Comparison of a New Multiplex Immunoassay for Measurement of Ferritin, Soluble Transferrin Receptor, Retinol-Binding Protein, C-Reactive Protein and α1-Acid-glycoprotein Concentrations against a Widely-Used s-ELISA Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the ELISA Methods
2.2. Description of the Gold Standard Malaria Test
2.3. Studied Population
2.4. Blood Collection Procedures
2.5. Data and Statistical Analysis
3. Results
3.1. Quality Control
3.2. Characterisitcs of the Women and Children Included in the Analysis
3.3. Trend Estimates for Each Analyte for Each Population Group and the Pooled Population
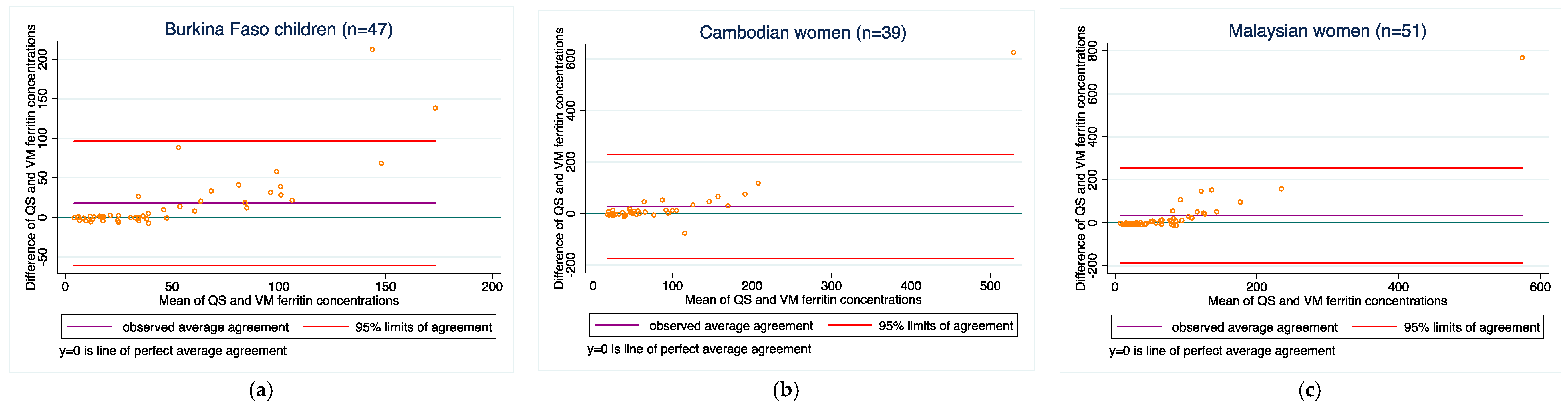
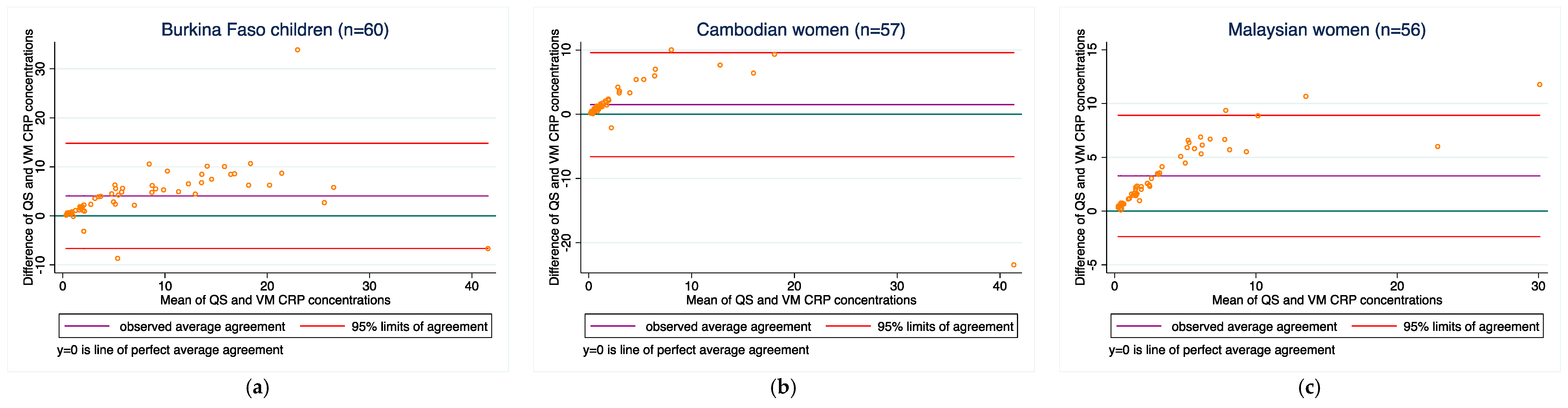
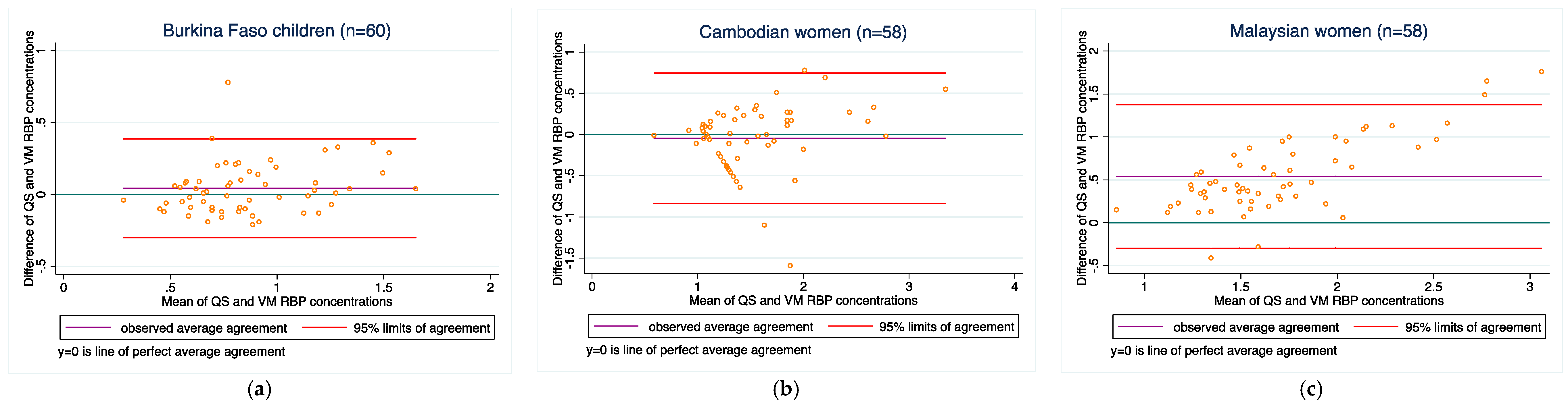
3.4. Method-Comparisons between the Two Methods for Each Analyte
3.5. Malaria Testing
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nutr. 2004, 134, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Supplement—Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA). Am. J. Clin. Nutr. 2017, 106, 327S–330S. Available online: https://academic.oup.com/ajcn/issue/106/suppl_1 (accessed on 1 January 2018).
- Erhardt, J.G.; VitMin Laboratory, Willstätt, Germany. Personal communication, 2017.
