An Integrated Imaging and Circulating Biomarker Approach for Secondary Tricuspid Regurgitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Measurements and Follow-Up
2.3. Laboratory Measurements and Hemodynamic Assessment
2.4. Echocardiographic Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Baseline Characteristics According to Severity of Tricuspid Regurgitation
3.3. Severity of Tricuspid Regurgitation and Outcome
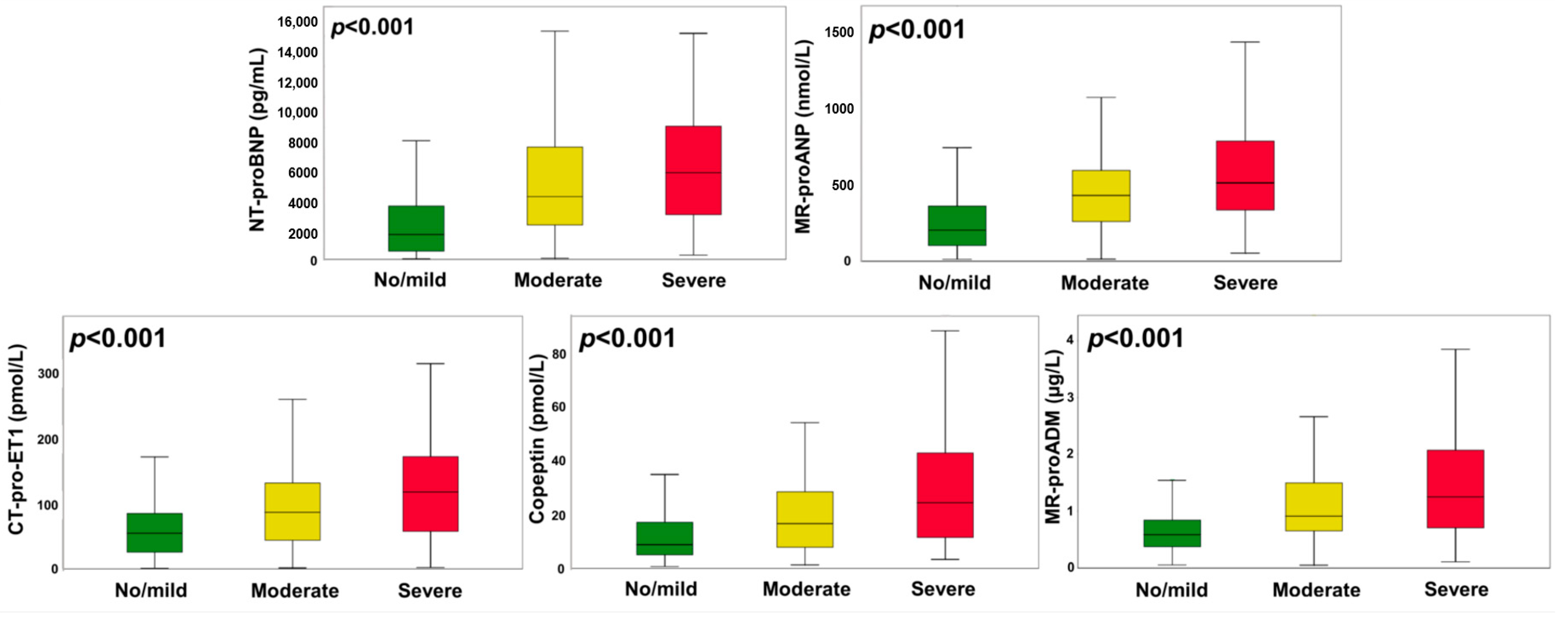
3.4. Activation of Neurohormones in Severe Tricuspid Regurgitation
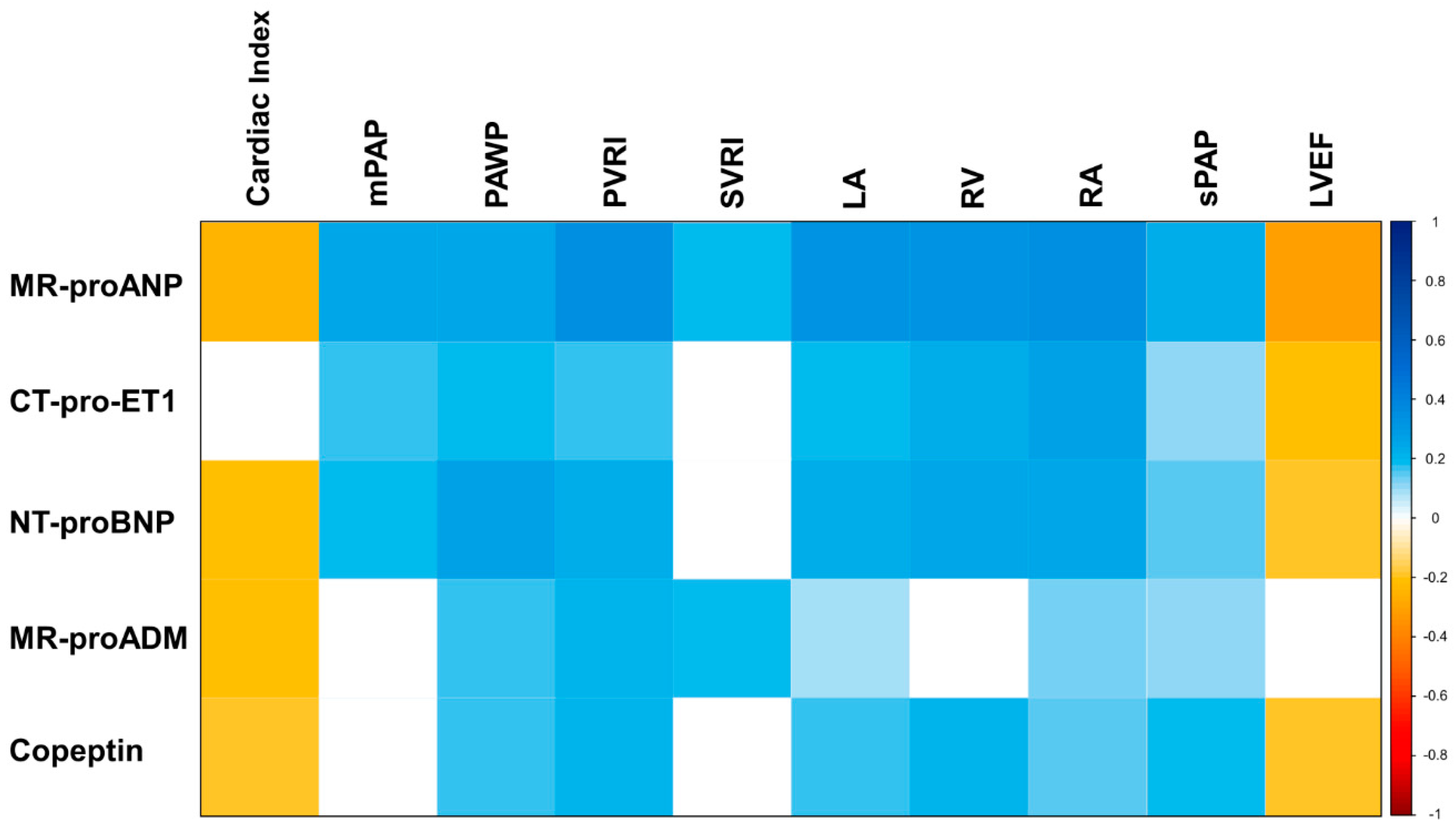
3.5. Association of Neurohumoral Activation with Hemodynamic and Morphologic Characteristics
4. Discussion
4.1. Neurohumoral Activation in Secondary Tricuspid Regurgitation
4.2. Neurohormones as Indicators of Morphologic and Hemodynamic Changes in sTR
4.3. Clinical Implications of Neurohumoral Activation in the Diagnosis of Severe sTR
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bartko, P.E.; Arfsten, H.; Frey, M.K.; Heitzinger, G.; Pavo, N.; Cho, A.; Neuhold, S.; Tan, T.C.; Strunk, G.; Hengstenberg, C.; et al. Natural history of functional tricuspid regurgitation: Implications of quantitative doppler assessment. JACC Cardiovasc. Imaging 2019, 12, 389–397. [Google Scholar] [CrossRef]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef]
- Badano, L.P.; Muraru, D.; Enriquez-Sarano, M. Assessment of functional tricuspid regurgitation. Eur. Heart J. 2013, 34, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Hahn, R.T.; Zamorano, J.L. The need for a new tricuspid regurgitation grading scheme. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1342–1343. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Kjær, A.; Hesse, B. Heart failure and neuroendocrine activation: Diagnostic, prognostic and therapeutic perspectives. Clin. Physiol. 2001, 21, 661–672. [Google Scholar] [CrossRef]
- Packer, M. The neurohormonal hypothesis: A theory to explain the mechanism of disease progression in heart failure. J. Am. Coll. Cardiol. 1992, 20, 248–254. [Google Scholar] [CrossRef]
