Personalisation of Molecular Radiotherapy through Optimisation of Theragnostics
Abstract
1. Introduction
2. Imaging Biomarkers
2.1. Differentiated Thyroid Cancer
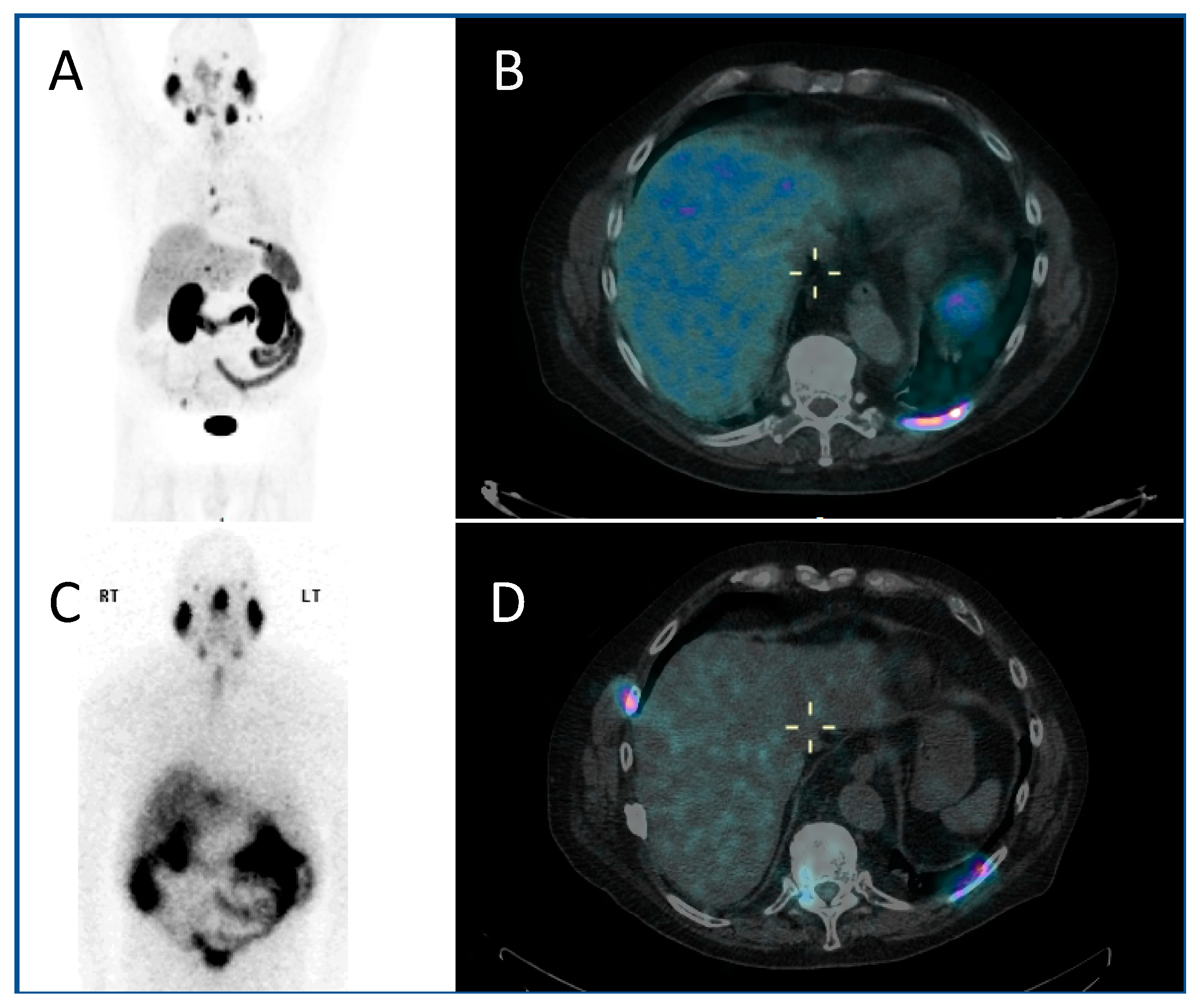
2.2. Neuroblastoma
- Neuroblastoma cells have been shown to be intrinsically highly radiosensitive, with a limited repair capacity [12];
- The disease is often metastatic to bone and bone marrow, and less commonly to other organs [13];
- Immunohistochemistry demonstrated that neuroblastoma cells express the noradrenaline transporter molecule, responsible for the uptake of mIBG, and the somatostatin receptor, responsible for uptake of somatostatin analogues such as DOTATATE [14].
