Ovarian Cancer Incidence Corrected for Oophorectomy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cancer Incidence Data
2.2. Kentucky Health Claims Data (KHCD)
2.3. Kentucky Behavioral Risk Factor Surveillance System (BRFSS) Data
2.4. Estimating Oophorectomy Prevalence
2.5. Age-Adjusted Incidence Rates for Ovary Cancer
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ries, L.A.G.; Kosary, C.L.; Hankey, B.F.; Miller, B.A.; Edwards, B.K. (Eds.) SEER Cancer Statistics Review 1973–1996. National Cancer Institute, 1999. Available online: https://seer.cancer.gov/archive/csr/1973_1996/overview.pdf (accessed on 21 March 2017).
- Merrill, R.M.; Feuer, E.J. Risk-adjusted cancer incidence rates (United States). Cancer Causes Control 1996, 7, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, L.S.; Koonin, L.M.; Pokras, R.; Strauss, L.T.; Xia, Z. Hysterectomy in the United States, 1988–1990. Obstet. Gynecol. 1994, 83, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lepine, L.A.; Hillis, S.D.; Marchbanks, P.A.; Koonin, L.M.; Morrow, B.; Kieke, B.A.; Wilcox, L.S. Hysterectomy Surveillance—United States, 1980–1993. MMWR CDC Surveill. Summ. 1997, 46, 1–15. [Google Scholar] [PubMed]
- Siegel, R.L.; Devesa, S.S.; Cokkinides, V.; Ma, J.; Jemal, A. State-level Uterine Corpus Cancer Incidence Rates Corrected for Hysterectomy Prevalence, 2004 to 2008. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Cabrero, I.; Navani, S.S.; Young, R.H.; Scully, R.E. Tumors of the fimbriated end of the fallopian tube: A clinicopathologic analysis of 20 cases, including nine carcinomas. Int. J. Gynecol. Pathol. 1997, 16, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Colgan, T.J.; Murphy, J.; Cole, D.E.; Narod, S.; Rosen, B. Occult carcinoma in prophylactic oophorectomy specimens: Prevalence and association with BRCA germline mutation status. Am. J. Surg. Pathol. 2001, 25, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Cass, I.; Holschneider, C.; Datta, N.; Barbuto, D.; Walts, A.E.; Karlan, B.Y. BRCA-mutation-associated fallopian tube carcinoma: A distinct clinical phenotype? Obstet. Gynecol. 2005, 106, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.; Muto, M.G.; Lee, Y.; Elvin, J.A.; Callahan, M.J.; Feltmate, C.; Garber, J.E.; Cramer, D.W.; Crum, C.P. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol. 2006, 30, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am. J. Surg. Pathol. 2007, 31, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Crum, C.R.; Drapkin, R.; Miron, A.; Ince, T.A.; Muto, M.; Kindelberger, D.W.; Lee, Y. The distal fallopian tube: A new model for pelvic serous carcinogenesis. Curr. Opin. Obstet. Gynecol. 2007, 19, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Landen, C.N.; Birrer, M.J.; Sood, A.K. Early events in the pathogenesis of epithelial ovarian cancer. J. Clin. Oncol. 