- Arfsten, H.; Bartko, P.E.; Pavo, N.; Heitzinger, G.; Mascherbauer, J.; Hengstenberg, C.; Hülsmann, M.; Goliasch, G. Phenotyping progression of secondary mitral regurgitation in chronic systolic heart failure. Eur. J. Clin. Investig. 2019, 49. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.M.; Stewart, R.A.H.; Gerber, I.L.; West, T.M.; Richards, A.M.; Yandle, T.G.; Kerr, A.J. Plasma natriuretic peptide levels increase with symptoms and severity of mitral regurgitation. J. Am. Coll. Cardiol. 2003, 41, 2280–2287. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Thomas, B.; Bergmann, A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin. Chem. 2004, 50, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chudasama, N.; Hayashi, Y.; Hawk, C.; Ramnauth, S.D.; Wong, K.Y.; Harxhi, A.; Onat, D.; Wakabayashi, M.; Uriel, N.; et al. Peripheral venous congestion causes time- and dose-dependent release of endothelin-1 in humans. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.T.; Tan, H.C.; Kritharides, L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am. J. Cardiol. 2002, 90, 1405–1409. [Google Scholar] [CrossRef]
- Papassotiriou, J.; Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Immunoluminometric assay for measurement of the C-terminal endothelin-I precursor fragment in human plasma. Clin. Chem. 2006, 52, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef]
- Neuhold, S.; Huelsmann, M.; Strunk, G.; Stoiser, B.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Moertl, D.; Berger, R.; Pacher, R. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: Prediction of death at different stages of the disease. J. Am. Coll. Cardiol. 2008, 52, 266–272. [Google Scholar] [CrossRef]
- Voors, A.A.; Kremer, D.; Geven, C.; Ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.D.; et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin. Chem. 2005, 51, 1823–1829. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Goliasch, G.; Bartko, P.E.; Pavo, N.; Neuhold, S.; Wurm, R.; Mascherbauer, J.; Lang, I.M.; Strunk, G.; Hülsmann, M. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur. Heart J. 2018, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American society of echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2016, 14, 30–38. [Google Scholar] [CrossRef]
