HER2 Heterogeneity in Personalized Therapy of Gastro-Oesophageal Malignancies: An Overview by Different Methodologies
Abstract
:1. Introduction
2. Methodological Procedures to Identify Human Epidermal Growth Factor Receptor-2 (HER2) Expression/Amplification
3. HER2 in Gastro-Oesophageal Dysplastic Conditions
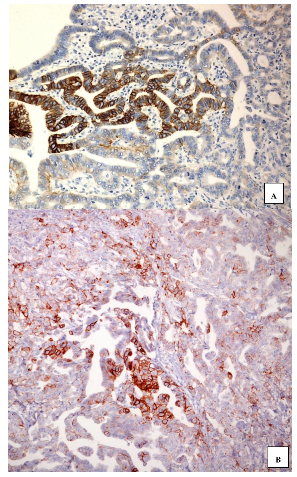
4. HER2 in Gastro-Oesophageal Malignant Lesions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsuno, K.; Ishihara, R.; Ohmori, M.; Iwagami, H.; Shichijyo, S.; Maekawa, A.; Kanesaka, T.; Yamamoto, S.; Takeuchi, Y.; Higashino, K.; et al. Time trends in the incidence of esophageal adenocarcinoma, gastric adenocarcinoma, and superficial esophagogastric junction adenocarcinoma. J. Gastroenterol. 2019, 54, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Crane, S.J.; Richard Locke, G., 3rd; Harmsen, W.S.; Diehl, N.N.; Zinsmeister, A.R.; Joseph Melton, L., 3rd; Romero, Y.; Talley, N.J. The changing incidence of esophageal and gastric adenocarcinoma by anatomic sub-site. Aliment. Pharm. 2007, 25, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.G.; Xie, S.H.; Lagergren, J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018, 154, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Devesa, S.S.; Chow, W.H. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J. Natl. Cancer Inst. 2008, 100, 1184–1187. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J. Epidemiology of Gastroesophageal Junction Adenocarcinoma in Korea. J. Gastric. Cancer 2018, 18, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; De Santis, C.E.; Jemal, A. Incidence Trends of Esophageal and Gastric Cancer Subtypes by Race, Ethnicity, and Age in the United States, 1997-2014. Clin. Gastroenterol. Hepatol. 2019, 17, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Natori, A.; Chan, B.A.; Sim, H.W.; Ma, L.; Yokom, D.W.; Chen, E.; Liu, G.; Darling, G.; Swallow, C.; Brar, S.; et al. Outcomes by treatment modality in elderly patients with localized gastric and esophageal cancer. Curr. Oncol. 2018, 25, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Pasini, F.; Fraccon, A.P.; Modena, Y.; Bencivenga, M.; Giacopuzzi, S.; La Russa, F.; Gusella, M.; de Manzoni, G. Targeted therapies for advanced and metastatic adenocarcinoma of the gastroesophageal junction: Is there something new? Gastric. Cancer 2017, 20, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Merrick, D.T.; Kittelson, J.; Winterhalder, R.; Kotantoulas, G.; Ingeberg, S.; Keith, R.L.; Kennedy, T.C.; Miller, Y.E.; Franklin, W.A.; Hirsch, F.R. Analysis of c-ErbB1/epidermal growth factor receptor and c-ErbB2/HER-2 expression in bronchial dysplasia: Evaluation of potential targets for chemoprevention of lung cancer. Clin. Cancer Res. 2006, 12, 2281–2288. [Google Scholar] [CrossRef] [Green Version]
- Lodato, R.F.; Maguire, H.C.; Greene, M.I.; Weiner, D.B.; LiVolsi, V.A. Immunohistochemical evaluation of c-erbB-2 oncogene expression in ductal carcinoma in situ and atypical ductal hyperplasia of the breast. Mod. Pathol. 1990, 3, 449–454. [Google Scholar] [PubMed]
- Van Bockstal, M.; Lambein, K.; Denys, H.; Braems, G.; Nuyts, A.; Van den Broecke, R.; Cocquyt, V.; De Wever, O.; Libbrecht, L. Histopathological characterization of ductal carcinoma in situ (DCIS) of the breast according to HER2 amplification status and molecular subtype. Virchows Archiv. 2014, 465, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets systems biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, G.; Ieni, A.; Barresi, V.; Caruso, R.A.; Tuccari, G. HER2 status in unusual histological variants of gastric adenocarcinomas. J. Clin. Pathol. 2012, 65, 237–241. [Google Scholar] [CrossRef]