- Brindle, E.; Stevens, D.; Crudder, C.; Levin, C.E.; Garrett, D.; Lyman, C.; Boyle, D.S. A multiplex immunoassay method for simultaneous quantification of iron, vitamin a and inflammation status markers. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Moody, A. Rapid Diagnostic Tests for Malaria Parasites. Clin. Microbiol. Rev. 2002, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. Assessing the Iron Status of Populations, 2nd ed.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of nutrition for development (BOND)—Vitamin A review. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 8, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Karakochuk, C.D.; Whitfield, K.C.; Rappaport, A.I.; Barr, S.I.; Vercauteren, S.M.; McLean, J.; Hou, K.; Talukder, A.; Houghton, L.A.; Bailey, K.B.; et al. Comparison of four immunoassays to measure serum ferritin concentrations and iron deficiency prevalence among non-pregnant Cambodian women and Congolese children. Clin. Chem. Lab. Med. 2017, 55, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Brindle, E.; Lillis, L.; Barney, R.; Hess, S.Y.; Wessells, K.R.; Ouédraogo, C.T.; Stinca, S.; Kalnoky, M.; Peck, R.; Tyler, A.; et al. Simultaneous assessment of iodine, iron, vitamin A, malarial antigenemia, and inflammation status biomarkers via a multiplex immunoassay method on a population of pregnant women from Niger. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Control and Prevention. Laboratory Procedure Manual: Ferritin; Centre for Disease Control and Prevention: Atlanta, GA, USA, 2007. [Google Scholar]


| Country | Population Group | Total n Available 1 | Total n Analysed 2 |
|---|---|---|---|
| Burkina Faso | Children aged 21–24 month | 2100 | 60 |
| Cambodia | Non-pregnant women aged 18–45 year | 809 | 60 |
| Malaysia | Non-pregnant women aged 18–45 year | 200 | 60 |
| Analyte | ULOQ 1 | LLOQ 2 | LOD 3 | Lowest Value (<) | Total n of Low Values | Highest Value (>) | Total n of High Values | |
|---|---|---|---|---|---|---|---|---|
| Assay 1 | Ferritin | 110.16 | 1.39 | 0.067 | <13.94 | 11/39 | - | - |
| n = 40 | STfR | 118.49 | 0.17 | 0.065 | - | - | - | - |
| AGP | 0.34 | 0.00059 | 0.00061 | - | - | - | - | |
| CRP | 17.87 | 0.029 | 0.0084 | <0.29 | 3/40 | - | - | |
| RBP | 0.39 | 0.0016 | 0.00053 | - | - | >3.9 | 2/40 | |
| HRP2 | 1.03 | 0.0015 | 0.00026 | <0.015 | 40/40 | - | - | |
| Assay 2 | Ferritin | 112.43 | 0.38 | 0.18 | <3.84 | 3/40 | - | - |
| n = 40 | STfR | 118.27 | 0.17 | 0.001 | <1.70 | 2/40 | - | - |
| AGP | 0.36 | 0.00053 | 0.00021 | - | - | - | - | |
| CRP | 6.97 | 0.029 | 0.0065 | <0.29 | 2/40 | - | - | |
| RBP | 0.39 | 0.0015 | 0.00027 | - | - | >3.86 | 2/40 | |
| HRP2 | 1.03 | 0.004 | 0.00092 | <0.040 | 40/40 | - | - | |
