An Implementation Science Framework to Develop a Clinical Decision Support Tool for Familial Hypercholesterolemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Physician Interviews and Usability Testing
2.3. Implementation Survey
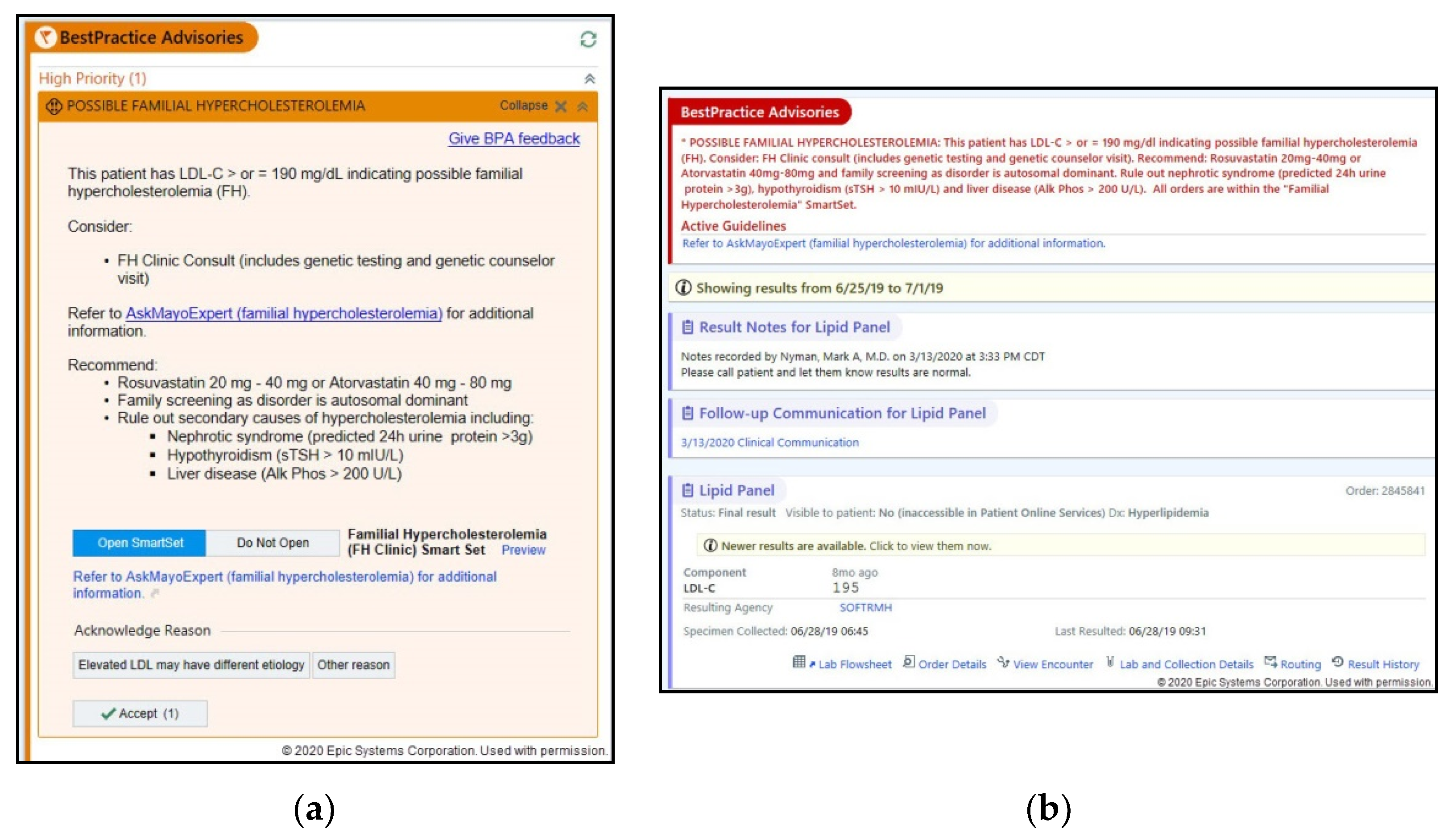
2.4. Deployment of CDS in Silent Mode
2.5. Data Analysis
3. Results
3.1. Physician Interview Findings
3.1.1. Understanding and Awareness of FH
- 1.