- Ieni, A.; Giuffrè, G.; Lanzafame, S.; Nuciforo, G.; Curduman, M.; Villari, L.; Roz, E.; Certo, G.; Cabibi, D.; Salomone, E.; et al. Morphological and biomolecular characteristics of subcentimetric invasive breast carcinomas in Sicily: A multicentre retrospective study in relation to trastuzumab treatment. Oncol. Lett. 2012, 3, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Ieni, A.; Barresi, V.; Giuffrè, G.; Caruso, R.A.; Lanzafame, S.; Villari, L.; Salomone, E.; Roz, E.; Cabibi., D.; Franco, V.; et al. HER2 status in advanced gastric carcinoma: A retrospective multicentric analysis from Sicily. Oncol. Lett. 2013, 6, 1591–1594. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.; Ying, J.; Lu, N. HER2 expression and relevant clinicopathological features in gastric and gastroesophageal junction adenocarcinoma in a Chinese population. Diagn. Pathol. 2013, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, R.M.; Campennì, A.; Giuffrè, G.; Giovanella, L.; Siracusa, M.; Simone, A.; Branca, G.; Scarfì, R.; Trimarchi, F.; Ieni, A.; et al. HER2 Analysis in Sporadic Thyroid Cancer of Follicular Cell Origin. Int. J. Mol. Sci. 2016, 17, 2040. [Google Scholar] [CrossRef] [Green Version]
- Gerson, J.N.; Skariah, S.; Denlinger, C.S.; Astsaturov, I. Perspectives of HER2-targeting in gastric and esophageal cancer. Expert Opin. Investig. Drugs 2017, 26, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Rüschoff, J.; Dietel, M.; Baretton, G.; Arbogast, S.; Walch, A.; Monges, G.; Chenard, M.P.; Penault-Llorca, F.; Nagelmeier, I.; Schlake, W.; et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010, 457, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Rüschoff, J.; Hanna, W.; Bilous, M.; Hofmann, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 testing in gastric cancer: A practical approach. Mod. Pathol. 2012, 25, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Pazo Cid, R.A.; Anton, A. Advanced HER2-positive gastric cancer: Current and future targeted therapies. Crit. Rev. Oncol. Hematol. 2013, 85, 350–362. [Google Scholar] [CrossRef]
- Albarello, L.; Pecciarini, L.; Doglioni, C. HER2 testing in gastric cancer. Adv. Anat. Pathol. 2011, 18, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Polydorides, A.D. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: A review of histopathology, diagnostic testing, and clinical implications. Arch. Pathol. Lab. Med. 2012, 136, 691–697. [Google Scholar] [CrossRef]
- Cho, E.Y.; Srivastava, A.; Park, K.; Kim, J.; Lee, M.H.; Do, I.; Lee, J.; Kim, K.M.; Sohn, T.S.; Kang, W.K.; et al. Comparison of four immunohistochemical tests and FISH for measuring HER2 expression in gastric carcinomas. Pathology 2012, 44, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Takehana, T.; Kunitomo, K.; Kono, K.; Kitahara, F.; Iizuka, H.; Matsumoto, Y.; Fujino, M.A.; Ooi, A. Status of c-erbB-2 in gastric adenocarcinoma: A comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int. J. Cancer 2002, 98, 833–837. [Google Scholar] [CrossRef]
- Kim, M.A.; Jung, E.J.; Lee, H.S.; Lee, H.E.; Jeon, Y.K.; Yang, H.K.; Kim, W.H. Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum. Pathol. 2007, 38, 1386–1393. [Google Scholar] [CrossRef]
- Tsapralis, D.; Panayiotides, I.; Peros, G.; Liakakos, T.; Karamitopoulou, E. Human epidermal growth factor receptor-2 gene amplification in gastric cancer using tissue microarray technology. World J. Gastroenterol. 2012, 18, 150–155. [Google Scholar] [CrossRef]
- Ieni, A.; Angelico, G.; Barresi, V.; Giuffrè, G.; Arena, F.; Caruso, R.A.; Tuccari, G. Human Epidermal Growth Factor Receptor 2 Status in Gastric Carcinomas with Distinctive Prevalent Cribriform Component. Dis. Markers 2018, 2018, 1505428. [Google Scholar] [CrossRef] [Green Version]
- Press, M.F.; Villalobos, I.; Santiago, A.; Guzman, R.; Cervantes, M.; Gasparyan, A.; Campeau, A.; Ma, Y.; Tsao-Wei, D.D.; Groshen, S. Assessing the New American Society of Clinical Oncology/College of American Pathologists Guidelines for HER2 Testing by Fluorescence In Situ Hybridization: Experience of an Academic Consultation Practice. Arch. Pathol. Lab. Med. 2016, 140, 1250–1258. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, A.S.; Sukov, W.R.; Eckel-Passow, J.E.; Sakai, Y.; Walsh, F.J.; Lonzo, M.; Wiktor, A.E.; Dogan, A.; Jenkins, R.B. Comparison of Fluorescence in Situ Hybridization (FISH) and dual-ISH (DISH) in the Determination of HER2 Status in Breast Cancer. Am. J. Clin. Pathol. 2013, 139, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, T.; Wood, M.; Wing, A.; Hnatovska, H.; Mendes, M.; Mullen, J.B.; Chang, M.C. Comparison of HER2 Dual-Color and Fluorescence In Situ Hybridization in Breast Cancer: A Cohort Study Emphasizing Equivocal Cases. Am. J. Clin. Pathol. 