| Assay 3 | Ferritin | 111.94 | 1.52 | 0.081 | <15.16 | 14/40 | - | - |
| n = 40 | STfR | 116.62 | 0.16 | 0.84 | - | - | - | - |
| AGP | 0.37 | 0.00059 | 0.0004 | - | - | - | - | |
| CRP | 19.86 | 0.03 | 0.001 | - | - | - | - | |
| RBP | 1.06 | 0.0043 | 0.001 | - | - | - | - | |
| HRP2 | 1.05 | 0.0014 | 0.0056 | <0.014 | 29/40 | >10.45 | 8/40 | |
| Assay 4 | Ferritin | 112.31 | 1.43 | 0.065 | <14.31 | 9/20 | - | - |
| n = 20 | STfR | 115.36 | 0.16 | 0.026 | - | - | - | - |
| AGP | 0.35 | 0.0015 | 0.00056 | - | - | - | - | |
| CRP | 18.45 | 0.26 | 0.0018 | <0.26 | - | - | - | |
| RBP | 0.11 | 0.0016 | 0.0016 | - | - | >1.08 | 18/20 | |
| HRP2 | 1.02 | 0.0015 | 0.00065 | <0.015 | 16/20 | - | - | |
| Assay 5 | Ferritin | 113.16 | 0.4 | 0.24 | <3.97 | 5/40 | - | - |
| n = 40 | STfR | 117.21 | 1.36 | 0.008 | - | - | - | - |
| AGP | 0.36 | 0.0014 | 0.00081 | - | - | - | - | |
| CRP | 18.74 | 0.027 | 0.01 | - | - | - | - | |
| RBP | 0.38 | 0.0042 | 0.002 | - | - | - | - | |
| HRP2 | 1.05 | 0.0045 | 0.000034 | <0.045 | 26/40 | >10.51 | 6/40 |
| Burkina Faso Children | Cambodian Women | Malaysian Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| s-ELISA n = 60 | Quansys_EXCL 2 n = 47–60 | Quansys_INCL 3 n = 60 | s-ELISA n = 60 | Quansys_EXCL 2 n = 39–60 | Quansys_INCL 3 n = 59–60 | s-ELISA n = 60 | Quansys_EXCL 2 n = 51–60 | Quansys_INCL 3 n = 60 | |
| Ferritin, μg/L | 20.5 (8.9, 44.8) | 34.6 (16.9, 91.0) | 22.5 (14.5, 62.6) | 29.3 (16.9, 59.8) | 54.9 (31.0, 105.7) | 31.0 (14.3, 73.5) | 47.1 (23.2, 81.9) | 62.6 (29.7, 118.6) | 54.6 (17.8, 96.0) |
| <12 or <15 μg/L 4, n (%) | 19/60 (32%) | 8/47 (17%) | 13/60 (22%) | 14/60 (23%) | 0/39 (0%) | 0/59 (0%) | 11/60 (18%) | 3/51 (6%) | 6/60 (10%) |
| STfR, mg/L | 10.4 (8.3, 15.8) | 30.7 (22.8, 49.5) | 30.7 (22.8, 49.5) | 6.6 (4.8, 9.6) | 8.5 (6.6, 14.6) | 8.5 (6.6, 14.6) | 5.2 (4.2, 6.0) | 4.7 (3.6, 5.8) | 4.7 (3.6, 5.8) |
| >8.3 mg/L, n (%) | 45/60 (75%) | 60/60 (100%) | 60/60 (100%) | 21/60 (35%) | 30/60 (50%) | 30/60 (50%) | 5/60 (8%) | 10/58 (17%) | 10/60 (10%) |
| AGP, g/L | 1.25 (0.86, 1.82) | 1.25 (0.97, 1.56) | 1.25 (0.97, 1.56) | 0.57 (0.44, 0.76) | 0.58 (0.50, 0.69) | 0.58 (0.50, 0.69) | 0.57 (0.49, 0.72) | 0.81 (0.65, 0.99) | 0.81 (0.65, 0.99) |
| >1 g/L, n (%) | 39/60 (65%) | 40/60 (67%) | 40/60 (67%) | 9/60 (15%) | 6/60 (10%) | 6/60 (10%) | 2/60 (3%) | 15/60 (25%) | 15/60 (25%) |
| CRP, mg/L | 3.2 (0.9, 9.2) | 7.3 (2.0, 16.3) | 7.3 (2.0, 16.3) | 0.4 (0.2, 0.8) | 1.4 (0.9, 3.0) | 1.4 (0.7, 2.8) | 0.78 (0.35, 2.16) | 3.0 (1.3, 8.5) | 2.7 (0.9, 8.3) |
| >5 g/L, n (%) | 25/60 (42%) | 35/60 (58%) | 35/60 (58%) | 4/60 (7%) | 10/57 (18%) | 10/60 (17%) | 6/60 (10%) | 19/56 (34%) | 19/60 (32%) |
