Evaluation of a Home Monitoring Application for Follow Up after Lung Transplantation—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Outcomes
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
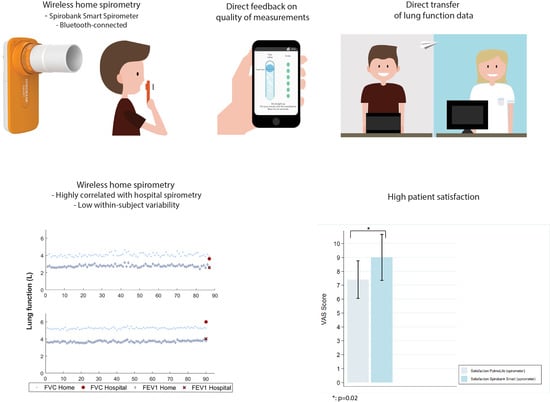
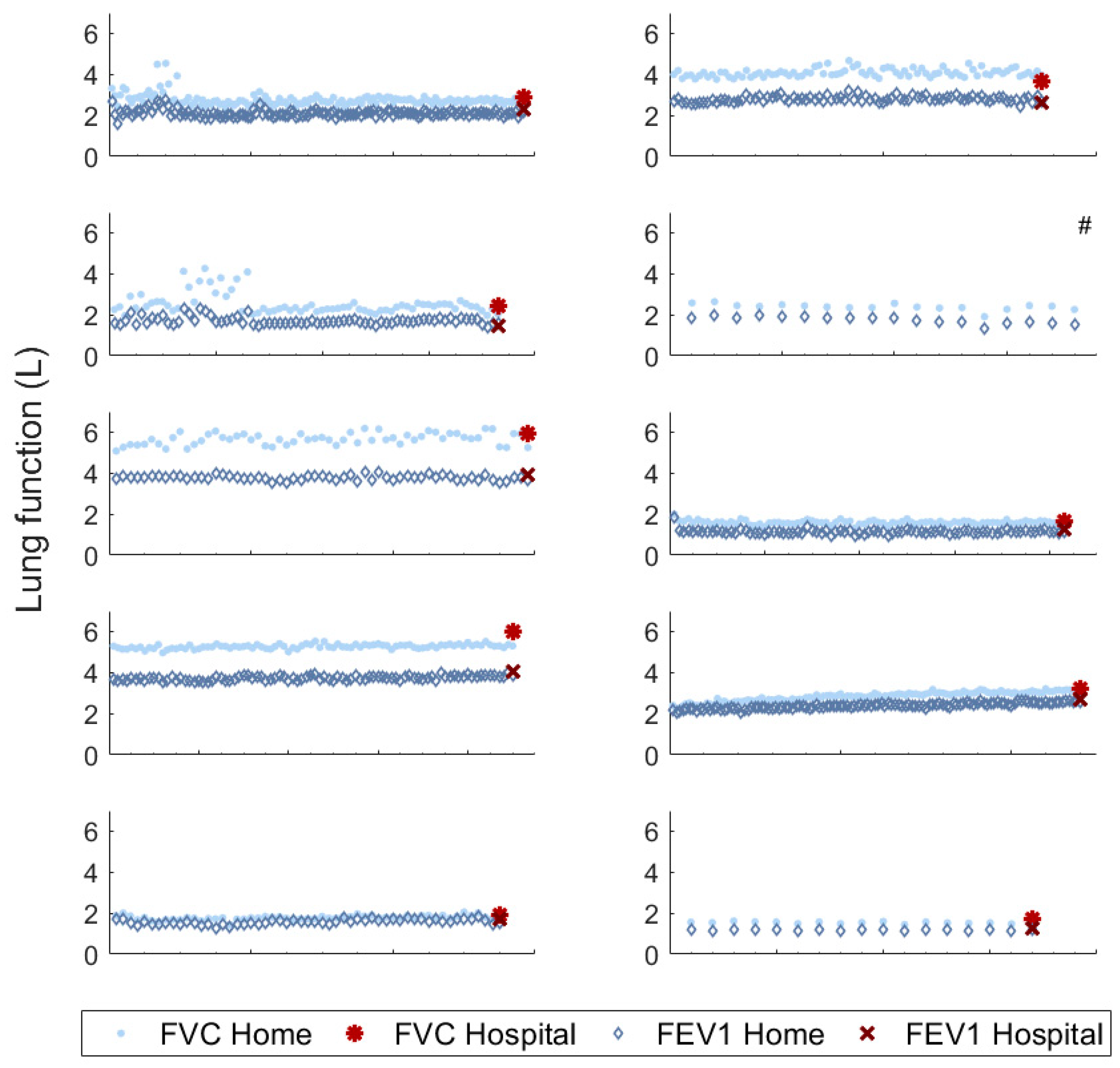
3.2. Feasibility and Reliability
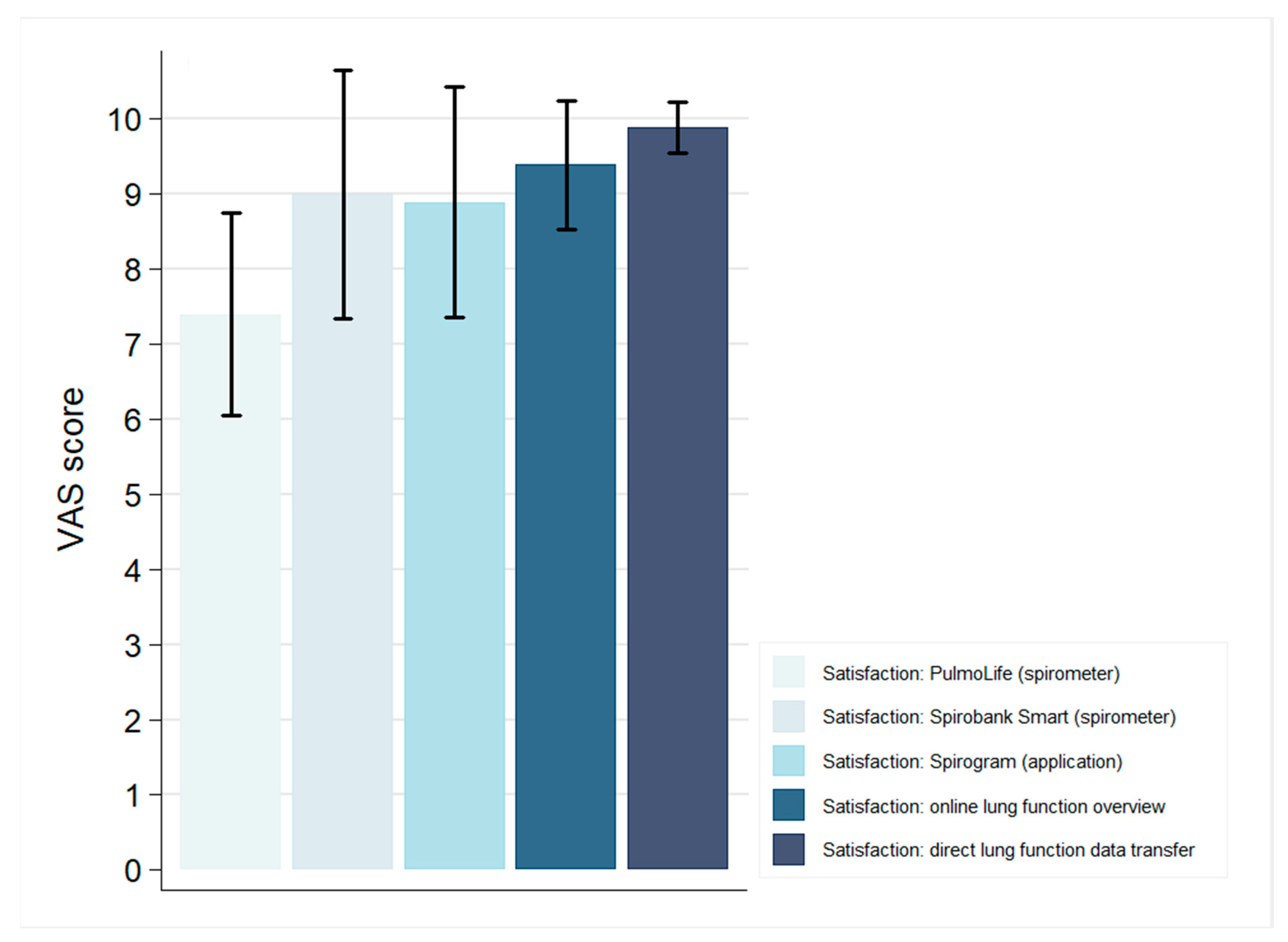
3.3. Patient Experiences
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Opelz, G.; Dohler, B.; Ruhenstroth, A.; Cinca, S.; Unterrainer, C.; Stricker, L.; Scherer, S.; Gombos, P.; Susal, C.; Daniel, V.; et al. The collaborative transplant study registry. Transpl. Rev. (Orlando) 2013, 27, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Verleden, S.E.; Vos, R.; Vanaudenaerde, B.M.; Verleden, G.M. Chronic lung allograft dysfunction phenotypes and treatment. J. Thorac. Dis. 2017, 9, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.M.; Theodore, J.; Dawkins, K.D.; Yousem, S.A.; Blank, N.; Billingham, M.E.; Van Kessel, A.; Jamieson, S.W.; Oyer, P.E.; Baldwin, J.C.; et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest 1984, 86, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Bertram, A.; Fuge, J.; Suhling, H.; Tudorache, I.; Haverich, A.; Welte, T.; Gottlieb, J. Adherence is associated with a favorable outcome after lung transplantation. PLoS ONE 2019, 14, e0226167. [Google Scholar] [CrossRef] [PubMed]
- Sengpiel, J.; Fuehner, T.; Kugler, C.; Avsar, M.; Bodmann, I.; Boemke, A.; Simon, A.; Welte, T.; Gottlieb, J. Use of telehealth technology for home spirometry after lung transplantation: A randomized controlled trial. Prog. Transpl. 2010, 20, 310–317. [Google Scholar] [CrossRef]