2016, 146, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layfield, L.J.; Wallander, M.L.; Tripp, S.R.; Redpath, S.; Banks, P.M. Comparison of Dual-ISH (DISH) With Fluorescence In Situ Hybridization (FISH) and Correlation With Immunohistochemical Findings for HER2/Neu Status in Breast Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Ban, H.; Ichikawa, H.; Sahara, S.; Otsuka, T.; Inatomi, O.; Bamba, S.; Furuta, T.; Andoh, A. Efficacy of the Kyoto classification of gastritis in identifying patients at high risk for gastric cancer. Intern. Med. 2017, 56, 579–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugge, M.; Fassan, M.; Pizzi, M.; Farinati, F.; Sturniolo, G.C.; Plebani, M.; Graham, D.Y. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J. Gastroenterol. 2011, 17, 4596–4601. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.-Y.; Zhang, J.-J.; Chen, X.-Y.; Ge, Z.-Z.; Li, X.-B. Operative link on gastritis assessment stage is an appropriate predictor of early gastric cancer. World J. Gastroenterol. 2016, 22, 3670–3678. [Google Scholar] [CrossRef]
- Rugge, M.; Genta, R.M. Staging and grading of chronic gastritis. Hum. Pathol. 2005, 36, 228–233. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B.; Wilson, K.T. Pathology of gastric intestinal metaplasia: Clinical implications. Am. J. Gastroenterol. 2010, 105, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Shichijo, S.; Hirata, Y.; Niikura, R.; Hayakawa, Y.; Yamada, A.; Ushiku, T.; Fukayama, M.; Koike, K. Histological intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest. Endosc. 2016, 84, 618–624. [Google Scholar] [CrossRef]
- Gonzalez, C.A.; Sanz-Anquela, J.M.; Companioni, O.; Bonet, C.; Berdasco, M.; Lopez, C.; Mendoza, J.; Martin-Arranz, M.D.; Rey, E.; Poves, E.; et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: Results of the spanish follow-up multicenter study. J. Gastroenterol. Hepatol. 2016, 31, 953–958. [Google Scholar] [CrossRef]
- Rugge, M.; Nitti, D.; Farinati, F.; di Mario, F.; Genta, R.M. Non-invasive neoplasia of the stomach. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuipers, E.J.; Spaander, M.C. Natural History of Barrett’s Esophagus. Dig. Dis. Sci. 2018, 63, 1997–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrift, A.P. Barrett’s Esophagus and Esophageal Adenocarcinoma: How Common Are They Really? Dig. Dis. Sci. 2018, 63, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Quante, M.; Leedham, S.; Jansen, M. The metaplastic mosaic of Barrett’s oesophagus. Virchows. Arch. 2018, 472, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voltaggio, L.; Montgomery, E.A.; Lam-Himlin, D. A clinical and histopathologic focus on Barrett esophagus and Barrett-related dysplasia. Arch. Pathol. Lab. Med. 2011, 135, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Grisanti, S.; Villanacci, V.; Della Casa, D.; Cengia, P.; Missale, G.; Minelli, L.; Buglione, M.; Cestari, R.; Bassotti, G. HER-2 overexpression/amplification in Barrett’s oesophagus predicts early transition from dysplasia to adenocarcinoma: A clinico-pathologic study. J. Cell Mol. Med. 2009, 13, 3826–3833. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.; Villanacci, V.; Bassotti, G.; Donato, F.; Festa, A.; Cengia, G.; Grisanti, S.; Cestari, R. TOPOIIalpha and HER-2/neu overexpression/amplification in Barrett’s oesophagus, dysplasia and adenocarcinoma. Histopathology 2010, 57, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Bandla, S.; Godfrey, T.E.; Tan, D.; Luketich, J.D.; Pennathur, A.; Qiu, X.; Hicks, D.G.; Peters, J.H.; Zhou, Z. HER2 amplification, overexpression and score criteria in esophageal adenocarcinoma. Mod. Pathol. 2011, 24, 899–907. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; de Boer, W.B.; Fermoyle, S.; Platten, M.; Kumarasinghe, M.P. Human epidermal growth factor receptor 2 testing in gastric carcinoma: Issues related to heterogeneity in biopsies and resections. Histopathology 2011, 59, 832–840. [Google Scholar] [CrossRef]