2008, 26, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, L.-M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Crum, C.P.; Mckeon, F.D.; Xian, X. The oviduct and ovarian cancer: Causality, clinical implications, and “targeted prevention”. Clin. Obstet. Gynecol. 2012, 55, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Friebel, T.M.; Garber, J.E.; Isaacs, C.; Matloff, E.; Eeles, R.; Evans, D.G.; Rubinstein, W.; Singer, C.F.; Rubin, S.; et al. Occult ovarian cancers identified at risk-reducing salpingo-oophorectomy in a prospective cohort of BRCA1/2 mutation carriers. Breast Cancer Res. Treat. 2010, 124, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.E.; Piedmonte, M.; Mai, P.L.; Ioffe, O.B.; Ronnett, B.M.; van Le, L.; Ivanov, I.; Bell, M.C.; Blank, S.V.; DiSilvestro, P.; et al. Pathologic findings at risk-reducing salpingo-oophorectomy: Primary results from Gynecologic Oncology Group Trial GOG-0199. J. Clin. Oncol. 2014, 32, 3275–3283. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Lynch, H.T.; Neuhausen, S.L.; Narod, S.A.; Van’t Veer, L.; Garber, J.E.; Evans, G.; Isaacs, C.; Daly, M.B.; Matloff, E.; et al. Prevention and Observation of Surgical End Points Study Group. N. Engl. J. Med. 2002, 346, 1616. [Google Scholar] [CrossRef] [PubMed]
- Kauff, N.D.; Satagopan, J.M.; Robson, M.E.; Scheuer, L.; Hensley, M.; Hudis, C.A.; Ellis, N.A.; Boyd, J.; Borgen, P.I.; Barakat, R.R.; et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N. Engl. J. Med. 2002, 346, 1609. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Ovary: SEER Stat Fact Sheets. Available online: http://seer.cancer.gov/statfacts/html/ovary.html (accessed on 27 December 2016).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Capocaccia, R.; de Angelis, R. Estimating the completeness of prevalence based on cancer registry data. Stat. Med. 1997, 16, 425–440. [Google Scholar] [CrossRef]
- Gail, M.H.; Kessler, L.; Midthune, D.; Scoppa, S. Two approaches for estimating disease prevalence from population-based registries of incidence and total mortality. Biometrics 1999, 55, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Marriotto, A.; Gigli, A.; Capocaccia, R.; Tavilla, A.; Clegg, L.X.; Depry, M.; Scoppa, S.; Ries, L.A.; Rowland, J.H.; Tesauro, G.; et al. Complete and Limited Duration Cancer Prevalence Estimates. SEER Cancer Statistics Review, 1973–1999, 2002. Available online: https://seer.cancer.gov/archive/csr/1973_1999/prevalence.pdf (accessed on 31 December 2016).
- Verdecchia, A.; de Angelis, G.; Capocaccia, R. Estimation and projections of cancer prevalence from cancer registry data. Stat. Med. 2002, 21, 3511–3526. [Google Scholar] [CrossRef] [PubMed]
