawa, M.; Kiyoi, H.; Takeo, T.; Sawamoto, A.; Iida, H.; Tsuzuki, M.; et al. Clinical significance of minimal residual disease in patients with t(8;21) acute myeloid leukemia in Japan. Int. J. Hematol. 2008, 88, 154–158. [Google Scholar] [CrossRef]
- Corbacioglu, A.; Scholl, C.; Schlenk, R.F.; Eiwen, K.; Du, J.; Bullinger, L.; Fröhling, S.; Reimer, P.; Rummel, M.; Derigs, H.-G.; et al. Prognostic impact of minimal residual disease inCBFB-MYH11–positive acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 3724–3729. [Google Scholar] [CrossRef]
- Döhner, K. Prognostic impact of minimal residual disease in RUNX1-RUNX1T1 acute myeloid leukemia (AML): A study of the german-austrian-AML study group (AMLSG). Haematologica 2012, 97, 216. [Google Scholar]
- Yin, J.A.L.; O’Brien, M.A.; Hills, R.K.; Daly, S.B.; Wheatley, K.; Burnett, A.K. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: Results of the United Kingdom MRC AML-15 trial. Blood 2012, 120, 2826–2835. [Google Scholar] [CrossRef]
- Hoyos, M.; Nomdedeu, J.F.; Esteve, J.; Duarte, R.; Ribera, J.M.; Llorente, A.; Escoda, L.; Bueno, J.; Tormo, M.; Gallardo, D.; et al. Core binding factor acute myeloid leukemia: The impact of age, leukocyte count, molecular findings, and minimal residual disease. Eur. J. Haematol. 2013, 91, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Y.; Lin, N.; Zhou, C.; Liu, B.; Qiu, S.; Gong, B.; Zhang, G.; Liu, K.; Wei, S.; et al. MRD is an independent prognostic factor in acute myeloid leukemia carrying t(8;21) chromosomal abnormalities. Blood 2016, 128, 1672. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Li, W.; Liu, B.; Wang, Y.; Lin, D.; Zhou, C.; Li, C.; Wang, J.; Mi, Y. Monitoring of minimal residual disease in acute myeloid leukemia with t(8;21)(q22;q22). Int. J. Hematol. 2013, 97, 786–792. [Google Scholar] [CrossRef]
- Wang, L.; Gao, L.; Xu, S.; Gong, S.; Liu, M.; Qiu, H.; Xu, X.; Ni, X.; Chen, L.; Lu, S.; et al. High prognostic value of minimal residual disease detected by flow-cytometry-enhanced fluorescence in situ hybridization in core-binding factor acute myeloid leukemia (CBF-AML). Ann. Hematol. 2014, 93, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Goswami, M.; Peng, J.; Zuo, Z.; Daver, N.; Borthakur, G.; Tang, G.; Medeiros, L.J.; Jorgensen, J.L.; Ravandi, F.; et al. Comparison of multiparameter flow cytometry immunophenotypic analysis and quantitative RT-PCR for the detection of minimal residual disease of core binding factor acute myeloid leukemia. Am. J. Clin. Pathol. 2016, 145, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Willekens, C.; Blanchet, O.; Renneville, A.; Cornillet-Lefebvre, P.; Pautas, C.; Guieze, R.; Ifrah, N.; Dombret, H.; Jourdan, E.; Preudhomme, C.; et al. Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: Results of the French CBF-2006 trial. Haematol 2015, 101, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Prabahran, A.; Tacey, M.; Fleming, S.; Wei, A.; Tate, C.; Marlton, P.; Ritchie, D. Prognostic markers in core-binding factor AML and improved survival with multiple consolidation cycles of intermediate-/high-dose cytarabine. Eur. J. Haematol. 2018, 101, 174–184. [Google Scholar] [CrossRef]
- Rothenberg-Thurley, M.; Amler, S.; Goerlich, D.; Köhnke, T.; Konstandin, N.P.; Schneider, S.; Sauerland, M.C.; Herold, T.; Hubmann, M.; Ksienzyk, B.; et al. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leuk 2018, 32, 1598–1608. [Google Scholar] [CrossRef]
- Kruse, A.; Abdel-Azim, N.; Kim, Y.-M.; Ruan, Y.; Phan, V.; Ogana, H.; Wang, W.; Lee, R.; Gang, E.J.; Khazal, S.J.; et al. Minimal residual disease detection in acute lymphoblastic leukemia. Int. J. Mol. Sci. 2020, 21, 1054. [Google Scholar] [CrossRef]
- Short, N.J.; Ravandi, F. How close are we to incorporating measurable residual disease into clinical practice for acute myeloid leukemia? Haematol 2019, 104, 1532–1541. [Google Scholar] [CrossRef]
- Versluis, J.; Cornelissen, J.J.; Craddock, C.; Sanz, M.Á.; Canaani, J.; Nagler, A. Acute myeloid leukemia in adults. In The EBMT Handbook; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 507–521. [Google Scholar]
- Estey, E.H. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol. 2018, 93, 1267–1291. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, G.; Liang, C.; Li, G.; Chen, X.; Ma, Q.; Zhai, W.; Yang, D.; He, Y.; Jiang, E.; et al. Combination of cytogenetic classification and MRD status correlates with outcome of autologous versus allogeneic stem cell transplantation in adults with primary acute myeloid leukemia in first remission. Leuk. Res. 2017, 55, 97–104. [Google Scholar] [CrossRef] [PubMed]