- Fassan, M.; Mastracci, L.; Grillo, F.; Zagonel, V.; Bruno, S.; Battaglia, G.; Pitto, F.; Nitti, D.; Celiento, T.; Zaninotto, G.; et al. Early HER2 dysregulation in gastric and oesophageal carcinogenesis. Histopathology 2012, 61, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Rocco, E.G.; Del Conte, C.; Pellegrini, C.; Bulfamante, G.; Di Nuovo, F.; Romagnoli, S.; Bosari, S. HER2 in gastric cancer: A digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod. Pathol. 2013, 26, 816–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, P.; Garrido, M.; Gullo, I.; Baldaia, H.; Marques, M.; Baldaque-Silva, F.; Lopes, J.; Carneiro, F. Epithelial dysplasia of the stomach with gastric immunophenotype shows features of biological aggressiveness. Gastric. Cancer. 2015, 18, 720–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ecker, B.L.; Taylor, L.; Zhang, P.J.; Furth, E.E.; Ginsberg, G.G.; McMillan, M.T.; Datta, J.; Czerniecki, B.J.; Roses, R.E. HER3 Expression Is a Marker of Tumor Progression in Premalignant Lesions of the Gastroesophageal Junction. PLoS ONE 2016, 11, e0161781. [Google Scholar] [CrossRef] [PubMed]
- Kawaura, Y.; Tatsuzawa, Y.; Wakabayashi, T.; Ikeda, N.; Matsuda, M.; Nishihara, S. Immunohistochemical study of p53, c-erbB-2, and PCNA in barrett’s esophagus with dysplasia and adenocarcinoma arising from experimental acid or alkaline reflux model. J. Gastroenterol. 2001, 36, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Rygiel, A.M.; Milano, F.; Ten Kate, F.J.; Bergman, J.J.; Krishnadath, K.K. Low Level of Her-2 Locus Amplification by Fluorescent In Situ Hybridization Does Not Correlate with Her-2 Protein Overexpression by Immunohistochemistry in Barrett’s Esophagus. J. Oncol. 2010, 2010, 382582. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Fujimura, A.; Ichimura, K.; Yanai, H.; Sato, Y.; Takata, K.; Okada, H.; Kawano, S.; Tanabe, S.; Yoshino, T. Clinicopathological characteristics of human epidermal growth factor receptor 2-positive Barrett’s adenocarcinoma. World J. Gastroenterol. 2012, 18, 6263–6268. [Google Scholar] [CrossRef]
- Gowryshankar, A.; Nagaraja, V.; Eslick, G.D. HER2 status in Barrett’s esophagus & esophageal cancer: A meta analysis. J. Gastrointest. Oncol. 2014, 5, 25–35. [Google Scholar]
- Realdon, S.; Dassie, E.; Fassan, M.; Dall’Olmo, L.; Hatem, G.; Buda, A.; Arcidiacono, D.; Diamantis, G.; Zhang, H.; Greene, M.I.; et al. In vivo molecular imaging of HER2 expression in a rat model of Barrett’s esophagus adenocarcinoma. Dis. Esophagus. 2015, 28, 394–403. [Google Scholar] [CrossRef]
- Yoon, H.H.; Shi, Q.; Sukov, W.R.; Wiktor, A.E.; Khan, M.; Sattler, C.A.; Grothey, A.; Wu, T.T.; Diasio, R.B.; Jenkins, R.B.; et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin. Cancer Res. 2012, 18, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Scott, G.K.; Goga, A.; Bhaumik, D.; Berger, C.E.; Sullivan, C.S.; Benz, C.C. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J. Biol. Chem. 2007, 282, 1479–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, N.; Mimori, K.; Fabbri, M.; Yokobori, T.; Sudo, T.; Tanaka, F.; Shibata, K.; Ishii, H.; Doki, Y.; Mori, M. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin. Cancer Res. 2011, 17, 2725–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassan, M.; Pizzi, M.; Realdon, S.; Balistreri, M.; Guzzardo, V.; Zagonel, V.; Castoro, C.; Mastracci, L.; Farinati, F.; Nitti, D.; et al. The HER2-miR125a5p/miR125b loop in gastric and esophageal carcinogenesis. Hum. Pathol. 2013, 44, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.R.; Buscaglia, B.; Koltz, B.R.; Henry, J.; McMahon, L.; Guo, J.; Hicks, D.G.; Whitney-Miller, C.L. Impact of Specimen Type and Specimen Number on HER2 Status in Gastroesophageal Junction and Gastric Adenocarcinoma. Am. J. Clin. Pathol. 2019, 151, 461–468. [Google Scholar] [CrossRef]
- Machlowska, J.; Maciejewski, R.; Sitarz, R. The Pattern of Signatures in Gastric Cancer Prognosis. Int. J. Mol. Sci. 2018, 19, 1658. [Google Scholar] [CrossRef] [Green Version]
- Wong, N.A.C.S.; Amary, F.; Butler, R.; Byers, R.; Gonzalez, D.; Haynes, H.R.; Ilyas, M.; Salto-Tellez, M.; Taniere, P. HER2 testing of gastro-oesophageal adenocarcinoma: A commentary and guidance document from the Association of Clinical Pathologists Molecular Pathology and Diagnostics Committee. J. Clin. Pathol. 2018, 71, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.Y.; Huang, J.Y.; Zhao, Q.R.; Jiang, N.; Xu, H.M.; Wang, Z.N.; Li, H.Q.; Zhang, S.B.; Sun, Z. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: A meta-analysis of literature. World J. Surg. Oncol. 2017, 15, 68. [Google Scholar] [CrossRef] [Green Version]