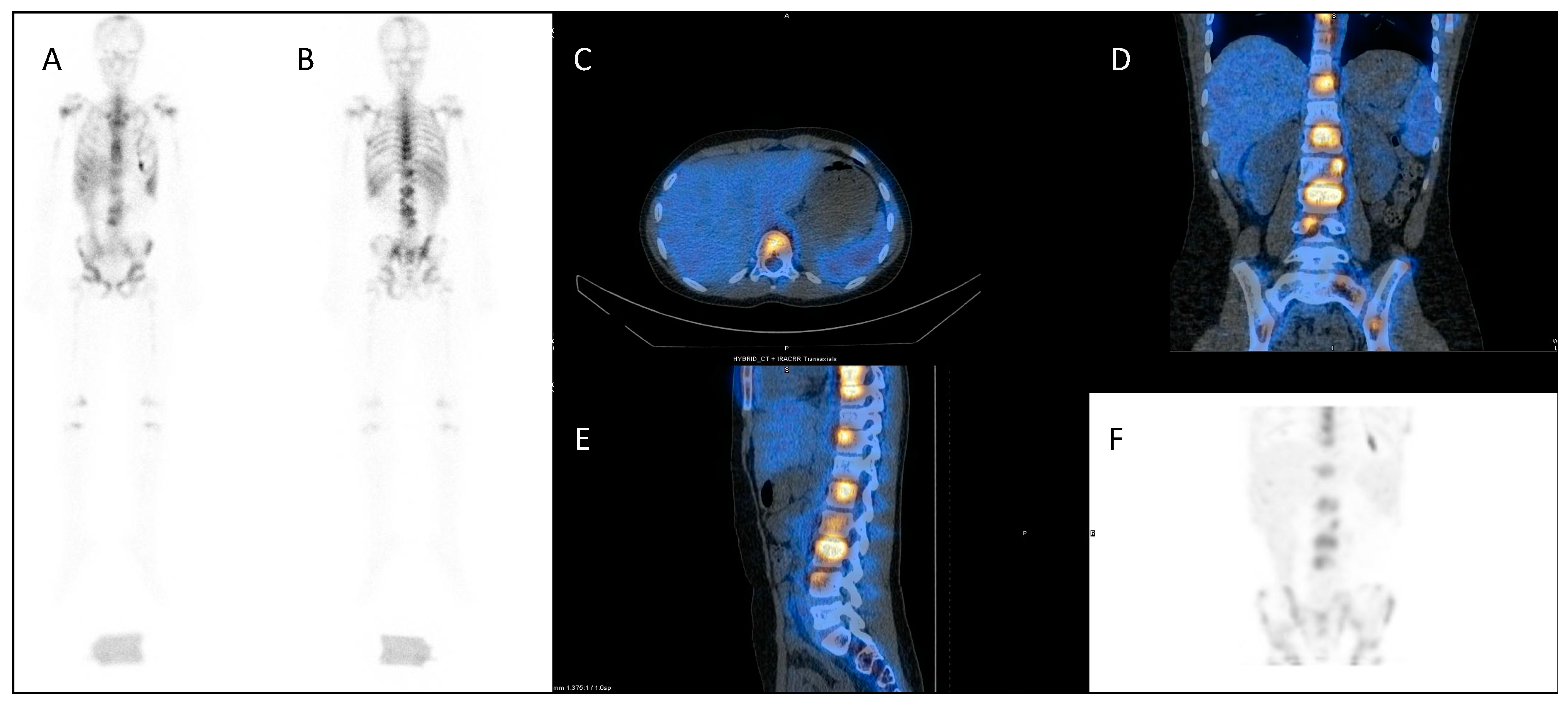
2.3. Metastatic Prostate Cancer
2.4. Liver Metastases
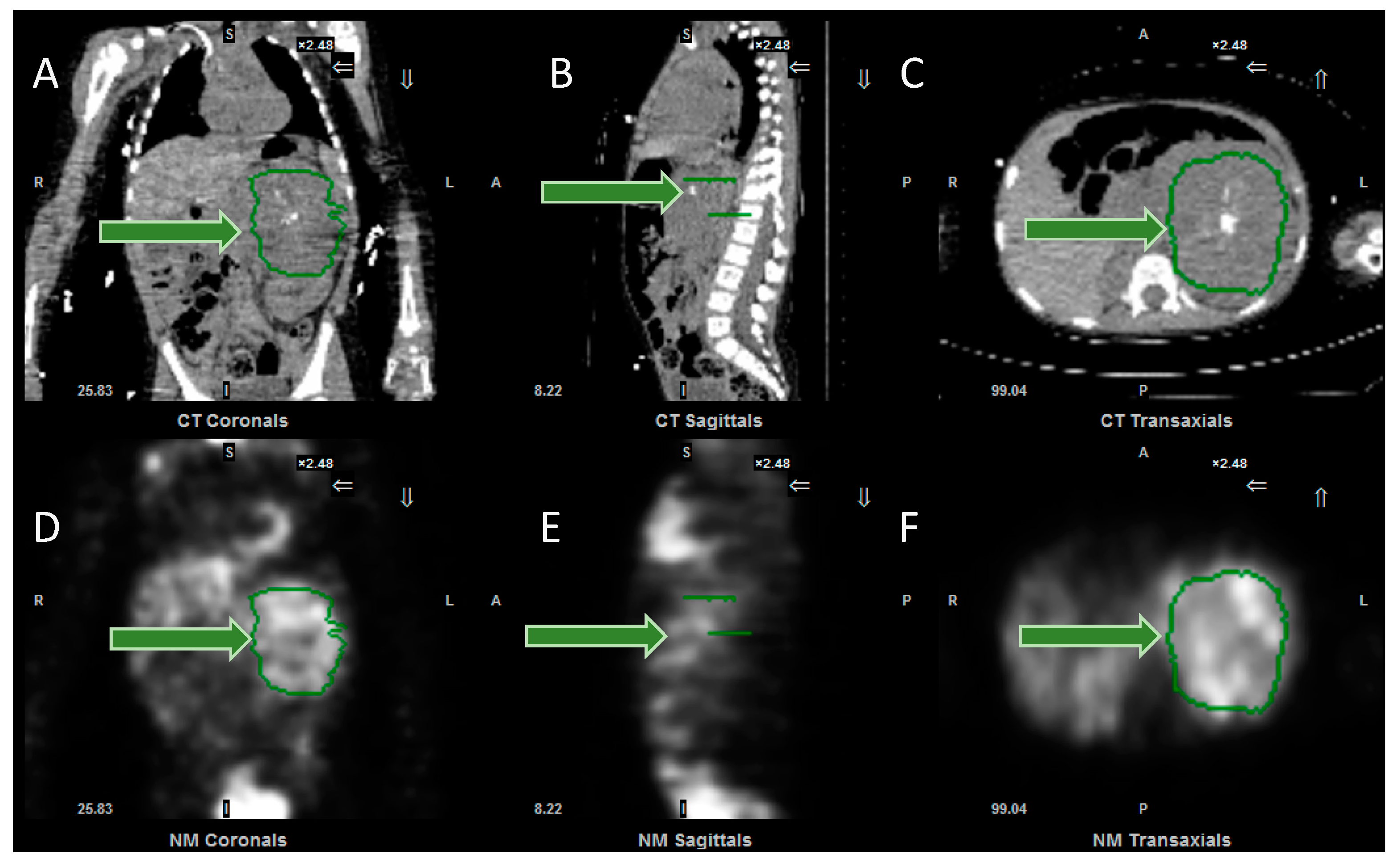
3. Dosimetry
4. Response Assessment
5. Individualised Decision Making and Communication
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Becker, D.V.; Sawin, C.T. Radioiodine and thyroid disease: The beginning. Semin. Nucl. Med. 1996, 26, 155–164. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. NETTER-1 trial investigators. Phase 3 trial of (177)Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. NETTER-1 study group. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with (177)Lu-Dotatate in the phase III NETTER-1 trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. NETTER-1 study group. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with (177)Lu-Dotatate: An analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Rudisile, S.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Böning, G.; Gildehaus, F.J.; Fendler, W.P.; Auernhammer, C.J.; Spitzweg, C.; Bartenstein, P.; et al. Salvage PRRT with 177Lu-DOTA-octreotate in extensively pretreated patients with metastatic neuroendocrine tumor (NET): Dosimetry, toxicity, efficacy, and survival. BMC Cancer 2019, 19, 788. [Google Scholar] [CrossRef] [PubMed]
- NICE. Lutetium (177Lu) Oxodotreotide for Treating Unresectable or Metastatic Neuroendocrine Tumours. 2018. Available online: https://www.nice.org.uk/guidance/ta539 (accessed on 5 September 2020).
- Hope, T.A.; Calais, J.; Zhang, L.; Dieckmann, W.; Millo, C. 111In-Pentetreotide Scintigraphy Versus 68Ga-DOTATATE PET: Impact on Krenning scores and effect of tumor burden. J. Nucl. Med. 2019, 60, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Boelaert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard Ba, G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. British Thyroid Association. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 81, 1–122. