A Web-Based Pharmacogenomics Search Tool for Precision Medicine in Perioperative Care
Abstract
1. Introduction
2. Aims
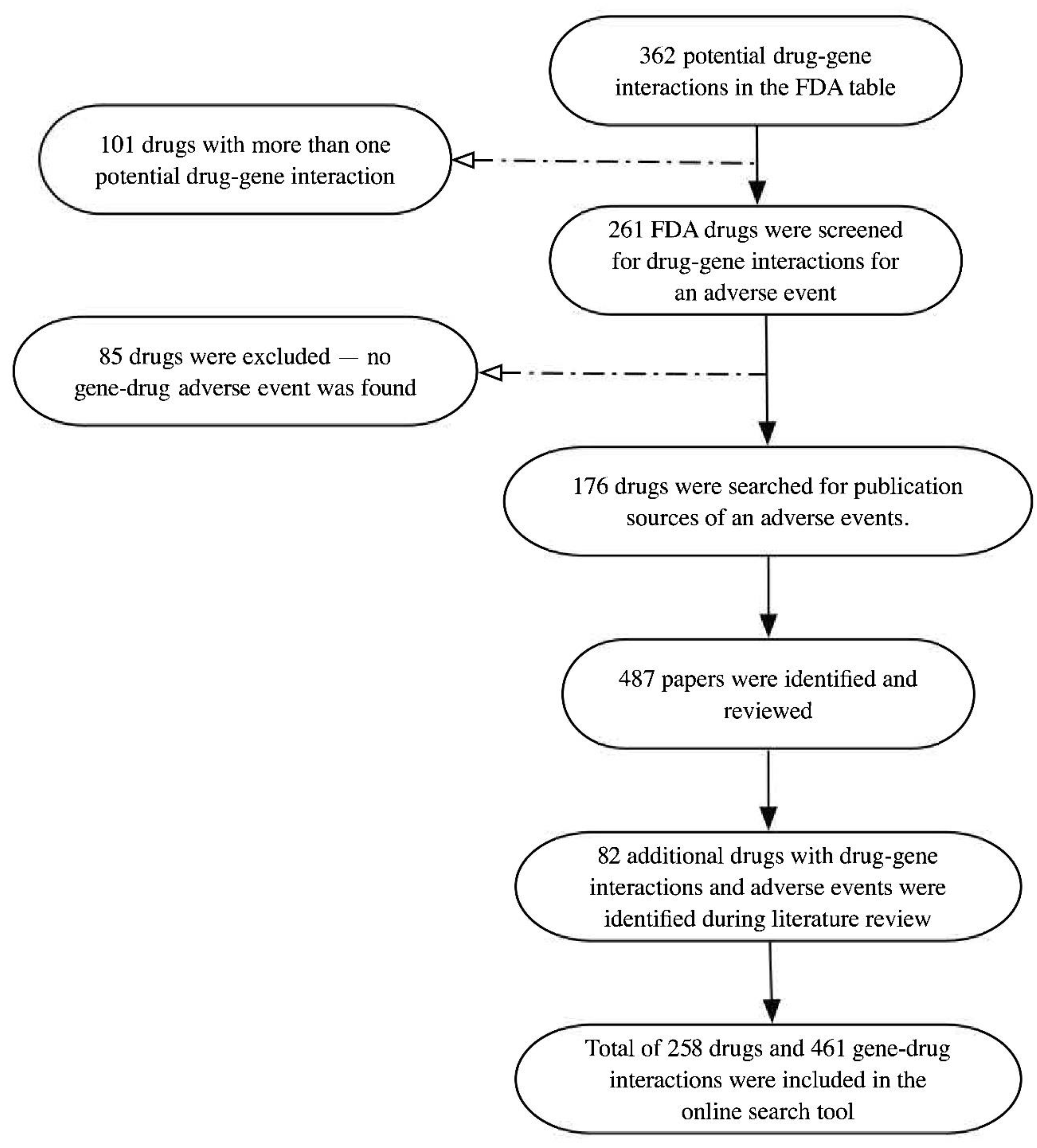
3. Methods
4. Result
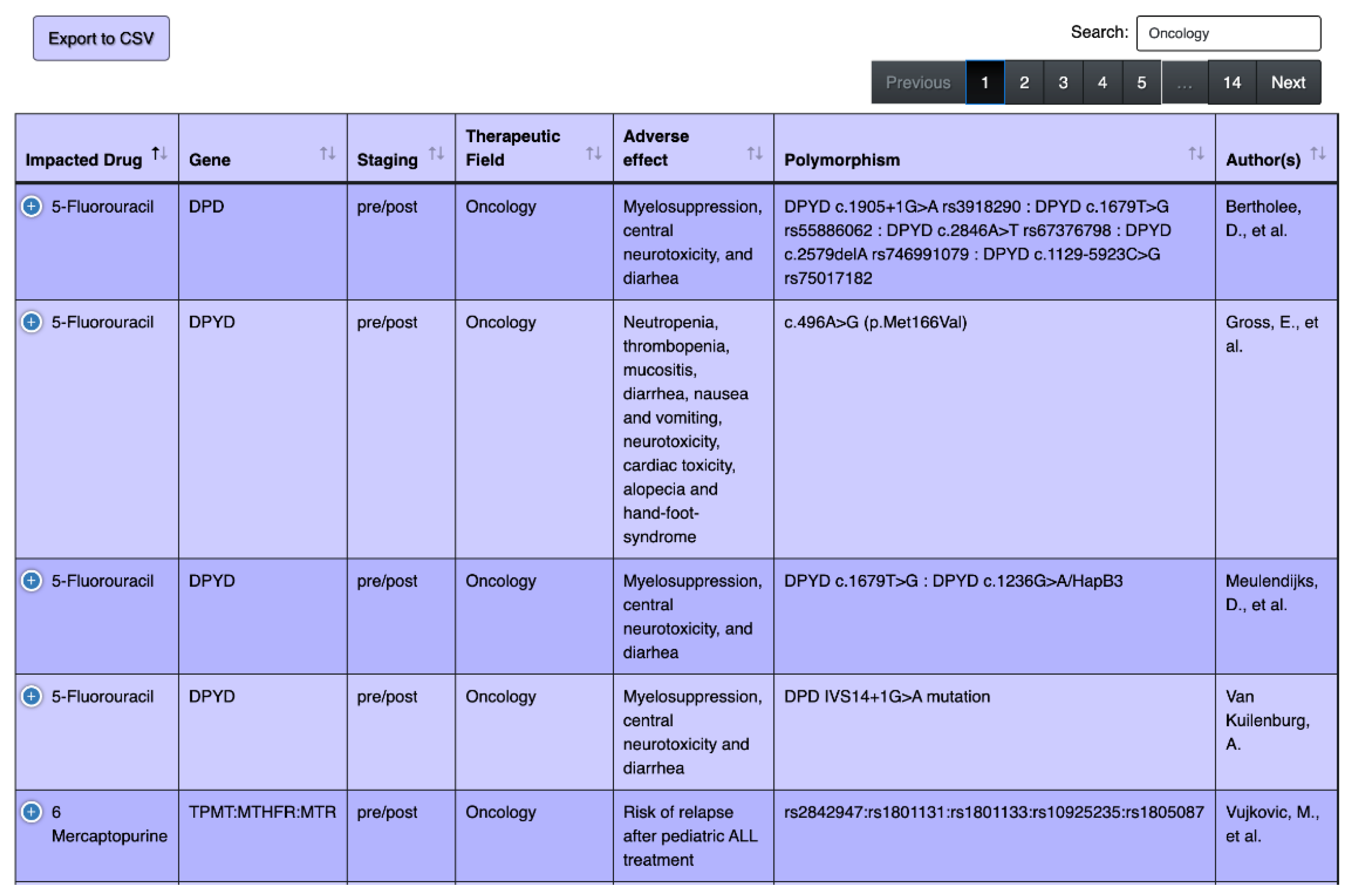
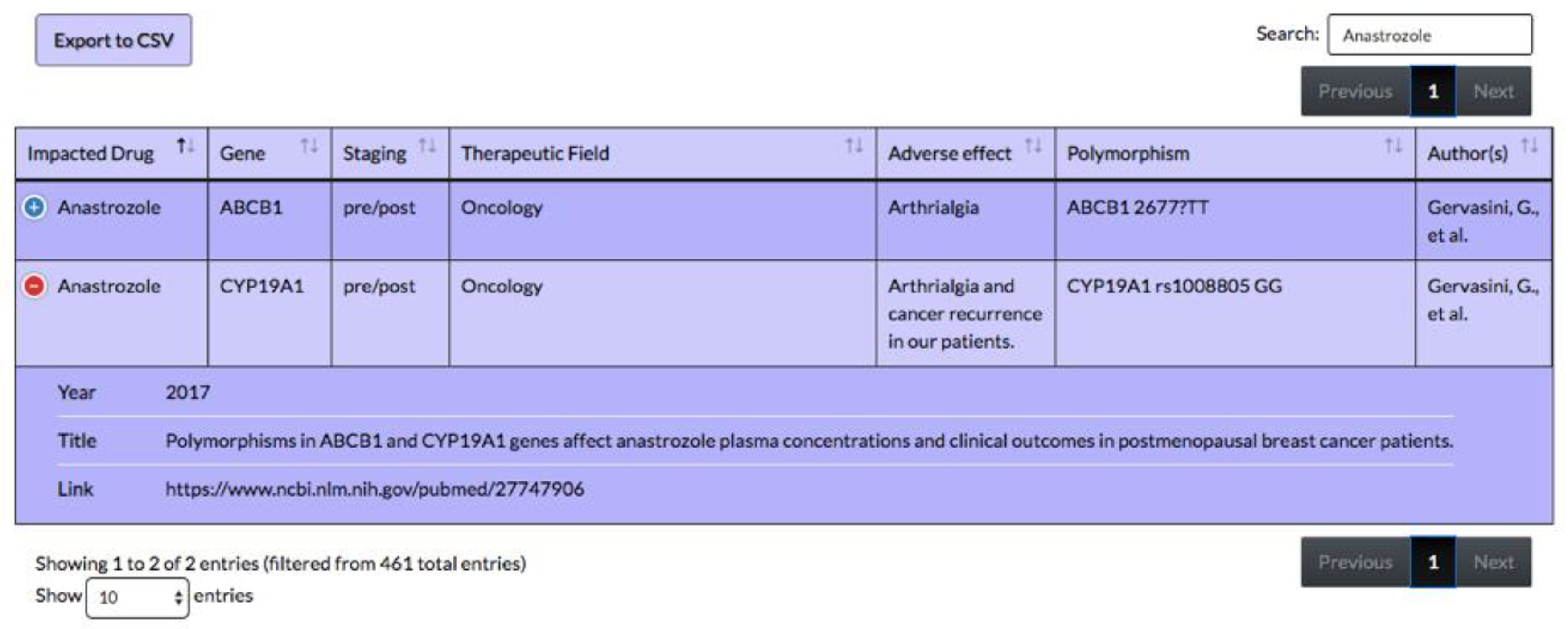
4.1. Overview of the Tool
4.2. Frequency of Drug–Gene Interactions
4.3. Frequencies by Speciality
4.4. Pharmacogenes
4.5. Intraoperative Precision Medicine: Anesthesiology
5. Discussion
5.1. Intraoperative Precision Medicine: Anesthesiology
5.2. Perioperative Precision Medicine: Fields Other Than Anesthesiology
5.3. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- What Is Precision Medicine?—Genetics Home Reference—NIH. Available online: https://ghr.nlm.nih.gov/primer/precisionmedicine/definition (accessed on 20 April 2019).