- Ieni, A.; Barresi, V.; Rigoli, L.; Caruso, R.A.; Tuccari, G. HER2 Status in Premalignant, Early, and Advanced Neoplastic Lesions of the Stomach. Dis. Markers 2015, 2015, 234851. [Google Scholar] [CrossRef] [Green Version]
- Abrahao-Machado, L.F.; Scapulatempo-Neto, C. HER2 testing in gastric cancer: An update. World J. Gastroenterol. 2016, 22, 4619–4625. [Google Scholar] [CrossRef]
- Vakiani, E. HER2 testing in gastric and gastroesophageal adenocarcinomas. Adv. Anat. Pathol. 2015, 22, 194–201. [Google Scholar] [CrossRef]
- Fujimoto, M.; Matsuzaki, I.; Nishino, M.; Iwahashi, Y.; Warigaya, K.; Kojima, F.; Ono, K.; Murata, S.I. HER2 is frequently overexpressed in hepatoid adenocarcinoma and gastric carcinoma with enteroblastic differentiation: A comparison of 35 cases to 334 gastric carcinomas of other histological types. J. Clin. Pathol. 2018, 71, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J. Advances in the management of HER2-positive advanced gastric and gastroesophageal junction cancer. J. Clin. Gastroenterol. 2012, 46, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.T. Role of human epidermal growth factor receptor 2 in gastric cancer: Biological and pharmacological aspects. World J. Gastroenterol. 2014, 20, 4526–4535. [Google Scholar] [CrossRef] [PubMed]
- Boku, N. HER2-positive gastric cancer. Gastric. Cancer 2014, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Laboissiere, R.S.; Buzelin, M.A.; Balabram, D.; De Brot, M.; Nunes, C.B.; Rocha, R.M.; Cabral, M.M.; Gobbi, H. Association between HER2 status in gastric cancer and clinicopathological features: A retrospective study using whole-tissue sections. Bmc Gastroenterol. 2015, 15, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.K.; Chen, Z.; Yun, T.; Li, C.Y.; Jiang, B.; Lv, X.X.; Chu, G.H.; Wang, S.N.; Yan, H.; Shi, L.F. Human epidermal growth factor receptor 2 expression in mixed gastric carcinoma. World J. Gastroenterol. 2015, 21, 4680–4687. [Google Scholar] [CrossRef]
- Koopman, T.; Louwen, M.; Hage, M.; Smits, M.M.; Imholz, A.L. Pathologic diagnostics of HER2 positivity in gastroesophageal adenocarcinoma. Am. J. Clin. Pathol. 2015, 143, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Hicks, D.G.; Whitney-Miller, C. HER2 testing in gastric and gastroesophageal junction cancers: A new therapeutic target and diagnostic challenge. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 506–508. [Google Scholar] [CrossRef]
- Hofmann, M.; Stoss, O.; Shi, D.; Büttner, R.; van de Vijver, M.; Kim, W.; Ochiai, A.; Rüschoff, J.; Henkel, T. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 2008, 52, 797–805. [Google Scholar] [CrossRef]
- Tajiri, R.; Ooi, A.; Fujimura, T.; Dobashi, Y.; Oyama, T.; Nakamura, R.; Ikeda, H. Intratumoral heterogeneous amplification of ERBB2 and subclonal genetic diversity in gastric cancers revealed by multiple ligation-dependent probe amplification and fluorescence in situ hybridization. Hum. Pathol. 2014, 45, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Treacy, A.D.; Karamchandani, J.R.; Streutker, C.J.; Grin, A. HER2 Genetic Heterogeneity in Gastric Cancer: Evaluation According to the College of American Pathologists Breast Cancer Criteria. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Bosari, S. HER2 aberrations and heterogeneity in cancers of the digestive system: Implications for pathologists and gastroenterologists. S. World J. Gastroenterol. 2016, 22, 7926–7937. [Google Scholar] [CrossRef]
- Grillo, F.; Fassan, M.; Sarocchi, F.; Fiocca, R.; Mastracci, L. HER2 heterogeneity in gastric/gastroesophageal cancers: From benchside to practice. World J. Gastroenterol. 2016, 22, 5879–5887. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, L.; Remotti, H.E.; Iuga, A.; Yang, H.M.; Lagana, S.M.; Sepulveda, A.R. HER2 Heterogeneity in Gastroesophageal Cancer Detected by Testing Biopsy and Resection Specimens. Arch. Pathol. Lab. Med. 2018, 142, 516–522. [Google Scholar] [CrossRef]