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, D.; Aiken, M.; Atkins, F.; Moreau, S.; Garcia, C.; Acio, E.; Burman, K.; Wartofsky, L. The utility of radioiodine scans prior to iodine 131 ablation in patients with well-differentiated thyroid cancer. Thyroid 2009, 19, 849–855. [Google Scholar] [CrossRef]
- Silberstein, E.B. Comparison of outcomes after (123)I vs. (131)I pre-ablation imaging before radioiodine ablation in differentiated thyroid carcinoma. J. Nucl. Med. 2007, 48, 1043–1046. [Google Scholar] [CrossRef]
- Rendl, G.; Rettenbacher, L.; Schweighofer-Zwink, G.; Hehenwarter, L.; Pirich, C. Pre-ablation rhTSH-stimulated F-18 FDG PET/CT changes patient management in increased-risk thyroid cancer. Horm. Metab. Res. 2020, 52, 158–167. [Google Scholar] [CrossRef]
- Deacon, J.M.; Wilson, P.A.; Peckham, M.J. The radiobiology of human neuroblastoma. Radiother. Oncol. 1985, 3, 201–209. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. INRG Task Force. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Gains, J.E.; Sebire, N.J.; Moroz, V.; Wheatley, K.; Gaze, M.N. Immunohistochemical evaluation of molecular radiotherapy target expression in neuroblastoma tissue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Matthay, K.K.; Shulkin, B.; Ladenstein, R.; Michon, J.; Giammarile, F.; Lewington, V.; Pearson, A.D.; Cohn, S.L. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the International Neuroblastoma Risk Group (INRG) Task Force. Br. J. Cancer 2010, 102, 1319–1326. [Google Scholar] [CrossRef]
- Lewington, V.; Lambert, B.; Poetschger, U.; Sever, Z.B.; Giammarile, F.; McEwan, A.J.B.; Castellani, R.; Lynch, T.; Shulkin, B.; Drobics, M.; et al. (123)I-mIBG scintigraphy in neuroblastoma: Development of a SIOPEN semi-quantitative reporting method by an international panel. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 234–241. [Google Scholar] [CrossRef]