| References | Numbers | CBF Types | Treatment | HSCT | Time of MRD Monitoring | MRD Cutoff | Source of MRD | Study Period | |
|---|---|---|---|---|---|---|---|---|---|
| MRD Positive | MRD Negative | ||||||||
| Leroy 2005 [16] | 6 | 15 | RUNX1-RUNX1T1 (n = 21) | Induction: daunorubicin, cytarabine and mitoxantrone Consolidation: mitoxantrone, cytarabine and idarubicin, cytarabine | Allo-HSCT | Pc | 10−5 | PB or BM | 1994–2001 |
| Perea 2006 [17] | 4 | 19 | RUNX1-RUNX1T1 or CBFB-MYH11 (n = 23) | Induction: idarubicin, etoposide, cytarabine Intensification: cytarabine and mitoxantrone Consolidation: HIDAC | Allo-HSCT (secondary AML) | Pc | 10−3 | BM | NA |
| Narimatsu 2008 [18] | 7 | 13 | RUNX1-RUNX1T1 (n = 20) | Induction: idarubicin, cytarabine or daunorubicin, cytarabine Consolidation: HIDAC, IDAC | NR | Pc (cycle1) | 10−3 | BM | 2000–2005 |
| Corbacloglu 2010 [19] | 20 | 29 | CBFB-MYH11 (n = 49) | Induction: ICEx2, ICE then S-HAM or HAM Consolidation: HIDAC | Auto-HSCT Allo-HSCT | Pc (cycle3) | 10−5 | BM | 1992–2006 |
| Dohner 2012 [20] | 13 | 20 | RUNX1-RUNX1T1 (n = 33) | Induction: ICEx2 Consolidation: HIDAC | Auto-HSCT Allo-HSCT | Pc1-Pc | 10−6 | BM | 1992–2004 |
| Liu Yin 2012 [21] (1) | 15 | 76 | RUNX1-RUNX1T1 (n = 91) | Induction: daunorubicin, cytarabine and/or etoposide or FLAG-Idarubicin and/or GO Consolidation: MACE or MIDAC or IDAC/HIDAC and/or GO | NR | Pc (cycle4) | 5 × 10−3 | BM | 2002–2009 |
| Liu Yin 2012 [21] (2) | 17 | 47 | CBFB-MYH11 (n = 64) | Induction: daunorubicin, cytarabine and/or etoposide or FLAG-Idarubicin and/or GO Consolidation: MACE or MIDAC or IDAC/HIDAC and/or GO | NR | Pc (cycle4) | 5 × 10−4 | BM | 2002–2009 |
| Hoyos 2013 [22] | 10 | 63 | CBFB-MYH11 (n = 73) | Induction: idarubicin, cytarabine and etoposide Consolidation: mitoxantone and cytarabine, HIDAC | Auto-HSCT | Pi | 10−2 | BM | 1999–2012 |
| Wei 2016 [23] | 31 | 60 | RUNX1-RUNX1T1 (n = 91) | Induction: homoharringtonine, cytarabine, daunorubicin Consolidation: HIDAC, IDAC | Allo-HSCT | Pi | 10−2 | NA | 2010–2016 |
| Zhang 2013 [24] | 9 | 23 | RUNX1-RUNX1T1 (n = 32) | Induction: cytarabine based chemotherapy Consolidation: HIDAC, IDAC | HSCT | Pi | 10−4 | BM | 2004–2011 |
| Wang 2014 [25] | 27 | 27 | RUNX1-RUNX1T1 or CBFB-MYH11 (n = 54) | Induction: cytarabine, daunorubicin/idarubicin Consolidation: IDAC | No | Pc (cycle4) | 10−3 | BM | NA–2013 |
| Ouyang 2016 [26] | 24 | 9 | RUNX1-RUNX1T1 or CBFB-MYH11 (n = 33) | Induction: FLAG-idarubicin Consolidation: FLAG or decitabine | HSCT (Relapse) | Pi | 10−3 | BM | 2012–2014 |
| Willekens 2016 [27] | 51 | 22 | RUNX1-RUNX1T1 (n = 73) | Induction: cytarabine, daunorubicin Consolidation: HIDAC | No | Pc (cycle3) | 10−5 | BM | 2007–2010 |
| Prabahran 2018 [28] | 26 | 11 | RUNX1-RUNX1T1 or CBFB-MYH11 (n = 37) | Induction: cytarabine, idarubicin/daunorubicin, HIDAC, ICE, FLAG, IDAC, MIDAC Consolidation: HIDAC, ICE, IDAC, FLAG, MIDAC | No | Pi | 10−3 | BM | 2001–2012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotchanapanya, W.; Hokland, P.; Tunsing, P.; Owattanapanich, W. Clinical Outcomes Based on Measurable Residual Disease Status in Patients with Core-Binding Factor Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. J. Pers. Med. 2020, 10, 250. https://doi.org/10.3390/jpm10040250
Rotchanapanya W, Hokland P, Tunsing P, Owattanapanich W. Clinical Outcomes Based on Measurable Residual Disease Status in Patients with Core-Binding Factor Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2020; 10(4):250. https://doi.org/10.3390/jpm10040250
Chicago/Turabian StyleRotchanapanya, Wannaphorn, Peter Hokland, Pattaraporn Tunsing, and Weerapat Owattanapanich. 2020. "Clinical Outcomes Based on Measurable Residual Disease Status in Patients with Core-Binding Factor Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 10, no. 4: 250. https://doi.org/10.3390/jpm10040250
APA StyleRotchanapanya, W., Hokland, P., Tunsing, P., & Owattanapanich, W. (2020). Clinical Outcomes Based on Measurable Residual Disease Status in Patients with Core-Binding Factor Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 10(4), 250. https://doi.org/10.3390/jpm10040250