- Bartko, P.E.; Hülsmann, M.; Hung, J.; Pavo, N.; Levine, R.A.; Pibarot, P.; Vahanian, A.; Stone, G.W.; Goliasch, G. Secondary valve regurgitation in patients with heart failure with preserved ejection fraction, heart failure with mid-range ejection fraction, and heart failure with reduced ejection fraction. Eur. Heart J. 2020, 41, 2799–2810. [Google Scholar] [CrossRef]
- Damman, K.; Valente, M.A.E.; Voors, A.A.; O’Connor, C.M.; Van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Chang, S.M.; Nabi, F.; Shah, D.J.; Estep, J.D. Imaging to diagnose and manage patients in heart failure with reduced ejection fraction. Circ. Cardiovasc. Imaging 2017, 10. [Google Scholar] [CrossRef]
- Bartko, P.E.; Pavo, N.; Pérez-Serradilla, A.; Arfsten, H.; Neuhold, S.; Wurm, R.; Lang, I.M.; Strunk, G.; Dal-Bianco, J.P.; Levine, R.A.; et al. Evolution of secondary mitral regurgitation. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 622–629. [Google Scholar] [CrossRef]
- Pacher, R.; Stanek, B.; Hülsmann, M.; Koller-Strametz, J.; Berger, R.; Schuller, M.; Hartter, E.; Ogris, E.; Frey, B.; Heinz, G.; et al. Prognostic impact of big endothelin-1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J. Am. Coll. Cardiol. 1996, 27, 633–641. [Google Scholar] [CrossRef]
- Kilic, A.; Saha-Chaudhuri, P.; Rankin, J.S.; Conte, J.V. Trends and outcomes of tricuspid valve surgery in north america: An analysis of more than 50,000 patients from the society of thoracic surgeons database. Ann. Thorac. Surg. 2013, 96, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Weber, M.; Schueler, R.; Hausleiter, J.; Näbauer, M.; von Bardeleben, R.S.; Sotiriou, E.; Schäfer, U.; Deuschl, F.; Kuck, K.H.; et al. 6-month outcomes of tricuspid valve reconstruction for patients with severe tricuspid regurgitation. J. Am. Coll. Cardiol. 2019, 73, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Total Study Population (n = 576) | No/Mild sTR (n = 377) | Moderate sTR (n = 136) | Severe sTR (n = 63) | p-Value |