- Ladenstein, R.; Lambert, B.; Pötschger, U.; Castellani, M.R.; Lewington, V.; Bar-Sever, Z.; Oudoux, A.; Śliwińska, A.; Taborska, K.; Biassoni, L.; et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: The SIOPEN/HR-NBL1 and COG-A3973 trials. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 292–305. [Google Scholar] [CrossRef]
- Jacobson, A.F.; Deng, H.; Lombard, J.; Lessig, H.J.; Black, R.R. 123I-metaiodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: Results of a meta-analysis. J. Clin. Endocrinol. Metab. 2010, 95, 2596–2606. [Google Scholar] [CrossRef]
- Wilson, J.S.; Gains, J.E.; Moroz, V.; Wheatley, K.; Gaze, M.N. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur. J. Cancer 2014, 50, 801–815. [Google Scholar] [CrossRef]
- Gains, J.E.; Bomanji, J.B.; Fersht, N.L.; Sullivan, T.; D’Souza, D.; Sullivan, K.P.; Aldridge, M.; Waddington, W.; Gaze, M.N. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J. Nucl. Med. 2011, 52, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Hofman, M.S.; Murray, W.K.; Wilson, S.; Wood, P.; Downie, P.; Super, L.; Hogg, A.; Eu, P.; Hicks, R.J. Initial experience with gallium-68 DOTA-octreotate PET/CT and peptide receptor radionuclide therapy for pediatric patients with refractory metastatic neuroblastoma. J. Pediatric Hematol. Oncol. 2016, 38, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gains, J.E.; Moroz, V.; Aldridge, M.D.; Wan, S.; Wheatley, K.; Laidler, J.; Peet, C.; Bomanji, J.B.; Gaze, M.N. A phase IIa trial of molecular radiotherapy with 177-lutetium DOTATATE in children with primary refractory or relapsed high-risk neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Gains, J.E.; Aldridge, M.D.; Mattoli, M.V.; Bomanji, J.B.; Biassoni, L.; Shankar, A.; Gaze, M.N. 68Ga-DOTATATE and 123I-mIBG as imaging biomarkers of disease localisation in metastatic neuroblastoma: Implications for molecular radiotherapy. Nucl. Med. Commun. 2020, 41, 1169–1177. [Google Scholar] [CrossRef]