- Ieni, A.; Cardia, R.; Lentini, M.; Tuccari, G. Intratumoral HER2 heterogeneity in early gastric carcinomas: Potential bias in therapeutic management. Virchows. Arch. 2019, 474, 401–402. [Google Scholar] [CrossRef]
- Yagi, S.; Wakatsuki, T.; Yamamoto, N.; Chin, K.; Takahari, D.; Ogura, M.; Ichimura, T.; Nakayama, I.; Osumi, H.; Shinozaki, E.; et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric. Cancer 2019, 22, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Ieni, A.; Angelico, G.; Giuffrè, G.; Tuccari, G. Discordance Rate of HER2 Status in Primary Gastric Cancer and Synchronous Lymph Node Metastases: Its Impact on Therapeutic Decision and Clinical Management. Pathol. Oncol. Res. 2018, 24, 695–696. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Yamamoto, N.; Sano, T.; Chin, K.; Kawachi, H.; Takahari, D.; Ogura, M.; Ichimura, T.; Nakayama, I.; Osumi, H.; et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J. Gastroenterol. 2018, 53, 1186–1195. [Google Scholar] [CrossRef] [Green Version]
- Ieni, A.; Angelico, G.; Zeppa, P.; Tuccari, G. Letter to the Editor regarding the paper by Park et al., Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: Results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur. J. Cancer 2017, 75, 190–191. [Google Scholar] [PubMed]
- Yoon, S.H.; Kim, Y.H.; Lee, Y.J.; Park, J.; Kim, J.W.; Lee, H.S.; Kim, B. Tumor Heterogeneity in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Advanced Gastric Cancer Assessed by CT Texture Analysis: Association with Survival after Trastuzumab Treatment. PLoS ONE 2016, 11, e0161278. [Google Scholar] [CrossRef]
- Qiu, M.Z.; Shi, S.M.; Chen, M.; Wang, J.; Wu, Q.N.; Sheng, H.; Zhang, H.Z.; Yun, J.P.; Zhou, Z.W.; Wang, F.H.; et al. Comparison of HER2 and Lauren Classification between Biopsy and Surgical Resection Samples, Primary and Metastatic Samples of Gastric Cancer. J. Cancer 2017, 8, 3531–3537. [Google Scholar] [CrossRef] [Green Version]
- Creemers, A.; Ter Veer, E.; de Waal, L.; Lodder, P.; Hooijer, G.K.J.; van Grieken, N.C.T.; Bijlsma, M.F.; Meijer, S.L.; van Oijen, M.G.H.; van Laarhoven, H.W.M. Discordance in HER2 Status in Gastro-esophageal Adenocarcinomas: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 3135. [Google Scholar] [CrossRef] [PubMed]
- Hedner, C.; Tran, L.; Borg, D.; Nodin, B.; Jirström, K.; Eberhard, J. Discordant human epidermal growth factor receptor 2 overexpression in primary and metastatic upper gastrointestinal adenocarcinoma signifies poor prognosis. Histopathology 2016, 68, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Ieni, A.; Barresi, V.; Caltabiano, R.; Caleo, A.; Bonetti, L.R.; Lanzafame, S.; Zeppa, P.; Caruso, R.A.; Tuccari, G. Discordance rate of HER2 status in primary gastric carcinomas and synchronous lymph node metastases: A multicenter retrospective analysis. Int. J. Mol. Sci. 2014, 15, 22331–22341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochi, M.; Fujii, M.; Masuda, S.; Kanamori, N.; Mihara, Y.; Funada, T.; Tamegai, H.; Watanabe, M.; Suda, H.; Takayama, T. Differing deregulation of HER2 in primary gastric cancer and synchronous related metastatic lymph nodes. Diagn. Pathol. 2013, 8, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassan, M.; Ludwig, K.; Pizzi, M.; Castoro, C.; Guzzardo, V.; Balistreri, M.; Zaninotto, G.; Ruol, A.; Giacomelli, L.; Ancona, E.; et al. Human epithelial growth factor receptor 2 (HER2) status in primary and metastatic esophagogastric junction adenocarcinomas. Hum. Pathol. 2012, 43, 1206–1212. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, H.J.; Yang, H.K.; Bang, Y.J.; Kim, W.H. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology 2011, 59, 822–831. [Google Scholar] [CrossRef] [Green Version]
- Bozzetti, C.; Negri, F.V.; Lagrasta, C.A.; Crafa, P.; Bassano, C.; Tamagnini, I.; Gardini, G.; Nizzoli, R.; Leonardi, F.; Gasparro, D.; et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br. J. Cancer 2011, 104, 1372–1376. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, B.; Liu, Z.; Gong, J.; Shao, L.; Ren, J.; Niu, Y.; Bo, S.; Li, Z.; Lai, Y.; et al. HER2 Copy Number of Circulating Tumour DNA Functions as a Biomarker to Predict and Monitor Trastuzumab Efficacy in Advanced Gastric Cancer. Eur. J. Cancer 2018, 88, 92–100. [Google Scholar] [CrossRef]