- Arias, E. United States Life Tables, 2010. National Vital Statistics Reports, 2014; Volume 63. Available online: https://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_11.pdf (accessed on 31 December 2016).
- Contemporary OB/GYN: Bilateral Oophorectomy: Solving the Risk/Benefit Equation—Choosing candidates, Monitoring Outcomes. Available online: http://contemporaryobgyn.modernmedicine.com/contemporary-obgyn/news/modernmedicine/modern-medicine-now/bilateral-oophorectomy-solving-riskbenefi?page=full (accessed on 31 December 2016).
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Ueland, F.R.; Depriest, P.D.; Desimone, C.P.; Pavlik, E.J.; Lele, S.M.; Kryscio, R.J.; van Nagell, J.R., Jr. The accuracy of examination under anesthesia and transvaginal sonography in evaluating ovarian size. Gynecol. Oncol. 2005, 99, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, E.P.; Vesco, K.K.; Eder, M.; Lin, J.S.; Senger, C.A.; Burda, B.U. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: A systematic review for the US Preventive Services Task Force. Ann. Intern. Med. 2011, 155. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.R.; Moorman, P.; Gierisch, J.M.; Havrilesky, L.J.; Grimm, L.J.; Ghate, S.; Davidson, B.; Mongtomery, R.C.; Crowley, M.J.; McCrory, D.C.; et al. Benefits and Harms of Breast Cancer Screening: A Systematic Review. J. Am. Med. Assoc. 2015, 314, 1615–1634. [Google Scholar] [CrossRef] [PubMed]
- Nagell, J.R., Jr.; Miller, R.W.; DeSimone, C.P.; Ueland, F.R.; Podzielinski, I.; Goodrich, S.T.; Elder, J.W.; Huang, B.; Kryscio, R.J.; Pavlik, E.J. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet. Gynecol. 2011, 118, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Menon, U.; Ryan, A.; Gentry-Maharaj, A.; Burnell, M.; Kalsi, J.K.; Amso, N.N.; Apostolidou, S.; Benjamin, E.; Cruickshank, D.; et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2016, 387, 945–956. [Google Scholar] [CrossRef]
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.C.; Lamerato, L.; Isaacs, C.; Reding, D.J.; Greenlee, R.T.; Yokochi, L.A.; Kessel, B. Effect of screening on ovarian cancer mortality—The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. J. Am. Med. Assoc. 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; Haruta, S.; Tsuji, Y.; et al. A randomized study of screening for ovarian cancer: A multicenter study in Japan. Int. J. Gynecol. Cancer 2008, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Nagell, J.R., Jr.; Miller, R.W. Evaluation and Management of Ultrasonographically Detected Ovarian Tumors in Asymptomatic Women. Obstet. Gynecol. 2016, 127, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Ueland, F.R.; DePriest, P.D.; Pavlik, E.J.; Kryscio, R.J.; van Nagell, J.R., Jr. Preoperative differentiation of malignant from benign ovarian tumors: The efficacy of morphology indexing and Doppler flow sonography. Gynecol. Oncol. 2003, 91, 46–50. [Google Scholar] [CrossRef]