- Firusian, N.; Mellin, P.; Schmidt, C.G. Results of 89 strontium Therapy in patients with carcinoma of the prostate and incurable pain from bone metastases: A preliminary report. J. Urol. 1976, 116, 764–768. [Google Scholar] [CrossRef]
- Reddy, E.K.; Robinson, R.G.; Mansfield, C.M. Strontium 89 for palliation of bone metastases. J. Natl. Med. Assoc. 1986, 78, 27–32. [Google Scholar] [PubMed]
- Bodei, L.; Lam, M.; Chiesa, C.; Flux, G.; Brans, B.; Chiti, A.; Giammarile, F. European Association of Nuclear Medicine. EANM procedure guideline for treatment of refractory metastatic bone pain. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.-L.; Wu, T.T.-L.; Vogelzang, N.J.; Huang, C.-Y.; Huang, S.-P.; Lin, C.-C.; Ou, Y.-C.; Pang, S.-T.; Shen, D.H.-Y.; Wu, W.-J.; et al. Y-H. Optimal usage of radium-223 in metastatic castration-resistant prostate cancer. J. Med. Assoc. 2017, 116, 825–836. [Google Scholar] [CrossRef]
- Suominen, M.I.; Wilson, T.; Käkönen, S.-M.; Scholz, A. The mode-of-action of targeted alpha therapy radium-223 as an enabler for novel combinations to treat patients with bone metastasis. Int. J. Mol. Sci. 2019, 20, 3899. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Flux, G.D. Imaging and dosimetry for radium-223: The potential for personalized treatment. Br. J. Radiol. 2017, 90, 20160748. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassman, M.; Klette, I.; Eiber, M.; et al. Wester, H.-J. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar] [CrossRef]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of (177)Lu-PSMA-617 in metastatic castration-resistant prostate cancer: Correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study of 177Lu-PSMA-617 in Metastatic Castrate-Resistant Prostate Cancer (VISION). Available online: https://clinicaltrials.gov/ct2/show/NCT03511664 (accessed on 6 September 2020).