| RBP, μmol/L, mean ± SD | 0.83 ± 0.29 | 0.88 ± 0.33 | 0.88± 0.33 | 1.62 ± 0.62 | 1.51 ± 0.59 | 1.59 ± 0.72 | 1.44 ± 0.32 | 1.97 ± 0.63 | 2.03 ± 0.71 |
| <0.7, μmol/L, n (%) | 12/60 (20%) | 23/60 (38%) | 23/60 (38%) | 1/60 (2%) | 1/58 (2%) | 1/60 (2%) | 0/60 (0%) | 0/58 (0%) | 0/60 (0%) |
| Burkina Faso | Cambodia | Malaysia | Pooled 1 | |
|---|---|---|---|---|
| Ferritin_EXCL 2 | 1.58x − 4.66 | 2.26x − 57.54 | 2.84x − 79.32 | 2.25x − 42.79 |
| Ferritin_INCL 3 | 1.55x − 2.78 | 2.00x − 30.93 | 2.56x − 54.31 | 2.08x − 27.59 |
| STfR 2 | 1.75x + 17.19 | 2.07x + 4.55 | 1.62x − 3.57 | 2.48x − 3.04 |
| RBP 2 | 0.97x + 0.07 | 0.96x − 0.02 | 1.55x − 0.25 | 1.12x + 0.04 |
| CRP 2 | 1.05x + 3.76 | 0.66x + 2.13 | 1.47x + 2.22 | 0.99x + 2.88 |
| AGP 2 | 0.54x + 0.56 | 0.56x + 0.26 | 0.86x + 0.30 | 0.64x + 0.36 |
| Participants | All Samples 1 | Only Samples Within LOD Range 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total, All | Bias | Limits of Agreement | Pearson’s Coefficient | Concordance (95% CI) | Within Range 1 | Bias | Limits of Agreement | Pearson’s Coefficient | Concordance (95% CI) | |
| n | mean ± SD | ±1.96 SD | r | ρc (±1.96 SD) | n | mean ± SD | ±1.96 SD | r | ρc (±1.96 SD) | |
| Ferritin, μg/L | ||||||||||
| Burkina Faso children | 60 | 14.7 ± 36.0 | −55.8, 85.3 | 0.81 | 0.62 (0.51, 0.71) | 47 | 17.8 ± 40.1 | −60.7, 96.3 | 0.78 | 0.57 (0.43, 0.68) |
| Cambodian women 3 | 59 | 18.3 ± 84.4 | −147.1, 183.7 | 0.81 | 0.55 (0.45, 0.63) | 39 | 27.1 ± 103.0 | −174.8, 229.0 | 0.80 | 0.48 (0.36, 0.59) |
| Malaysian women | 60 | 29.3 ± 104.6 | −175.8, 234.3 | 0.76 | 0.40 (0.31, 0.48) | 51 | 34.1 ± 112.9 | −187.3, 255.4 | 0.76 | 0.36 (0.27, 0.45) |
| STfR, mg/L | ||||||||||
| Burkina Faso children | 60 | 26.5 ± 21.8 | −16.1, 69.1 | 0.42 | 0.09 (0.03, 0.14) | 60 | 26.5 ± 21.8 | −16.1, 69.1 | 0.42 | 0.09 (0.03, 0.14) |
| Cambodian women | 60 | 5.2 ± 10.4 | −15.2, 25.6 | 0.91 | 0.62 (0.54, 0.69) | 60 | 5.2 ± 10.4 | −15.2, 25.6 | 0.91 | 0.62 (0.54, 0.69) |
| Malaysian women | 60 | 0.1 ± 3.3 | −6.3, 6.5 | 0.92 | 0.78 (0.72, 0.83) | 58 | 0.2 ± 3.3 | −6.3, 6.6 | 0.91 | 0.78 (0.72, 0.83) |
| AGP, g/L | ||||||||||
| Burkina Faso children | 60 | −0.1 ± 0.4 | −0.9, 0.8 | 0.67 | 0.65 (0.49, 0.77) | 60 | −0.1 ± 0.4 | −0.9, 0.8 | 0.67 | 0.65 (0.49, 0.77) |
| Cambodian women | 60 | −0.04 ± 0.2 | −0.5, 0.4 | 0.94 | 0.82 (0.77, 0.86) | 60 | −0.04 ± 0.2 | −0.5, 0.4 | 0.94 | 0.82 (0.77, 0.86) |
| Malaysian women | 60 | 0.2 ± 0.2 | −0.1, 0.6 | 0.66 | 0.41 (0.27, 0.54) | 60 | 0.2 ± 0.2 | −0.1, 0.6 | 0.66 | 0.41 (0.27, 0.54) |
| CRP, mg/L | ||||||||||
| Burkina Faso children | 60 | 4.1 ± 5.5 | −6.7, 14.8 | 0.83 | 0.73 (0.61, 0.82) | 60 | 4.1 ± 5.5 | −6.7, 14.8 | 0.83 | 0.73 (0.61, 0.82) |