- Pavlik, E.J.; Ueland, F.R.; Miller, R.W.; Ubellacker, J.M.; Desimone, C.P.; Elder, J.; Hoff, J.; Baldwin, L.; Kryscio, R.J.; Nagell, J.R., Jr. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet. Gynecol. 2013, 122, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.E.; Carreon, J.D.; Lacey, J.V., Jr.; Devesa, S.S. Impact of hysterectomy on endometrial carcinoma rates in the United States. J. Natl. Cancer Inst. 2005, 97, 1700–1702. [Google Scholar] [CrossRef] [PubMed]
- Temkin, S.M.; Minasian, L.; Noone, A.M. The End of the Hysterectomy Epidemic and Endometrial Cancer Incidence: What Are the Unintended Consequences of Declining Hysterectomy Rates? Front. Oncol. 2016, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- ACOG. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet. Gynecol. 2009. [Google Scholar] [CrossRef]
- Whiteman, M.K.; Hillis, S.D.; Jamieson, D.J.; Morrow, B.; Podgornik, M.N.; Brett, K.M.; Marchbanks, P.A. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am. J. Obstet. Gynecol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Herzog, T.J.; Tsui, J.; Ananth, C.V.; Lewin, S.N.; Lu, Y.-S.; Neugut, A.I.; Hershman, D.L. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet. Gynecol. 2013, 122, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, E.; Salemi, J.L.; Mogos, M.F.; Hart, S.; Salihu, H.M.; Imudia, A.N. National trends of adnexal surgeries at the time of hysterectomy for benign indication, United States, 1998–2011. Am. J. Obstet. Gynecol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H.; Feskanich, D.; Broder, M.S.; Chang, E.; Shoupe, D.; Farquhar, C.M. Long-term mortality associated with oophorectomy versus ovarian conservation in the nurses’ health study. Obstet. Gynecol. 2013, 121, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Bandera, C.A.; Muto, M.G.; Schorge, J.O.; Berkowitz, R.S.; Rubin, S.C.; Mok, S.C. BRCA1 gene mutations in women with papillary serous carcinoma of the peritoneum. Obstet. Gynecol. 1998, 92, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.V.; Kuller, L.; Langer, R.; Manson, J.E.; Allen, C.; Assaf, A.; Cochrane, B.B.; Larson, J.C.; Lasser, N.; Rainford, M.; et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: The Women’s Health Initiative Observational Study. Circulation 2005, 111, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
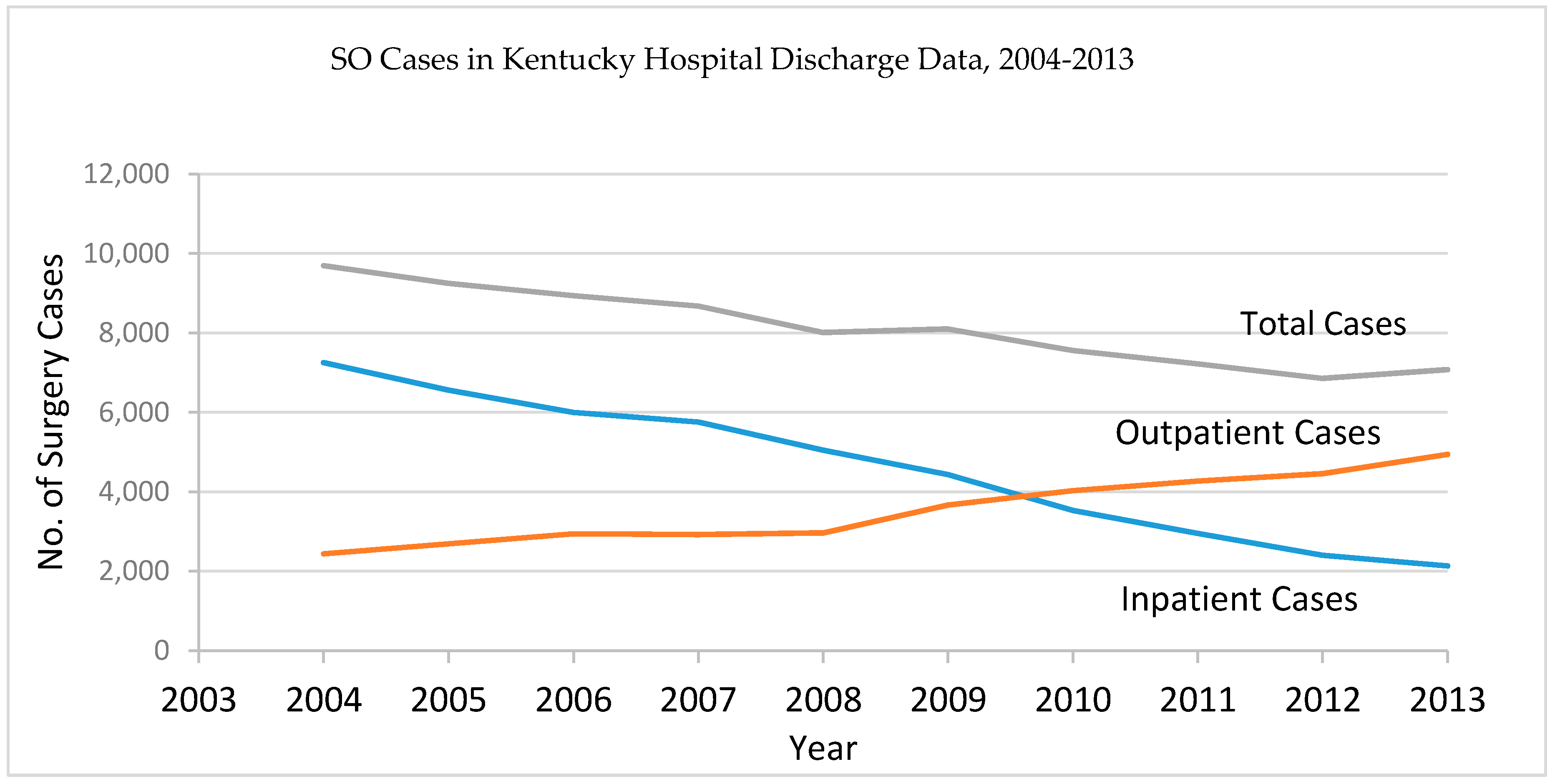
| Year | Age Group | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1–5 | 6–10 | 11–15 | 16–20 | 21–25 | 26–30 | 31–35 | 36–40 | 41–45 | 46–50 | 51–55 | 56–60 | 61–65 | 66–70 | 71–75 | 76–80 | 81 and over | Total | |
| 2004 | 1 | 1 | 0 | 6 | 24 | 179 | 561 | 966 | 1482 | 2077 | 1919 | 987 | 445 | 356 | 265 | 202 | 142 | 79 | 9692 |
| 2005 | 0 | 1 | 1 | 7 | 24 | 191 | 542 | 887 | 1342 | 1999 | 1874 | 935 | 501 | 323 | 242 | 179 | 121 | 80 | 9249 |