- ClinicalTrials.gov. A Trial of 177Lu-PSMA-617 Theranostic Vs. Cabazitaxel in Progressive Metastatic Castration Resistant Prostate Cancer (TheraP). Available online: https://clinicaltrials.gov/ct2/show/NCT03392428 (accessed on 6 September 2020).
- Wasan, H.S.; Gibbs, P.; Sharma, N.K.; Taieb, J.; Heinemann, V.; Ricke, J.; Peeters, M.; Findlay, M.; Weaver, A.; Mills, J.; et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): A combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017, 18, 1159–1171. [Google Scholar] [CrossRef]
- SIRtex.com. Further Indications. Available online: https://www.sirtex.com/eu/clinicians/further-indications (accessed on 23 September 2020).
- Abbott, E.M.; Falzone, N.; Lee, B.Q.; Kartsonaki, C.; Winter, H.; Greenhalgh, T.A.; McGowan, D.R.; Syed, N.; Denis-Bacelar, A.M.; Boardman, P.; et al. The Impact of radiobiologically-informed dose prescription on the clinical benefit of yttrium-90 sirt in colorectal cancer patients. J. Nucl. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Terumo-Europe.com. QuiremSpheres-Microspheres. Available online: https://www.terumo-europe.com/en-emea/products/quiremspheres®-microspheres (accessed on 23 September 2020).
- Dehbi, H.M.; Mallick, U.; Wadsley, J.; Newbold, K.; Harmer, C.; Hackshaw, A. Recurrence after low-dose radioiodine ablation and recombinant human thyroid-stimulating hormone for differentiated thyroid cancer (HiLo): Long-term results of an open-label, non-inferiority randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 44–51. [Google Scholar] [CrossRef]
- Matthay, K.K.; Weiss, B.; Villablanca, J.G.; Maris, J.M.; Yanik, G.A.; Dubois, S.G.; Stubbs, J.; Groshen, S.; Tsao-Wei, D.; Hawkins, R.; et al. Dose escalation study of no-carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory neuroblastoma: New approaches to neuroblastoma therapy consortium trial. J. Nucl. Med. 2012, 53, 1155–1163. [Google Scholar] [CrossRef]
- Gov.uk. H.M. Government. Lonising Radiation (Medical Exposure) Regulations. 2017. Available online: https://www.legislation.gov.uk/uksi/2017/1322/contents/made (accessed on 6 September 2020).
- Gov.uk Administration of Radioactive Substances Advisory Committee. Notes for Guidance on the Clinical Administration of Radiopharmaceuticals and Use of Sealed Radioactive Sources. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/912979/ARSAC_NfG_Sept_2020_FINAL_DRAFT_280820.pdf (accessed on 6 September 2020).