- Osanlou, O.; Pirmohamed, M.; Daly, A.K. Pharmacogenetics of Adverse Drug Reactions. In Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2018; pp. 155–190. [Google Scholar]
- Flaten, H.K.; Kim, H.S.; Campbell, J.; Hamilton, L.; Monte, A.A. CYP2C19 drug-drug and drug-gene interactions in ED patients. Am. J. Emerg. Med. 2016, 34, 245–249. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Dunnenberger, H.M.; Hicks, J.K.; Caudle, K.E.; Carrillo, M.W.; Freimuth, R.R.; Williams, M.S.; Klein, T.E.; Peterson, J.F. Developing knowledge resources to support precision medicine: Principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J. Am. Med. Inform. Assoc. 2016, 23, 796. [Google Scholar] [CrossRef]
- Gonsalves, S.G.; Dirksen, R.T.; Sangkuhl, K.; Pulk, R.; Alvarellos, M.; Vo, T.; Hikino, K.; Roden, D.; Klein, T.E.; Poler, S.M.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. Clin. Pharmacol. Ther. 2019, 105, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Sachidanandam, R.; Weissman, D.; Schmidt, S.C.; Kakol, J.M.; Stein, L.D.; Marth, G.; Sherry, S.; Mullikin, J.C.; Mortimore, B.J.; Willey, D.L.; et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 2001, 409, 928–933. [Google Scholar] [PubMed]
- Mooney, S.D. Progress towards the integration of pharmacogenomics in practice. Hum. Genet. 2015, 134, 459–465. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25238897 (accessed on 18 April 2020). [CrossRef] [PubMed][Green Version]
- Pulley, J.M.; Denny, J.C.; Peterson, J.F.; Bernard, G.R.; Vnencak-Jones, C.L.; Ramirez, A.H.; Delaney, J.T.; Bowton, E.; Brothers, K.; Johnson, K.; et al. Operational Implementation of Prospective Genotyping for Personalized Medicine: The Design of the Vanderbilt PREDICT Project. Clin. Pharmacol. Ther. 2012, 92, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Crews, K.R.; Hicks, J.K.; Pui, C.-H.; Relling, M.V.; Evans, W.E. Pharmacogenomics and individualized medicine: Translating science into practice. Clin. Pharmacol. Ther. 2012, 92, 467–475. [Google Scholar] [CrossRef]
- Berinstein, E.; Levy, A. Recent developments and future directions for the use of pharmacogenomics in cardiovascular disease treatments. Expert Opin. Drug Metab. Toxicol. 2017, 13, 973–983. [Google Scholar] [CrossRef]
- Lanning, R.K.; Zai, C.C.; Müller, D.J. Pharmacogenetics of tardive dyskinesia: An updated review of the literature. Pharmacogenomics 2016, 17, 1339–1351. [Google Scholar] [CrossRef]
- Behrooz, A. Pharmacogenetics and anaesthetic drugs: Implications for perioperative practice. Ann. Med. Surg. 2015, 4, 470–474. [Google Scholar] [CrossRef]
- Bainbridge, D.; Martin, J.; Arango, M.; Cheng, D. Perioperative and anaesthetic-related mortality in developed and developing countries: A systematic review and meta-analysis. Lancet 2012, 380, 1075–1081. [Google Scholar] [CrossRef]
- Rosenberg, H.; Pollock, N.; Schiemann, A.; Bulger, T.; Stowell, K. Malignant hyperthermia: A review. Orphanet. J. Rare Dis. 2015, 10, 93. [Google Scholar] [CrossRef]
- US Food & Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. Center for Drug Evaluation and Research. Available online: https://www.fda.gov/drugs/science-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 21 August 2018).
- Lesko, L.J.; Zineh, I. DNA, drugs and chariots: On a decade of pharmacogenomics at the US FDA. Pharmacogenomics 2010, 11, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Codeine Therapy and CYP2D6 Genotype. In Medical Genetics Summaries; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Alvarellos, M.L.; McDonagh, E.M.; Patel, S.; McLeod, H.L.; Altman, R.B.; Klein, T.E. PharmGKB summary: Succinylcholine pathway, pharmacokinetics/pharmacodynamics. Pharm. Genom. 