- Subtheme: Physicians awareness of FHAmongst both PCPs and specialists, there was a lack of FH awareness—physicians did not always distinguish between hypercholesterolemia of a genetic etiology versus hypercholesterolemia due to other causes. In some instances, both were perceived as being similar and requiring similar management.
- 2.
- Subtheme: Physicians perceived scope of workPCPs and specialists agreed that all physicians were responsible for diagnosing FH; however, they differed in their views on who was responsible for the management of FH patients. Some physicians preferred to manage FH patients themselves, while others preferred referral to a specialist.
3.1.2. Clinical Workflow
- 1.
- Subtheme: Diagnosis in workflowDetermining how physicians established a diagnosis in workflow centered on understanding when tasks such as ordering laboratory tests and reviewing results were performed. Physicians identified CDS alert relevance as being based on both encounter type and patient type. PCPs with high patient volumes were likely to have limited time to review alerts.
- 2.
- Subtheme: Next steps for physiciansFor a patient with suspected FH, the next steps for physicians included prescribing medication, counseling on lifestyle changes, ordering tests and ruling out secondary causes of hypercholesterolemia. Most physicians were willing to refer patients to the institutional FH Clinic if this was the appropriate next step in management.
3.1.3. Physician Preferences and Value of CDS Tools
- 1.
- Subtheme: CDS tool preferencesMost physicians had previously used one or more CDS tools and viewed them positively. However, a physician’s personal style or prior knowledge of a patient was likely to influence their decision on whether or not to use a specific CDS tool. With a relatively recent transition to a new EHR system at the time of interviews, some physicians continued to face challenges in interacting with the new EHR and highlighted the need to find more efficient ways to carry out routine tasks.
- 2.
- Subtheme: Cognitive loadA common feedback from all physicians was that the increasing number of CDS alerts and in-basket messages were contributing to rising levels of alert fatigue and information overload, subsequently resulting in CDS alerts being ignored or bypassed.
- 3.
- Subtheme: Value of FH Clinic referral to physiciansPhysicians agreed that having the option to refer patients with ‘possible FH’ to the institutional FH Clinic was likely going to reduce their cognitive burden and would be useful for complex cases such as those where: (a) goal LDL-cholesterol was not being attained on maximum statin therapy; (b) a patient had statin intolerance or (c) cases where genetic testing was warranted.
3.1.4. Perspectives on Patient Needs and Values
- 1.
- Subtheme: Patient engagement through the use of digital toolsPhysicians indicated that there was a need for patient education materials that could be printed and attached to messages as well as videos that could be shared directly through the institutional patient portal. Most physicians agreed that there was value in shared decision-making tools such as patient decision aids, especially if these were available on the internet and easily accessible. Physicians also highlighted the importance of being able to share the computer screen displaying the decision aid with patients, so as to engage them during clinical encounters.
- 2.
- Subtheme: Value of FH Clinic referral to patientsPhysicians highlighted that the value of a referral to the institutional FH Clinic for patients and their family members centered on: (a) obtaining access to resources available specifically in the FH Clinic; (b) cascade testing of family members; (c) receiving access to new medications such as PCSK9 inhibitors and (d) facilitating appointment scheduling for genetic testing.
3.1.5. Dissemination and Implementation
- 1.
- Subtheme: Facilitators to Dissemination and ImplementationPhysicians suggested that departmental meetings, conferences, grand rounds as well as newsletters could be used to promote awareness of the CDS tool and facilitate its uptake in practice. They also highlighted the need to obtain support from institutional champions, opinion leaders, early adopters and others likely to influence the attitudes and behaviors of providers.
- 2.
- Subtheme: Barriers to Dissemination and ImplementationThe main barriers identified by physicians that were likely going to impact utilization of the CDS tool in practice included use of pre-existing CDS tools that may prevent adoption of new tools, limited time in patient encounters to view and act upon CDS alerts and lack of institutional guidance and education on new CDS availability.