- DeVito Dabbs, A.; Song, M.K.; Myers, B.A.; Li, R.; Hawkins, R.P.; Pilewski, J.M.; Bermudez, C.A.; Aubrecht, J.; Begey, A.; Connolly, M.; et al. A randomized controlled trial of a mobile health intervention to promote self-management after lung transplantation. Am. J. Transpl. 2016, 16, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Fadaizadeh, L.; Najafizadeh, K.; Shajareh, E.; Shafaghi, S.; Hosseini, M.; Heydari, G. Home spirometry: Assessment of patient compliance and satisfaction and its impact on early diagnosis of pulmonary symptoms in post-lung transplantation patients. J. Telemed. Telecare 2016, 22, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Morlion, B.; Knoop, C.; Paiva, M.; Estenne, M. Internet-based home monitoring of pulmonary function after lung transplantation. Am. J. Respir. Crit. Care Med. 2002, 165, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Moor, C.C.; Wapenaar, M.; Miedema, J.R.; Geelhoed, J.J.M.; Chandoesing, P.P.; Wijsenbeek, M.S. A home monitoring program including real-time wireless home spirometry in idiopathic pulmonary fibrosis: A pilot study on experiences and barriers. Respir. Res. 2018, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Moor, C.C.; Mostard, R.L.M.; Grutters, J.C.; Bresser, P.; Aerts, J.; Chavannes, N.H.; Wijsenbeek, M.S. Home monitoring in patients with idiopathic pulmonary fibrosis: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2020, 202, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Moor, C.C.; van den Berg, C.A.L.; Visser, L.S.; Aerts, J.G.J.V.; Cottin, V.; Wijsenbeek, M.S. Diurnal variation in forced vital capacity in patients with fibrotic interstitial lung disease using home spirometry. ERJ Open Res. 2020, 6, 00054–02020. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Moor, C.C.; Gür-Demirel, Y.; Wijsenbeek, M.S. Feasibility of a comprehensive home monitoring program for sarcoidosis. J. Pers. Med. 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Geramita, E.M.; DeVito Dabbs, A.J.; DiMartini, A.F.; Pilewski, J.M.; Switzer, G.E.; Posluszny, D.M.; Myaskovsky, L.; Dew, M.A. Impact of a mobile health intervention on long-term nonadherence after lung transplantation: Follow-up after a randomized controlled trial. Transplantation 2020, 104, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Kugler, C.; Fuehner, T.; Dierich, M.; DeWall, C.; Haverich, A.; Simon, A.; Welte, T.; Gottlieb, J. Effect of adherence to home spirometry on bronchiolitis obliterans and graft survival after lung transplantation. Transplantation 2009, 88, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Robson, K.S.; West, A.J. Improving survival outcomes in lung transplant recipients through early detection of bronchiolitis obliterans: Daily home spirometry versus standard pulmonary function testing. Can. J. Respir. Ther. 2014, 50, 17–22. [Google Scholar] [PubMed]
- de Vries, A.P.J.; Alwayn, I.P.J.; Hoek, R.A.S.; van den Berg, A.P.; Ultee, F.C.W.; Vogelaar, S.M.; Haase-Kromwijk, B.J.J.M.; Heemskerk, M.B.A.; Hemke, A.C.; Nijboer, W.N.; et al. Immediate impact of covid-19 on transplant activity in the netherlands. Transpl. Immunol. 2020, 61, 101304. [Google Scholar] [CrossRef] [PubMed]
| Variables | Patient (n = 10) | ||
|---|---|---|---|
| Age | (Years (range)) | 67 | (58–78) |
| Gender | Male | 5 | (50%) |
| Female | 5 | (50%) | |