2015, 25, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Ma, W.; Guo, Q.; Liu, J.; Li, W.; Mcleod, H.L.; He, Y. The pharmacogenetics of medications used in general anesthesia. Pharmacogenomics 2018, 19, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Frueh, F.W.; Amur, S.; Mummaneni, P.; Epstein, R.S.; Aubert, R.E.; DeLuca, T.M.; Verbrugge, R.R.; Burckart, G.J.; Lesko, L.J. Pharmacogenomic Biomarker Information in Drug Labels Approved by the United States Food and Drug Administration: Prevalence of Related Drug Use. Pharmacotherapy 2008, 28, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Vivot, A.; Boutron, I.; Ravaud, P.; Porcher, R. Guidance for pharmacogenomic biomarker testing in labels of FDA-approved drugs. Genet. Med. 2015, 17, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Tutton, R. Pharmacogenomic biomarkers in drug labels: What do they tell us? Pharmacogenomics 2014, 15, 297–304. [Google Scholar] [CrossRef]
- Kaye, A.D.; Mahakian, T.; Kaye, A.J.; Pham, A.A.; Hart, B.M.; Gennuso, S.; Cornett, E.M.; Gabriel, R.A.; Urman, R.D. Pharmacogenomics, precision medicine, and implications for anesthesia care. Best Pract. Res. Clin. Anaesthesiol. 2018, 32, 61–81. [Google Scholar] [CrossRef]
- Gabriel, R.A.; Burton, B.N.; Urman, R.D.; Waterman, R.S. Genomics Testing and Personalized Medicine in the Preoperative Setting. Anesthesiol. Clin. 2018, 36, 639–652. [Google Scholar] [CrossRef]
- Todd, J.J.; Razaqyar, M.S.; Witherspoon, J.W.; Lawal, T.A.; Mankodi, A.; Chrismer, I.C.; Allen, C.; Meyer, M.D.; Kuo, A.; Shelton, M.S.; et al. Novel Variants in Individuals with RYR1-Related Congenital Myopathies: Genetic, Laboratory, and Clinical Findings. Front. Neurol. 2018, 9, 118. [Google Scholar] [CrossRef]
- Halliday, N.J. Malignant hyperthermia. J. Craniofac. Surg. 2003, 14, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative council. J. Pain 2016, 17, 131–157. [Google Scholar] [PubMed]
- Krasowski, M.D. Pharmacogenomics|CURRENT Diagnosis & Treatment: Family Medicine; Access Medicine|McGraw-Hill Medical: New York, NY, USA, 2015. [Google Scholar]
- Wei, C.-Y.; Michael Lee, M.-T.; Chen, Y.-T. Pharmacogenomics of adverse drug reactions: Implementing personalized medicine. Hum. Mol. Genet. 2012, 21, R58–R65. [Google Scholar] [CrossRef] [PubMed]
- Barash, M.; Reich, K.; Rademaker, D. Lidocaine-Induced Methemoglobinemia: A Clinical Reminder. J. Am. Osteopath. Assoc. 2015, 115, 94–98. [Google Scholar] [CrossRef]
- Elyassi, A.R.; Rowshan, H.H. Perioperative management of the glucose-6-phosphate dehydrogenase deficient patient: A review of literature. Anesth. Prog. 2009, 56, 86–91. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers (accessed on 21 August 2019).
- Flockhart, D.A. Drug Interactions: Cytochrome P450 Drug Interaction Table Indiana University School of Medicine. 2007. Available online: https://drug-interactions.medicine.iu.edu (accessed on 29 May 2018).
- MacKenzie, M.; Hall, R. Pharmacogenomics and pharmacogenetics for the intensive care unit: A narrative review. Can. J. Anesth. Can. Anesth. 2017, 64, 45–64. [Google Scholar] [CrossRef]
- Hicks, J.K.; Swen, J.J.; Thorn, C.F.; Sangkuhl, K.; Kharasch, E.D.; Ellingrod, V.L.; Skaar, T.C.; Müller, D.J.; Gaedigk, A.; Stingl, J.C. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin. Pharmacol. Ther. 2013, 93, 402–408. [Google Scholar] [CrossRef]
- Candiotti, K.; Yang, Z.; Xue, L.; Zhang, Y.; Rodriguez, Y.; Wang, L.; Hao, S.; Gitlin, M. Single-Nucleotide Polymorphism C3435T in the ABCB1 Gene is Associated with Opioid Consumption in Postoperative Pain. Pain Med. 2013, 14, 1977–1984. [Google Scholar] [CrossRef]
- Bertholee, D.; Maring, J.G.; van Kuilenburg, A.B.P. Genotypes Affecting the Pharmacokinetics of Anticancer Drugs. Clin. Pharm. 2017, 56, 317–337. [Google Scholar] [CrossRef]
- NHLBI Trans-Omics for Precision Medicine WGS-About TOPMed. Available online: https://www.nhlbiwgs.org/ (accessed on 30 September 2019).