|---|---|---|---|---|---|
| Age, median years (IQR) | 58 (50–64) | 57 (50–63) | 58 (50–65) | 60 (50–65) | 0.391 |
| Male sex, n (%) | 476 (83) | 317 (84) | 110 (81) | 49 (78) | 0.391 |
| BMI, kg/m2 (IQR) | 26 (24–29) | 27 (24–29) | 26 (24–28) | 26 (22–28) | 0.001 |
| Systolic blood pressure, mmHg (IQR) | 115 (100–130) | 120 (105–135) | 109 (95–120) | 100 (90–115) | <0.001 |
| Ischemic etiology of HF, n (%) | 225 (39) | 160 (42) | 49 (36) | 16 (25) | 0.026 |
| Hypertension, n (%) | 284 (49) | 210 (56) | 57 (42) | 17 (27) | <0.001 |
| Diabetes, n (%) | 130 (23) | 92 (24) | 27 (20) | 11 (17) | 0.326 |
| Hypercholesterolemia, n (%) | 234 (41) | 175 (46) | 46 (34) | 13 (21) | <0.001 |
| Left bundle branch block, n (%) | 112 (19) | 80 (21) | 23 (17) | 9 (14) | 0.872 |
| Atrial fibrillation, n (%) | 119 (21) | 58 (15) | 45 (33) | 16 (25) | <0.001 |
| NYHA functional class | <0.001 | ||||
| NYHA II, n (%) | 153 (27) | 121 (32) | 24 (18) | 8 (13) | |
| NYHA III, n (%) | 236 (41) | 151 (40) | 62 (46) | 23 (37) | |
| NYHA IV, n (%) | 121 (21) | 49 (13) | 42 (31) | 30 (48) | |
| Creatinine, mg/dL (IQR) | 1.2 (1.0–1.4) | 1.2 (1.0–1.3) | 1.2 (1.0–1.5) | 1.3 (1.1–1.7) | <0.001 |
| Blood urea nitrogen, mg/dL (IQR) | 20 (17–30) | 20 (15–26) | 23 (18–37) | 30 (20–38) | <0.001 |
| Echocardiographic characteristics | |||||
| Left ventricular end-diastolic diameter, mm (IQR) | 64 (58–71) | 63 (56–70) | 64 (59–70) | 66 (61–73) | 0.048 |
| Left ventricular function | |||||
| Moderately reduced (EF 30–40%), n (%) | 159 (28) | 120 (32) | 32 (24) | 7 (11) | 0.001 |
| Severely reduced (EF < 30%), n (%) | 325 (56) | 177 (47) | 93 (68) | 55 (87) | <0.001 |
| Left ventricular ejection fraction, % (IQR) | 27 (20–35) | 30 (22–37) | 26 (20–35) | 22 (14–26) | <0.001 |
| Left atrial diameter, mm (IQR) | 64 (57–71) | 61 (55–68) | 69 (64–74) | 72 (66–77) | <0.001 |
| Right ventricular end-diastolic diameter, mm (IQR) | 36 (31–42) | 34 (30–38) | 41 (35–45) | 44 (40–49) | <0.001 |
| Right ventricular function | |||||
| Moderately reduced, n (%) | 23 (4) | 4 (1) | 6 (4) | 13 (21) | <0.001 |