3.2. Usability Recommendations
3.3. Implementation Survey Results
3.4. Silent Mode Metrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Safarova, M.S.; Kullo, I.J. My approach to the patient with familial hypercholesterolemia. Mayo Clin. Proc. 2016, 91, 770–786. [Google Scholar] [CrossRef] [PubMed]
- Safarova, M.S.; Liu, H.; Kullo, I.J. Rapid identification of familial hypercholesterolemia from electronic health records: The SEARCH study. J. Clin. Lipidol. 2016, 10, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Rivera-Valerio, M.; Bangash, H.; Prokop, L.; Kullo, I.J. New case detection by cascade testing in familial hypercholesterolemia: A systematic review of the literature. Circ. Genom. Precis. Med. 2019, 12, e002723. [Google Scholar] [CrossRef]
- Gidding, S.S.; Champagne, M.A.; de Ferranti, S.D.; Defesche, J.; Ito, M.K.; Knowles, J.W.; McCrindle, B.; Raal, F.; Rader, D.; Santos, R.D.; et al. The agenda for familial hypercholesterolemia. Circulation 2015, 132, 2167–2192. [Google Scholar] [CrossRef]
- Bangash, H.; Khan, F.; He, B.; Arce, M.; Kullo, I.J. Use of twitter to promote awareness of familial hypercholesterolemia. Circ. Genom. Precis. Med. 2019, 12, e002550. [Google Scholar] [CrossRef] [PubMed]
- Degoma, E.M.; Ahmad, Z.S.; O’Brien, E.C.; Kindt, I.; Shrader, P.; Newman, C.B.; Pokharel, Y.; Baum, S.J.; Hemphill, L.C.; Hudgins, L.C.; et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: Data from the CASCADE-FH registry. Circ. Cardiovasc. Genet. 2016, 9, 240–249. [Google Scholar] [CrossRef]
- Manolio, T.A.; Abramowicz, M.; Al-Mulla, F.; Anderson, W.; Balling, R.; Berger, A.C.; Bleyl, S.; Chakravarti, A.; Chantratita, W.; Chisholm, R.L.; et al. Global implementation of genomic medicine: We are not alone. Sci. Transl. Med. 2015, 7, 290ps13. [Google Scholar] [CrossRef]
- Bryan, C.; Boren, S.A. The use and effectiveness of electronic clinical decision support tools in the ambulatory/primary care setting: A systematic review of the literature. Inform. Prim. Care 2008, 16, 79–91. [Google Scholar] [CrossRef]
- Hasnie, A.A.; Kumbamu, A.; Safarova, M.S.; Caraballo, P.J.; Kullo, I.J. A clinical decision support tool for familial hypercholesterolemia based on physician input. Mayo Clin. Proc. Innov. Qual. Outcomes 2018, 2, 103–112. [Google Scholar] [CrossRef]
- Lobach, D.; Sanders, G.D.; Bright, T.J.; Wong, A.; Dhurjati, R.; Bristow, E.; Bastian, L.; Coeytaux, R.; Samsa, G.; Hasselblad, V.; et al. Enabling health care decision making through clinical decision support and knowledge management. Evid. Rep. Technol. Assess. 2012, 203, 1–784. [Google Scholar]
- Ash, J.S.; Sittig, D.F.; Campbell, E.M.; Guappone, K.P.; Dykstra, R.H. Some unintended consequences of clinical decision support systems. AMIA Annu. Symp. Proc. 2007, 26–30. [Google Scholar]
- Carroll, C.; Marsden, P.; Soden, P.; Naylor, E.; New, J.; Dornan, T. Involving users in the design and usability evaluation of a clinical decision support system. Comput. Methods Programs Biomed. 2002, 69, 123–135. [Google Scholar] [CrossRef]
- Ancker, J.S.; Edwards, A.; Nosal, S.; Hauser, D.; Mauer, E.; Kaushal, R.; with the HITEC Investigators. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med. Inform. Decis. Mak. 2017, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Linder, J.A.; Schnipper, J.L.; Tsurikova, R.; Melnikas, A.J.; Volk, L.A.; Middleton, B. Barriers to electronic health record use during patient visits. AMIA Annu. Symp. Proc. 2006, 2006, 499–503. [Google Scholar]
- Sittig, D.F.; Wright, A.; Osheroff, J.A.; Middleton, B.; Teich, J.M.; Ash, J.S.; Campbell, E.; Bates, D.W. Grand challenges in clinical decision support. J. Biomed. Inform. 2008, 41, 387–392. [Google Scholar] [CrossRef]