- Nayak, L.; Ray, I.; De, R.K. Precision medicine with electronic medical records: From the patients and for the patients. Ann. Transl. Med. 2016, 4 (Suppl. 1), S61. [Google Scholar] [CrossRef] [PubMed]


| Field of Medicine | Most Common Gene Associated with Adverse Effect in an Interaction with Drugs |
|---|---|
| Oncology | ABCB1, BCR-ABL, BRAF, CYP3A4, DPD, ESR, PGR, TPMT, UGT1A1 |
| Infectious Disease | CYP2C8, CYP2C19, G6PD, HLA-B, HLA-A, IFNL3/ IFNL4, UGT1A1 |
| Cardiology | ABCG2, CYP2D6, SLCO1B1 |
| Psychiatry | CYP1A2, CYP2C19, CYP2D6 |
| Anesthesiology | BCHE, CYP2D6, CYP3A4, G6PD, RYR1 |
| Neurology | CYP2C19, CYP2D6, GST M1, HLA-B |
| Hematology | CYP2C9, CYP2C19, VKORC1 |
| Field of Medicine | % Drugs with Pharmacogenetic Information |
|---|---|
| Oncology | 27.88% |
| Infectious Disease | 14.95% |
| Cardiology | 13.94% |
| Psychiatry | 9.29% |
| Anesthesiology | 7.75% |
| Neurology | 6.67% |
| Hematology | 6.06% |
| Gastroenterology | 4.85% |
| Rheumatology | 3.64% |
| Endocrinology | 2.22% |
| Dermatology | 0.81% |
| Gynecology | 0.81% |
| Pulmonary | 0.81% |
| Urology | 0.61% |
| Genetic Medicine | 0.40% |
| Ophthalmology | 0.20% |
| Genes | % Drug–Gene Interactions |
|---|---|
| CYP | 44% |
| HLA | 11% |
| G6PD | 10% |
| UGT1A1 | 6% |
| TPMT | 4% |
| ABCB1 | 4% |
| GST | 3% |
| SLCO1B1 | 3% |
| BCR-ABL | 3% |
| DPYD | 2% |
| IFNL | 2% |
| VKORC1 | 2% |
| RYR1 | 2% |
| ESR | 2% |
| BRAF | 2% |
| PGR | 1% |
| CACNA1S | 1% |
| BCHE | 1% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei, S.; Costas, Y.; Orozco, G.; Zaydlin, M.; Mirtar, A.; Abouali, M.; Diaz-Marty, C.; Akhlaghipour, G.; Fernandez Altamirano, P.; Gonzalez Cardona, A.R.; et al. A Web-Based Pharmacogenomics Search Tool for Precision Medicine in Perioperative Care. J. Pers. Med. 2020, 10, 65. https://doi.org/10.3390/jpm10030065
Zarei S, Costas Y, Orozco G, Zaydlin M, Mirtar A, Abouali M, Diaz-Marty C, Akhlaghipour G, Fernandez Altamirano P, Gonzalez Cardona AR, et al. A Web-Based Pharmacogenomics Search Tool for Precision Medicine in Perioperative Care. Journal of Personalized Medicine. 2020; 10(3):65. https://doi.org/10.3390/jpm10030065
Chicago/Turabian StyleZarei, Sara, Yensea Costas, Gloria Orozco, Michelle Zaydlin, Ali Mirtar, Mohammad Abouali, Cristina Diaz-Marty, Golnoush Akhlaghipour, Pablo Fernandez Altamirano, Anel R. Gonzalez Cardona, and et al. 2020. "A Web-Based Pharmacogenomics Search Tool for Precision Medicine in Perioperative Care" Journal of Personalized Medicine 10, no. 3: 65. https://doi.org/10.3390/jpm10030065
APA StyleZarei, S., Costas, Y., Orozco, G., Zaydlin, M., Mirtar, A., Abouali, M., Diaz-Marty, C., Akhlaghipour, G., Fernandez Altamirano, P., Gonzalez Cardona, A. R., Reiley, L. E., & Mirzakhani, H. (2020). A Web-Based Pharmacogenomics Search Tool for Precision Medicine in Perioperative Care. Journal of Personalized Medicine, 10(3), 65. https://doi.org/10.3390/jpm10030065