| Severely reduced, n (%) | 13 (2) | 4 (1) | 6 (4) | 3 (5) | 0.030 |
| Right atrial diameter, mm (IQR) | 58 (52–66) | 55 (50–62) | 65 (58–71) | 70 (60–77) | <0.001 |
| Mitral regurgitation (≥moderate), n (%) | 193 (34) | 71 (19) | 73 (54) | 49 (78) | <0.001 |
| Systolic pulmonary artery pressure, mmHg (IQR) | 46 (39–56) | 41 (35–50) | 50 (43–59) | 54 (46–60) | <0.001 |
| Medication | |||||
| RAS-antagonist, n (%) | 551 (96) | 363 (96) | 128 (94) | 60 (95) | 0.559 |
| Percent of maximal recommended dose, median % | 100 | 100 | 100 | 100 | 0.15 |
| Beta blockers, n (%) | 410 (71) | 274 (73) | 96 (71) | 40 (63) | 0.324 |
| Percent of maximal recommended dose, median % | 50 | 50 | 50 | 44 | 0.66 |
| Mineral corticoid receptor antagonist, n (%) | 189 (33) | 100 (27) | 62 (46) | 27 (42) | <0.001 |
| Furosemide, n (%) | 429 (75) | 253 (67) | 118 (87) | 58 (92) | <0.001 |
| Device therapy | |||||
| Implanted cardioverter defibrillator, n (%) | 69 (12) | 40 (11) | 19 (14) | 10 (16) | 0.352 |
| Pacemaker, n (%) | 100 (17) | 50 (13) | 33 (24) | 17 (27) | 0.002 |
| Cardiac resynchronization therapy, n (%) | 55 (10) | 41 (11) | 11 (8) | 13 (21) | 0.250 |
| Hemodynamic characteristics | N = 150 | N = 150 | |||
| mPAP, mmHg (IQR) | 38 (31–43) | 39 (33–46) | 36 (31–40) | 38 (33–42) | 0.194 |
| PAWP, mmHg (IQR) | 23 (20–26) | 24 (21–26) | 22 (20–26) | 23 (21–28) | 0.543 |
| Cardiac Index, l/min/m2 (IQR) | 1.8 (1.5–2.1) | 1.8 (1.5–2.1) | 1.9 (1.6–2.1) | 1.7 (1.5–2.0) | 0.512 |
| Pulmonary vascular resistance, dyn∙s/cm5 (IQR) | 635 (480–811) | 699 (499–898) | 595 (395–722) | 600 (467–770) | 0.142 |
| Systemic vascular resistance, dyn∙s/cm5 (IQR) | 2766 (2360–3368) | 2905 (2480–3721) | 2641 (2282–3057) | 2746 (2452–3095) | 0.131 |
| Neurohormones | |||||
| NT-proBNP, pg/mL (IQR) | 2360 (867–5163) | 1632 (541–3510) | 4131 (2262–7408) | 5700 (2875–9083) | <0.001 |
| MR-proANP, pmol/L (IQR) | 275 (131–479) | 202 (102–359) | 429 (258–592) | 510 (329–799) | <0.001 |
| MR-proADM, nmol/L (IQR) | 0.67 (0.42–1.06) | 0.59 (0.37–0.84) | 0.91 (0.65–1.50) | 1.25 (0.62–2.12) | <0.001 |