- Shiffman, R.N. Towards effective implementation of a pediatric asthma guideline: Integration of decision support and clinical workflow support. Proc. Annu. Symp. Comput. Appl. Med. Care 1994, 797–801. [Google Scholar]
- Brownson, R.C.; Colditz, G.A.; Proctor, E.K. Dissemination and Implementation Research in Health: Translating Science to Practice; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Glasgow, R.E.; Vinson, C.; Chambers, D.; Khoury, M.J.; Kaplan, R.M.; Hunter, C. National Institutes of Health approaches to dissemination and implementation science: Current and future directions. Am. J. Public Health 2012, 102, 1274–1281. [Google Scholar] [CrossRef]
- Elwyn, G.; Scholl, I.; Tietbohl, C.; Mann, M.; Edwards, A.G.; Clay, C.; Légaré, F.; van der Weijden, T.; Lewis, C.L.; Wexler, R.M.; et al. ‘Many miles to go …’: A systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med. Inform. Decis. Mak. 2013, 13 (Suppl. 2), S14. [Google Scholar] [CrossRef]
- Hartzler, A.; McCarty, C.A.; Rasmussen, L.V.; Williams, M.S.; Brilliant, M.; Bowton, E.A.; Clayton, E.W.; Faucett, W.A.; Ferryman, K.; Field, J.R.; et al. Stakeholder engagement: A key component of integrating genomic information into electronic health records. Genet. Med. 2013, 15, 792–801. [Google Scholar] [CrossRef]
- Downing, G.J.; Boyle, S.N.; Brinner, K.M.; Osheroff, J.A. Information management to enable personalized medicine: Stakeholder roles in building clinical decision support. BMC Med. Inform. Decis. Mak. 2009, 9, 44. [Google Scholar] [CrossRef]
- Devaraj, S.; Sharma, S.K.; Fausto, D.J.; Viernes, S.; Kharrazi, H. Barriers and facilitators to clinical decision support systems adoption: A systematic review. JBAR 2014, 3, 36. [Google Scholar] [CrossRef]
- Virzi, R.A. Refining the test phase of usability evaluation: How many subjects is enough? Hum. Factors 1992, 34, 457–468. [Google Scholar] [CrossRef]
- Kastner, M.; Lottridge, D.; Marquez, C.; Newton, D.; Straus, S.E. Usability evaluation of a clinical decision support tool for osteoporosis disease management. Implement. Sci. 2010, 5, 96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grando, A.; Manataki, A.; Furniss, S.K.; Duncan, B.; Solomon, A.; Kaufman, D.; Hirn, S.; Sunday, R.; Bouchereau, J.; Doebbeling, B.; et al. Multi-method study of electronic health records workflows. AMIA Annu. Symp. Proc. 2018, 2018, 498–507. [Google Scholar] [PubMed]
- Abouzahra, M.; Tan, J. The multi-level impact of clinical decision support system: A framework and a call for mixed methods evaluation. In Proceedings of the Pacific Asia Conference on Information Systems (PACIS), Chengdu, China, 24–28 June 2014. [Google Scholar]
- Curran, G.M.; Bauer, M.; Mittman, B.; Pyne, J.M.; Stetler, C. Effectiveness-implementation hybrid designs: Combining elements of clinical effectiveness and implementation research to enhance public health impact. Med. Care 2012, 50, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, L.; Williams, C.M.; Wiggers, J.; Nathan, N.; Yoong, S.L. Improving the translation of health promotion interventions using effectiveness-implementation hybrid designs in program evaluations. Health Promot. J. Austr. 2016, 27, 204–207. [Google Scholar] [CrossRef]
- Li, A.C.; Kannry, J.L.; Kushniruk, A.; Chrimes, D.; McGinn, T.G.; Edonyabo, D.; Mann, D.M. Integrating usability testing and think-aloud protocol analysis with ‘near-live’ clinical simulations in evaluating clinical decision support. Int. J. Med. Inform. 2012, 81, 761–772. [Google Scholar] [CrossRef]
- Fonteyn, M.E.; Kuipers, B.; Grobe, S.J. A description of think aloud method and protocol analysis. Qual. Health Res. 1993, 3, 430–441. [Google Scholar] [CrossRef]