- Gaze, M.N.; Chang, Y.-C.; Flux, G.D.; Mairs, R.J.; Saran, F.H.; Meller, S.T. Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother. Radiopharm. 2005, 20, 195–199. [Google Scholar] [CrossRef]
- Buckley, S.E.; Saran, F.H.; Gaze, M.N.; Chittenden, S.; Partridge, M.; Lancaster, D.; Pearson, A.; Flux, G.D. Dosimetry for fractionated (131)I-mIBG therapies in patients with primary resistant high-risk neuroblastoma: Preliminary results. Cancer Biother. Radiopharm. 2007, 22, 105–112. [Google Scholar] [CrossRef]
- Buckley, S.E.; Chittenden, S.J.; Saran, F.H.; Meller, S.T.; Flux, G.D. Whole-body dosimetry for individualized treatment planning of 131I-MIBG radionuclide therapy for neuroblastoma. J. Nucl. Med. 2009, 50, 1518–1524. [Google Scholar] [CrossRef]
- Huang, S.Y.; Bolch, W.E.; Lee, C.; Van Brocklin, H.F.; Pampaloni, M.H.; Hawkins, R.A.; Sznewajs, A.; DuBois, S.G.; Matthay, K.K.; Seo, Y. Patient-specific dosimetry using pretherapy [¹²⁴I]m-iodobenzylguanidine ([¹²⁴I]mIBG) dynamic PET/CT imaging before [¹³¹I]mIBG targeted radionuclide therapy for neuroblastoma. Mol. Imaging Biol. 2015, 17, 284–294. [Google Scholar] [CrossRef]
- Seo, Y.; Huh, Y.; Huang, S.Y.; Hernandez-Pampaloni, J.M.; Hawkins, R.A.; Gustafson, W.C.; Vo, K.T.; Matthay, K.K. Technical note: Simplified and practical pretherapy tumor dosimetry—A feasibility study for 131 I-MIBG therapy of neuroblastoma using 124 I-MIBG PET/CT. Med. Phys. 2019, 46, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Aboian, M.; Huang, S.Y.; Pampaloni, M.H.; Hawkins, R.A.; Huh, Y.; Vo, K.; Gustafson, C.; Matthay, K.; Seo, Y. 124I-MIBG PET-CT to monitor metastatic disease in children with relapsed neuroblastoma. J. Nucl. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- EU Clinical Trials register: 90-Yttrium-labelled anti-CD66 Monoclonal Antibody as Part of a Reduced intensity Conditioning Regimen Prior to Allogeneic Haematopoietic Stem Cell Transplantation: An Open Label, Dose Escalating Phase I Study in Children with Relapsed/Refractory Leukaemia. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-000015-24/GB (accessed on 9 August 2020).
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Bagatell, R.; Cohn, S.L.; Pearson, A.D.; Villablanca, J.G.; Berthold, F.; Burchill, S.; Boubaker, A.; McHugh, K.; Nuchtern, J.G.; et al. Revisions to the International neuroblastoma response criteria: A consensus statement from the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 2017, 35, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Trial Evaluating and Comparing Two Intensification Treatment Strategies for Metastatic Neuroblastoma Patients with a Poor Response to Induction Chemotherapy (VERITAS). Available online: https://clinicaltrials.gov/ct2/show/NCT03165292 (accessed on 9 August 2020).