| Copeptin, pmol/L (IQR) | 11.3 (5.8–21.8) | 8.8 (5.0–17.2) | 16.8 (7.9–28.7) | 24.6 (11.4–43.9) | <0.001 |
| CT-pro-ET1, pmol/L (IQR) | 62 (31–106) | 55 (26–85) | 87 (44–132) | 118 (56–173) | <0.001 |
| Univariable Model | Selected Neurohormones | |||||||
|---|---|---|---|---|---|---|---|---|
| SD | OR | 95% CI | p-Value | ROC | Adj. HR 1 | 95% CI | p-Value | |
| Neurohormones | ||||||||
| CT-pro-ET1 | 65.3 | 2.08 | 1.65–2.63 | <0.001 | 0.70 | 1.46 | 1.11–1.91 | 0.006 |
| MR-proANP | 347 | 1.80 | 1.44–2.24 | <0.001 | 0.77 | 1.45 | 1.13–1.87 | 0.004 |
| NT-proBNP | 5452 | 1.63 | 1.34–1.98 | <0.001 | 0.75 | |||
| Copeptin | 23.7 | 1.60 | 1.31–1.95 | <0.001 | 0.73 | |||
| MR-proADM | 2.19 | 1.17 | 0.96–1.43 | 0.121 | 0.72 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinka, G.; Bartko, P.E.; Heitzinger, G.; Teo, E.; Prausmüller, S.; Arfsten, H.; Pavo, N.; Winter, M.-P.; Mascherbauer, J.; Hengstenberg, C.; et al. An Integrated Imaging and Circulating Biomarker Approach for Secondary Tricuspid Regurgitation. J. Pers. Med. 2020, 10, 233. https://doi.org/10.3390/jpm10040233
Spinka G, Bartko PE, Heitzinger G, Teo E, Prausmüller S, Arfsten H, Pavo N, Winter M-P, Mascherbauer J, Hengstenberg C, et al. An Integrated Imaging and Circulating Biomarker Approach for Secondary Tricuspid Regurgitation. Journal of Personalized Medicine. 2020; 10(4):233. https://doi.org/10.3390/jpm10040233
Chicago/Turabian StyleSpinka, Georg, Philipp E. Bartko, Gregor Heitzinger, Eliza Teo, Suriya Prausmüller, Henrike Arfsten, Noemi Pavo, Max-Paul Winter, Julia Mascherbauer, Christian Hengstenberg, and et al. 2020. "An Integrated Imaging and Circulating Biomarker Approach for Secondary Tricuspid Regurgitation" Journal of Personalized Medicine 10, no. 4: 233. https://doi.org/10.3390/jpm10040233
APA StyleSpinka, G., Bartko, P. E., Heitzinger, G., Teo, E., Prausmüller, S., Arfsten, H., Pavo, N., Winter, M.-P., Mascherbauer, J., Hengstenberg, C., Hülsmann, M., & Goliasch, G. (2020). An Integrated Imaging and Circulating Biomarker Approach for Secondary Tricuspid Regurgitation. Journal of Personalized Medicine, 10(4), 233. https://doi.org/10.3390/jpm10040233