- Diaz, L.A.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Shoda, K.; Masuda, K.; Ichikawa, D.; Arita, T.; Miyakami, Y.; Watanabe, M.; Konishi, H.; Imoto, I.; Otsuji, E. HER2 amplification detected in the circulating DNA of patients with gastric cancer: A retrospective pilot study. Gastric. Cancer 2015, 18, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H. Is “liquid biopsy” useful for assessing HER2 status in gastric cancer? J. Gastroenterol. 2015, 50, 119–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishima, Y.; Matsusaka, S.; Chin, K.; Mikuniya, M.; Minowa, S.; Takayama, T.; Shibata, H.; Kuniyoshi, R.; Ogura, M.; Terui, Y. Detection of HER2 Amplification in Circulating Tumor Cells of HER2-Negative Gastric Cancer Patients. Target. Oncol. 2017, 12, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Shoda, K.; Ichikawa, D.; Fujita, Y.; Masuda, K.; Hiramoto, H.; Hamada, J.; Arita, T.; Konishi, H.; Komatsu, S.; Shiozaki, A. Monitoring the HER2 copy number status in circulating tumor DNA by droplet digital PCR in patients with gastric cancer. Gastric. Cancer 2017, 20, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Uguen, A. About HER2 monitoring using liquid biopsies in patients with gastric cancer. Gastric. Cancer 2017, 20, 1011–1012. [Google Scholar] [CrossRef]
- Shen, L. Liquid biopsy: A powerful tool to monitor trastuzumab resistance in HER2-positive metastatic gastric cancer. Cancer Commun. 2018, 38, 72. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Franovic, A.; Shiotsu, Y.; Kim, S.T.; Kim, K.M.; Banks, K.C.; Raymond, V.M.; Lanman, R.B. Detection of ERBB2 (HER2) Gene Amplification Events in Cell-Free DNA and Response to Anti-HER2 Agents in a Large Asian Cancer Patient Cohort. Front. Oncol. 2019, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Iwatsuki, M.; Toyoshima, K.; Watanabe, M.; Hayashi, N.; Ishimoto, T.; Eto, K.; Iwagami, S.; Baba, Y.; Yoshida, N.; Hayashi, A.; et al. Frequency of HER2 expression of circulating tumour cells in patients with metastatic or recurrent gastrointestinal cancer. Br. J. Cancer 2013, 109, 2829–2832. [Google Scholar] [CrossRef]
- Wang, D.S.; Liu, Z.X.; Lu, Y.X.; Bao, H.; Wu, X.; Zeng, Z.L.; Liu, Z.; Zhao, Q.; He, C.Y.; Lu, J.H.; et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 2018, 68, 1152–1161. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Fucà, G.; Morano, F.; Gloghini, A.; Corso, S.; Aprile, G.; Perrone, F.; De Vita, F.; Tamborini, E.; Tomasello, G.; et al. Biomarkers of Primary Resistance to Trastuzumab in HER2-Positive Metastatic Gastric Cancer Patients: The AMNESIA Case-Control Study. Clin. Cancer Res. 2018, 24, 1082–1089. [Google Scholar] [CrossRef] [Green Version]
- Pietrantonio, F.; Caporale, M.; Morano, F.; Scartozzi, M.; Gloghini, A.; De Vita, F.; Giommoni, E.; Fornaro, L.; Aprile, G.; Melisi, D.; et al. HER2 Loss in HER2-positive Gastric or Gastroesophageal Cancer After Trastuzumab Therapy: Implication for Further Clinical Research. Int. J. Cancer 2016, 139, 2859–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Samuel, J.; Klempner, S.J.; Chao, J. Progress and Challenges in HER2-positive Gastroesophageal Adenocarcinoma. J. Hematol. Oncol. 2019, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, F.; Naseem, M.; Puccini, A.; Lenz, H.J. Molecular biomarkers in gastro-esophageal cancer: Recent developments, current trends and future directions. Cancer Cell Int. 2018, 18, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Ahn, S.; Son, D.S.; Kim, N.K.; Lee, K.W.; Kim, S.; Lee, J.; Park, S.H.; Park, J.O.; Kang, W.K.; et al. Bridging genomics and phenomics of gastric carcinoma. Int. J. Cancer 2019, 145, 2407–2417. [Google Scholar] [CrossRef]