| Cambodian women | 60 | 1.4 ± 4.0 | −6.5, 9.3 | 0.83 | 0.79 (0.68, 0.86) | 57 | 1.5 ± 4.1 | −6.6, 9.6 | 0.83 | 0.79 (0.68, 0.86) |
| Malaysian women | 60 | 3.1 ± 2.9 | −2.6, 8.7 | 0.95 | 0.74 (0.65, 0.81) | 56 | 3.3 ± 2.9 | −2.4, 8.9 | 0.95 | 0.73 (0.64, 0.80) |
| RBP, μmol/L | ||||||||||
| Burkina Faso children | 60 | 0.04 ± 0.2 | −0.3, 0.4 | 0.85 | 0.83 (0.74, 0.90) | 60 | 0.04 ± 0.2 | −0.3, 0.4 | 0.85 | 0.83 (0.74, 0.90) |
| Cambodian women | 60 | −0.04 ± 0.4 | −0.8, 0.8 | 0.83 | 0.82 (0.72, 0.89) | 58 | −0.05 ± 0.4 | −0.8, 0.7 | 0.74 | 0.72 (0.58, 0.82) |
| Malaysian women | 60 | 0.6 ± 0.5 | −0.3, 1.6 | 0.79 | 0.38 (0.27, 0.48) | 58 | 0.5 ± 0.4 | −0.3, 1.4 | 0.79 | 0.40 (0.28, 0.50) |
| Malaria Diagnosis Confirmed by the Rapid Diagnostic Test (Reference) | ||||
|---|---|---|---|---|
| Quansys HRP2 | Yes—Malaria | No—Malaria | Total | |
| Positive (HRP2 > 1) | True-positive 18/25 (72%) | False-positive 7/35 (20%) | 25 | |
| Negative (HRP2 < 1) | False-negative 7/25 (28%) | True-negative 28/35 (80%) | 35 | |
| Total | 25 | 35 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakochuk, C.D.; Henderson, A.M.; Samson, K.L.I.; Aljaadi, A.M.; Devlin, A.M.; Becquey, E.; Wirth, J.P.; Rohner, F. Comparison of a New Multiplex Immunoassay for Measurement of Ferritin, Soluble Transferrin Receptor, Retinol-Binding Protein, C-Reactive Protein and α1-Acid-glycoprotein Concentrations against a Widely-Used s-ELISA Method. Diagnostics 2018, 8, 13. https://doi.org/10.3390/diagnostics8010013
Karakochuk CD, Henderson AM, Samson KLI, Aljaadi AM, Devlin AM, Becquey E, Wirth JP, Rohner F. Comparison of a New Multiplex Immunoassay for Measurement of Ferritin, Soluble Transferrin Receptor, Retinol-Binding Protein, C-Reactive Protein and α1-Acid-glycoprotein Concentrations against a Widely-Used s-ELISA Method. Diagnostics. 2018; 8(1):13. https://doi.org/10.3390/diagnostics8010013
Chicago/Turabian StyleKarakochuk, Crystal D., Amanda M. Henderson, Kaitlyn L. I. Samson, Abeer M. Aljaadi, Angela M. Devlin, Elodie Becquey, James P. Wirth, and Fabian Rohner. 2018. "Comparison of a New Multiplex Immunoassay for Measurement of Ferritin, Soluble Transferrin Receptor, Retinol-Binding Protein, C-Reactive Protein and α1-Acid-glycoprotein Concentrations against a Widely-Used s-ELISA Method" Diagnostics 8, no. 1: 13. https://doi.org/10.3390/diagnostics8010013
APA StyleKarakochuk, C. D., Henderson, A. M., Samson, K. L. I., Aljaadi, A. M., Devlin, A. M., Becquey, E., Wirth, J. P., & Rohner, F. (2018). Comparison of a New Multiplex Immunoassay for Measurement of Ferritin, Soluble Transferrin Receptor, Retinol-Binding Protein, C-Reactive Protein and α1-Acid-glycoprotein Concentrations against a Widely-Used s-ELISA Method. Diagnostics, 8(1), 13. https://doi.org/10.3390/diagnostics8010013