| Time after transplantation | (Years (range)) | 8.04 | (0.4–19) |
| Type of transplantation | Unilateral | 1 | (10%) |
| Bilateral | 9 | (90%) | |
| Underlying disease | COPD | 3 | (30%) |
| A1AT deficiency-related emphysema | 4 | (40%) | |
| ILD | 3 | (30%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijbenga, N.; Hoek, R.A.S.; Mathot, B.J.; Seghers, L.; van Weezel, J.J.; den Ouden, J.; Wijsenbeek, M.S.; Aerts, J.G.J.V.; Hellemons, M.E.; Moor, C.C. Evaluation of a Home Monitoring Application for Follow Up after Lung Transplantation—A Pilot Study. J. Pers. Med. 2020, 10, 240. https://doi.org/10.3390/jpm10040240
Wijbenga N, Hoek RAS, Mathot BJ, Seghers L, van Weezel JJ, den Ouden J, Wijsenbeek MS, Aerts JGJV, Hellemons ME, Moor CC. Evaluation of a Home Monitoring Application for Follow Up after Lung Transplantation—A Pilot Study. Journal of Personalized Medicine. 2020; 10(4):240. https://doi.org/10.3390/jpm10040240
Chicago/Turabian StyleWijbenga, Nynke, Rogier A. S. Hoek, Bas J. Mathot, Leonard Seghers, Jan J. van Weezel, José den Ouden, Marlies S. Wijsenbeek, Joachim G. J. V. Aerts, Merel E. Hellemons, and Catharina C. Moor. 2020. "Evaluation of a Home Monitoring Application for Follow Up after Lung Transplantation—A Pilot Study" Journal of Personalized Medicine 10, no. 4: 240. https://doi.org/10.3390/jpm10040240
APA StyleWijbenga, N., Hoek, R. A. S., Mathot, B. J., Seghers, L., van Weezel, J. J., den Ouden, J., Wijsenbeek, M. S., Aerts, J. G. J. V., Hellemons, M. E., & Moor, C. C. (2020). Evaluation of a Home Monitoring Application for Follow Up after Lung Transplantation—A Pilot Study. Journal of Personalized Medicine, 10(4), 240. https://doi.org/10.3390/jpm10040240