- Kim, C.; Lee, C.K.; Chon, H.J.; Kim, J.H.; Park, H.S.; Heo, S.J.; Kim, H.J.; Kim, T.S.; Kwon, W.S.; Chung, H.C.; et al. PTEN loss and level of HER2 amplification is associated with trastuzumab resistance and prognosis in HER2-positive gastric cancer. Oncotarget 2017, 8, 113494–113501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janjigian, Y.Y.; Sanchez-Vega, F.; Jonsson, P.; Chatila, W.K.; Hechtman, J.F.; Ku, G.Y.; Riches, J.C.; Tuvy, Y.; Kundra, R.; Bouvier, N.; et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018, 8, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Pectasides, E.; Stachler, M.D.; Derks, S.; Liu, Y.; Maron, S.; Islam, M.; Alpert, L.; Kwak, H.; Kindler, H.; Polite, B.; et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 2018, 8, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, H.; Zang, W.; Li, B.; Rao, G.; Li, L.; Yu, Y.; Li, Z.; Dong, B.; Lu, Z.; et al. Circulating tumor DNA functions as an alternative for tissue to overcome tumor heterogeneity in advanced gastric cancer. Cancer Sci. 2017, 108, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Kankeu Fonkoua, L.; Yee, N.S. Molecular Characterization of Gastric Carcinoma: Therapeutic Implications for Biomarkers and Targets. Biomedicines 2018, 6, 32. [Google Scholar] [CrossRef] [Green Version]
| HER2 Score | HER2 IHC in Surgical Specimens | HER2 IHC in Biopsy Specimens | HER2 Assessment |
|---|---|---|---|
| 0 | No membranous reactivity in <10% of neoplastic elements | No membranous reactivity in any cancer cell | Negative |
| 1+ | Faint or barely membranous immunoreactivity in ≥10% of neoplastic elements | Neoplastic cluster (≥5 neoplastic cells) characterized by a faint or barely membranous immunoreactivity, irrespective of percentage of stained cells | Negative |
| 2+ | Weak to moderate complete, basolateral or lateral membranous immunoreactivity in ≥10% of neoplastic elements | Neoplastic cluster (≥5 neoplastic elements) characterized by a weak to moderate complete, basolateral or lateral membranous immunoreactivity, irrespective of percentage of stained cells | Equivocal |
| 3+ | Strong complete, basolateral or lateral membranous immunoreactivity in ≥10% of neoplastic elements | Neoplastic cluster (≥5 neoplastic elements) characterized by a strong complete, basolateral or lateral membranous immunoreactivity, irrespective of percentage of stained cells | Positive |
| Negative for Intraepithelial Neoplasia/Dysplasia |
|---|
| Indefinite for intraepithelial neoplasia/dysplasia (Reactive/regenerative aspect of chronic atrophic gastritis and intestinal metaplasia) |
| Low-grade intraepithelial neoplasia/dysplasia/LG-IEN (Minimal architectural disarray and only mild-to-moderate cytological atypia) |
| High-grade intraepithelial neoplasia/dysplasia/HG-IEN (Prominent architectural disarray, mitoses, high nucleus/cytoplasm ratio and nucleoli) |
| Intramucosal invasive neoplasia/intramucosal carcinoma (Marked glandular crowding, excessive branching and fused/cribriform glands). |
| Negative for dysplasia Preserved surface maturation, lack stratification and cytologic atypia is incomplete to the basal part of glands. |
| Indefinite for dysplasia (ID) Modifications in deeper glands indicative but not diagnostic dysplasia with surface maturation and occasional cytologic atypia. |
| Low-grade dysplasia (LGD) Loss of surface maturation and architectural alteration with glandular crowding and mild cytologic atypia. |
| High-grade dysplasia (HGD) Loss of surface maturation and glandular crowding with hyperchromatic nuclei, mitoses and marked cytologic atypia. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ieni, A.; Cardia, R.; Pizzimenti, C.; Zeppa, P.; Tuccari, G. HER2 Heterogeneity in Personalized Therapy of Gastro-Oesophageal Malignancies: An Overview by Different Methodologies. J. Pers. Med. 2020, 10, 10. https://doi.org/10.3390/jpm10010010
Ieni A, Cardia R, Pizzimenti C, Zeppa P, Tuccari G. HER2 Heterogeneity in Personalized Therapy of Gastro-Oesophageal Malignancies: An Overview by Different Methodologies. Journal of Personalized Medicine. 2020; 10(1):10. https://doi.org/10.3390/jpm10010010
Chicago/Turabian StyleIeni, Antonio, Roberta Cardia, Cristina Pizzimenti, Pio Zeppa, and Giovanni Tuccari. 2020. "HER2 Heterogeneity in Personalized Therapy of Gastro-Oesophageal Malignancies: An Overview by Different Methodologies" Journal of Personalized Medicine 10, no. 1: 10. https://doi.org/10.3390/jpm10010010
APA StyleIeni, A., Cardia, R., Pizzimenti, C., Zeppa, P., & Tuccari, G. (2020). HER2 Heterogeneity in Personalized Therapy of Gastro-Oesophageal Malignancies: An Overview by Different Methodologies. Journal of Personalized Medicine, 10(1), 10. https://doi.org/10.3390/jpm10010010