- Kushniruk, A.W.; Patel, V.L.; Cimino, J.J. Usability testing in medical informatics: Cognitive approaches to evaluation of information systems and user interfaces. Proc. AMIA Annu. Fall Symp. 1997, 218–222. [Google Scholar]
- Richardson, S.; Mishuris, R.; O’Connell, A.; Feldstein, D.; Hess, R.; Smith, P.; McCullagh, L.; McGinn, T.; Mann, D. ‘Think aloud’ and ‘Near live’ usability testing of two complex clinical decision support tools. Int. J. Med. Inform. 2017, 106, 1–8. [Google Scholar] [CrossRef]
- Weiner, B.J.; Lewis, C.C.; Stanick, C.; Powell, B.J.; Dorsey, C.N.; Clary, A.S.; Boynton, M.H.; Halko, H. Psychometric assessment of three newly developed implementation outcome measures. Implement. Sci. 2017, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.E.; Crosson, J.C.; O’Malley, A.S.; Cromp, D.; Taylor, E.F. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: A rapid-cycle evaluation approach to improving implementation. Implement. Sci. 2017, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Damschroder, L.; Hall, C.; Gillon, L.; Reardon, C.; Kelley, C.; Sparks, J.; Lowery, J. The Consolidated Framework for Implementation Research (CFIR): Progress to date, tools and resources, and plans for the future. Implement. Sci. 2015, 10, A12. [Google Scholar] [CrossRef]
- Gale, N.K.; Heath, G.; Cameron, E.; Rashid, S.; Redwood, S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med. Res. Methodol. 2013, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Firth, J. Qualitative data analysis: The framework approach. Nurse Res. 2011, 18, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Safarova, M.S.; Kullo, I.J. Using the electronic health record for genomics research. Curr. Opin. Lipidol. 2020, 31, 85–93. [Google Scholar] [CrossRef]
- Sinsky, C.; Colligan, L.; Li, L.; Prgomet, M.; Reynolds, S.; Goeders, L.; Westbrook, J.; Tutty, M.; Blike, G. Allocation of physician time in ambulatory practice: A time and motion study in 4 specialties. Ann. Intern. Med. 2016, 165, 753–760. [Google Scholar] [CrossRef]
- Arndt, B.G.; Beasley, J.W.; Watkinson, M.D.; Temte, J.L.; Tuan, W.J.; Sinsky, C.A.; Gilchrist, V.J. Tethered to the EHR: Primary care physician workload assessment using EHR event log data and time-motion observations. Ann. Fam. Med. 2017, 15, 419–426. [Google Scholar] [CrossRef]
- The Office of the National Coordinator for Health Information Technology. Strategy on Reducing Regulatory and Administrative Burden Relating to the Use of Health IT and EHRs Final Report 2020. Available online: https://www.healthit.gov/sites/default/files/page/2020-02/BurdenReport_0.pdf (accessed on 25 February 2020).
- Trivedi, M.H.; Daly, E.J.; Kern, J.K.; Grannemann, B.D.; Sunderajan, P.; Claassen, C.A. Barriers to implementation of a computerized decision support system for depression: An observational report on lessons learned in ‘real world’ clinical settings. BMC Med. Inform. Decis. Mak. 2009, 9, 6. [Google Scholar] [CrossRef]
- Lyerla, F. Design and implementation of a nursing clinical decision support system to promote guideline adherence. Comput. Inform. Nurs. 2008, 26, 227–233. [Google Scholar] [CrossRef]
- North, F.; Fox, S.; Chaudhry, R. Clinician time used for decision making: A best case workflow study using cardiovascular risk assessments and Ask Mayo Expert algorithmic care process models. BMC Med. Inform. Decis. Mak. 2016, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Evans, P. EHR Implementation with Minimal Practice Disruption in Primary Care Settings: The Experience of the Washington & Idaho Regional Extension Center. 2012. Available online: https://www.healthit.gov/sites/default/files/ehr-implementation-wirec.pdf (accessed on 20 March 2020).