- Aide, N.; Lasnon, C.; Veit-Haibach, P.; Sera, T.; Sattler, B.; Boellaard, R. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Islam, M. Treat patient, not just the disease: Holistic needs assessment for haematological cancer patients. Oncol. Rev. 2018, 12, 374. [Google Scholar] [CrossRef]
- Gains, J.E.; Walker, C.; Sullivan, T.M.; Waddington, W.A.; Fersht, N.L.; Sullivan, K.P.; Armstrong, E.; D′Souza, D.P.; Aldridge, M.D.; Bomanji, J.B.; et al. Radiation exposure to comforters and carers during paediatric molecular radiotherapy. Pediatric Blood Cancer 2015, 62, 235–239. [Google Scholar] [CrossRef]


| Disease to be Treated | Molecular Imaging Radiopharmaceutical | Molecular Radiotherapy Radiopharmaceutical |
|---|---|---|
| Differentiated thyroid cancer | 123I sodium iodide | 131I sodium iodide |
| Neuroblastoma | 123I mIBG 1 | 131I mIBG |
| Neuroendocrine cancers | 68Ga DOTATATE 2,3 111In Pentetreotide 4 | 177Lu DOTATATE 90Y DOTATOC 5 |
| 85Sr strontium chloride | 89Sr strontium chloride | |
| Metastatic prostate cancer | 99mTc HDP 6 | 153Sm lexidronam 186Re etidronate 223Ra radium dichloride |
| 68Ga PMSA 7 | 177Lu PMSA | |
| Acute leukaemia | 111In anti-CD66 monoclonal antibody | 90Y anti-CD66 8 monoclonal antibody |
| Disease to be Treated | Molecular Radiotherapy Radiopharmaceutical | Dosimetry Notes |
|---|---|---|
| Differentiated thyroid cancer | 131I sodium iodide | Routinely fixed administered activity. Two means of personalised dosimetry: the lesion/remnant disease-based method; and the bone marrow dose method |
| Neuroblastoma | 131I mIBG | Administered activity scaled to weight or body surface area. Can be tailored to individual patients based on whole-body absorbed dose. |
| Neuroendocrine cancers | 177Lu DOTATATE 90Y DOTATOC | Routinely fixed administered activity. 177Lu DOTATATE dosimetry can be calculated using the gamma emissions to obtain post therapy planar images; 90Y DOTATOC not routinely possible. |
| 89Sr strontium chloride | Fixed administered activity. Dosimetry not routinely performed | |
| Metastatic prostate cancer | 153Sm lexidronam 186Re etidronate 223Ra radium dichloride | 153Sm lexidronam weight-based administered activity; 186Re etidronate fixed administered activity; no more detailed dosimetry routinely performed. 223Ra photons allow for post-treatment imaging. Low-dose pre-treatment administration also allow potential for greater dosimetric accuracy. |
| 177Lu PMSA | 68Ga PSMA diagnostic imaging used for pre-therapeutic dosimetric calculation; 177Lu gamma emission allows dosimetric calculation for subsequent therapies. | |
| Acute leukaemia | 90Y anti-CD66 monoclonal antibody | 111In labeled anti-CD66 monoclonal antibody single photon emission computed tomography used for dosimetric calculation prior to therapy to ensure bone marrow limit not exceeded. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, L.; Smith, A.-L.; Aldridge, M.D.; Foulkes, J.; Peet, C.; Wan, S.; Gains, J.E.; Bomanji, J.B.; Gaze, M.N. Personalisation of Molecular Radiotherapy through Optimisation of Theragnostics. J. Pers. Med. 2020, 10, 174. https://doi.org/10.3390/jpm10040174
Davis L, Smith A-L, Aldridge MD, Foulkes J, Peet C, Wan S, Gains JE, Bomanji JB, Gaze MN. Personalisation of Molecular Radiotherapy through Optimisation of Theragnostics. Journal of Personalized Medicine. 2020; 10(4):174. https://doi.org/10.3390/jpm10040174
Chicago/Turabian StyleDavis, LauraMay, April-Louise Smith, Matthew D. Aldridge, Jack Foulkes, Connie Peet, Simon Wan, Jennifer E. Gains, Jamshed B. Bomanji, and Mark N. Gaze. 2020. "Personalisation of Molecular Radiotherapy through Optimisation of Theragnostics" Journal of Personalized Medicine 10, no. 4: 174. https://doi.org/10.3390/jpm10040174
APA StyleDavis, L., Smith, A.-L., Aldridge, M. D., Foulkes, J., Peet, C., Wan, S., Gains, J. E., Bomanji, J. B., & Gaze, M. N. (2020). Personalisation of Molecular Radiotherapy through Optimisation of Theragnostics. Journal of Personalized Medicine, 10(4), 174. https://doi.org/10.3390/jpm10040174