| Physician Characteristics | n (%) |
|---|---|
| Gender | |
| Females | 7 (53.8) |
| Males | 6 (46.2) |
| Age | |
| <40 years | 4 (30.8) |
| 40–60 years | 7 (53.8) |
| >60 years | 2 (15.4) |
| Race/Ethnicity | |
| Non-Hispanic white | 10 (76.9) |
| Black | 1 (7.7) |
| Asian | 1 (7.7) |
| Hispanic | 1 (7.7) |
| Specialties | |
| Community Internal Medicine | 3 (23.1) |
| Family Medicine | 3 (23.1) |
| Family Medicine/Obstetrics | 1 (7.7) |
| Cardiology | 5 (38.4) |
| Vascular Medicine | 1 (7.7) |
| Years in Practice | |
| 0–5 | 3 (23.1) |
| 6–10 | 1 (7.7) |
| 11–15 | 2 (15.4) |
| 16–20 | 1 (7.7) |
| More than 20 | 6 (46.1) |
| Theme | Quotation |
|---|---|
| Understanding & Awareness of FH | “But the initial management as far as I know is the same as anybody else.” (PCP) |
| “…I mean, I see people that carry that diagnosis [FH], but I don’t think I would, sort of, come up with it myself.” (Specialist) | |
| Clinical Workflow | “And to be honest, if I’m seeing them for strep throat, I might ignore it [CDS] or mention to them that they need to talk to their doctor about it. I will try to mention to a patient ‘hey you’re due for your lipids, you’re due for your colonoscopy, may I order those things for you?’ and if they say ‘yes’, I will, but if I have a 15-min appointment for their broken leg… I don’t know that I’m going to get into a huge discussion…” (PCP) |
| “I would counsel them, and then I would want to send them to the FH Clinic if they’re around or local. If not, you know, of course I’d start them on a statin, and then you’re kind of stuck because I wouldn’t feel comfortable ordering the genetic tests for them. That’s really the logical next step, in my opinion. In which case, you’d try to send them to Genetics and/or the FH Clinic.” (Specialist) | |
| Physician Preferences & Value of CDS Tools | “I would suggest one area that we constantly struggle with is finding the right orders in Epic when it’s orders that we haven’t done a lot of… so the more detail… showing you exactly how to find the right order would be very helpful… Because we have really struggled… with the amount of different things that we order, that has been a real hard spot…” (PCP) |
| “Personally, I guess that’s why I’m here, I would really prefer passive alerts… I think, where it was an active alert… people get alert fatigue, and they’re just going to click it to bypass it.” (PCP) | |
| Perspectives on Patient Needs & Values | “Because I’ve learned when I do risk counseling and I use the shared decision-making aid, which includes all the risk percentages…I’m learning as I teach my own patients, like, oh, yeah, that’s right, smoking does increase by this much, and so if you have something like that for FH, if you really want to drive home the point of how much greater risk, people who use that will start educating themselves in addition to their patients.” (Specialist) |
| Dissemination & Implementation | “I think whenever anything significantly new like this [CDS] is deployed; some type of communication is useful. I mean either in the EHR update, which many of us are actually reading now, or in like communication from leadership or through multiple approaches.” (PCP) |
| “The problem with BPA is, again, that just so many of them don’t apply. You really have to sort it out from them. You’d have to suppress everything that didn’t apply to me and patients…I’m just going to see a bunch of yellow and I’m going to ignore it because it’s not… it doesn’t apply to me, I’m therefore going to ignore everything and I’m going to miss the important alarms.” (Specialist) |
| Recommendation | Description | Quotation |
|---|---|---|
| Format & Placement | Having both the BPA and in-basket alert formats embedded in the EHR would likely increase the probability of providers viewing the CDS | “And if you could somehow attach to the results in-basket that would allow us to see that alert at the same time that we’re seeing the result, which would be pretty awesome rather than having more in-baskets on it.”(PCP) |
| Physicians indicated preference for the in-basket format linked to a lipid panel report | ||
| Content | Physicians highlighted the need for BPA and in-basket content to be more concise and clear | “There’s a lot of dense text. A lot of dense text…don’t be afraid of white space…and be telegraphic. So I would… critically looking at this message, I think I would look at individual words. This patient has an… those are not useful words yet. So LDL greater equal 190. That could be a line. Warning, possible familial hypercholesterolemia. Next line…Consider high-intensity… I wouldn’t even say high intensity… consider Rosuvastatin 20 or Atorvastatin 40 mg taken by mouth…Recommended laboratory testing could be another line.” (PCP) |
| Only important information should be displayed | ||
| Have few clicks to access knowledge resources and a relevant order set | ||
| Have a reminder present to rule out secondary causes of hypercholesterolemia | ||
| Timing & Frequency | Have ‘reasons not to use’ at the end of the CDS so that providers can explain their decision to not act upon the alert | “…but if I’m done with it [in-basket message] or I feel like I’ve addressed it, maybe I’ve put in the orders for the FH Clinic, I’d like to get it out of there, because otherwise things get too cluttered, and I’ll get very frustrated if there’s no way to dismiss it and if it just stays there forever.” (Specialist) |
| Viewing one alert should turn off all other alerts for the same provider | ||
| Prioritization | Most physicians described FH as being an important condition and agreed that color coding the CDS red would likely get their attention | “I think keep it red, because the people with this condition have significant events, and it’s something that you can prevent in their family members, so I would keep it red.”(Specialist) |
| Measures and Constructs Assessed | Question | Completely Agree/Agree N (%) | Other 1 N (%) |
|---|---|---|---|
| Acceptability of Intervention Measure (AIM) | This tool meets my approval | 11 (84.6) | 2 (15.4) |
| This tool is appealing to me | 10 (76.9) | 3 (23.1) | |
| I like this tool | 11 (84.6) | 2 (15.4) | |
| I welcome this tool | 12 (92.3) | 1 (7.7) | |
| Intervention Appropriateness Measure (IAM) | This tool seems fitting | 12 (92.3) | 1 (7.7) |
| This tool seems suitable | 12 (92.3) | 1 (7.7) | |
| This tool seems applicable | 12 (92.3) | 1 (7.7) | |
| This tool seems like a good match | 10 (76.9) | 3 (23.1) | |
| Feasibility of Intervention Measure (FIM) | This tool seems implementable | 11 (84.6) | 2 (15.4) |
| This tool seems possible | 12 (92.3) | 1 (7.7) | |
| This tool seems doable | 12 (92.3) | 1 (7.7) | |
| This tool seems easy to use | 11 (84.6) | 2 (15.4) | |
| Intervention Characteristics | I trust the quality and validity of evidence supporting this intervention | 12 (92.3) | 1 (7.7) |
| Implementing this tool is a good option to identify FH patients at Mayo | 11 (84.6) | 2 (15.4) | |
| This tool will improve early diagnosis of patients with FH | 11 (84.6) | 2 (15.4) | |
| Outer Setting | This tool meets my needs to provide needed resources to my patients | 9 (69.2) | 4 (30.8) |
| Inner Setting | This tool is appropriate for ECH clinicians | 11 (84.6) | 2 (15.4) |
| This tool fits within my existing workflow | 10 (76.9) | 3 (23.1) | |
| This tool will not increase the time needed with a patient | 4 (30.8) | 9 (69.2) | |
| The implementation of this intervention within Mayo is important | 12 (92.3) | 1 (7.7) | |
| I recognize the importance of implementing this tool into the practice | 13 (100) | 0 (0.0) | |
| This tool appears easy to access and incorporate into my workflow | 10 (76.9) | 3 (23.1) | |
| Characteristics of Individuals | This is a valuable tool for ECH clinicians | 10 (76.9) | 3 (23.1) |
| This tool will help me identify and refer or manage FH patients | 12 (92.3) | 1 (7.7) | |
| Process | It is important to me that the cardiologists embedded in ECH continue to vet this tool | 7 (53.8) | 6 (46.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bangash, H.; Pencille, L.; Gundelach, J.H.; Makkawy, A.; Sutton, J.; Makkawy, L.; Dikilitas, O.; Kopecky, S.; Freimuth, R.; Caraballo, P.J.; et al. An Implementation Science Framework to Develop a Clinical Decision Support Tool for Familial Hypercholesterolemia. J. Pers. Med. 2020, 10, 67. https://doi.org/10.3390/jpm10030067
Bangash H, Pencille L, Gundelach JH, Makkawy A, Sutton J, Makkawy L, Dikilitas O, Kopecky S, Freimuth R, Caraballo PJ, et al. An Implementation Science Framework to Develop a Clinical Decision Support Tool for Familial Hypercholesterolemia. Journal of Personalized Medicine. 2020; 10(3):67. https://doi.org/10.3390/jpm10030067
Chicago/Turabian StyleBangash, Hana, Laurie Pencille, Justin H. Gundelach, Ahmed Makkawy, Joseph Sutton, Lenae Makkawy, Ozan Dikilitas, Stephen Kopecky, Robert Freimuth, Pedro J. Caraballo, and et al. 2020. "An Implementation Science Framework to Develop a Clinical Decision Support Tool for Familial Hypercholesterolemia" Journal of Personalized Medicine 10, no. 3: 67. https://doi.org/10.3390/jpm10030067
APA StyleBangash, H., Pencille, L., Gundelach, J. H., Makkawy, A., Sutton, J., Makkawy, L., Dikilitas, O., Kopecky, S., Freimuth, R., Caraballo, P. J., & Kullo, I. J. (2020). An Implementation Science Framework to Develop a Clinical Decision Support Tool for Familial Hypercholesterolemia. Journal of Personalized Medicine, 10(3), 67. https://doi.org/10.3390/jpm10030067