| 2006 | 0 | 0 | 0 | 6 | 24 | 184 | 536 | 954 | 1308 | 1798 | 1662 | 894 | 500 | 365 | 277 | 201 | 142 | 87 | 8938 |
| 2007 | 1 | 0 | 1 | 5 | 31 | 193 | 500 | 833 | 1299 | 1669 | 1625 | 923 | 574 | 371 | 233 | 200 | 119 | 96 | 8673 |
| 2008 | 0 | 0 | 1 | 6 | 26 | 172 | 462 | 769 | 1119 | 1549 | 1512 | 832 | 486 | 382 | 306 | 179 | 124 | 86 | 8011 |
| 2009 | 1 | 2 | 0 | 8 | 38 | 147 | 500 | 771 | 1123 | 1530 | 1509 | 851 | 500 | 436 | 291 | 182 | 119 | 89 | 8097 |
| 2010 | 0 | 0 | 0 | 10 | 32 | 162 | 454 | 783 | 1075 | 1327 | 1373 | 828 | 479 | 384 | 265 | 198 | 112 | 75 | 7557 |
| 2011 | 2 | 1 | 2 | 12 | 40 | 155 | 411 | 764 | 1008 | 1241 | 1288 | 777 | 473 | 416 | 310 | 182 | 72 | 67 | 7221 |
| 2012 | 0 | 0 | 0 | 6 | 31 | 130 | 363 | 699 | 941 | 1185 | 1205 | 781 | 488 | 391 | 274 | 191 | 94 | 72 | 6851 |
| 2013 | 0 | 0 | 0 | 9 | 27 | 122 | 366 | 726 | 1031 | 1256 | 1188 | 748 | 518 | 425 | 307 | 186 | 103 | 63 | 7075 |
| Total | 5 | 5 | 5 | 75 | 297 | 1635 | 4695 | 8152 | 11,728 | 15,631 | 15,155 | 8556 | 4964 | 3849 | 2770 | 1900 | 1148 | 794 | 81,364 |
| Age Group | Prob. of Survival | 2009 | 2013 | ||||
|---|---|---|---|---|---|---|---|
| Population | Prevalence Count | Prevalence Rate | Population | Prevalence Count | Prevalence Rate | ||
| 0 | 0.989 | 27,302 | 1.0 | 0.000 | 26,905 | 0.0 | 0.000 |
| 1–5 | 0.995 | 138,333 | 2.2 | 0.000 | 135,862 | 1.0 | 0.000 |
| 6–10 | 0.995 | 137,647 | 4.8 | 0.000 | 138,692 | 3.8 | 0.000 |
| 11–15 | 0.995 | 137,317 | 23.2 | 0.000 | 139,204 | 32.4 | 0.000 |
| 16–20 | 0.995 | 146,960 | 128.7 | 0.001 | 138,987 | 134.5 | 0.001 |
| 21–25 | 0.995 | 139,412 | 689.7 | 0.005 | 154,076 | 596.8 | 0.004 |
| 26–30 | 0.994 | 143,447 | 2624.1 | 0.018 | 137,250 | 2201.0 | 0.016 |
| 31–35 | 0.994 | 135,456 | 6206.3 | 0.046 | 142,561 | 5680.1 | 0.040 |
| 36–40 | 0.994 | 145,489 | 11,649.2 | 0.080 | 135,750 | 10,662.2 | 0.079 |
| 41–45 | 0.993 | 150,939 | 19,138.6 | 0.127 | 146,947 | 17,331.1 | 0.118 |
| 46–50 | 0.992 | 164,356 | 27,186.7 | 0.165 | 154,584 | 25,180.5 | 0.163 |
| 51–55 | 0.991 | 159,697 | 31,613.2 | 0.198 | 163,369 | 31,226.4 | 0.191 |
| 56–60 | 0.989 | 142,234 | 32,472.1 | 0.228 | 153,709 | 33,146.2 | 0.216 |
| 61–65 | 0.987 | 118,111 | 32,339.0 | 0.274 | 134,958 | 32,887.2 | 0.244 |
| 66–70 | 0.982 | 92,015 | 31,612.5 | 0.344 | 106,051 | 31,852.8 | 0.300 |
| 71–75 | 0.974 | 71,219 | 30,014.6 | 0.421 | 78,893 | 30,093.7 | 0.381 |
| 76–80 | 0.960 | 58,333 | 27,272.4 | 0.468 | 58,703 | 27,192.7 | 0.463 |
| 81+ | 0.911 | 84,869 | 39,805.1 | 0.469 | 87,494 | 39,812.0 | 0.455 |
| Age Group | Hysterectomy Prevalence Rate by BRFSS ^ | Ratio of SO vs. Hysterectomy * | SO Prevalence Rate by BRFSS |
|---|---|---|---|
| 21–25 | 0.000 | 0.902 | 0.000 |
| 26–30 | 0.024 | 0.723 | 0.018 |
| 31–35 | 0.076 | 0.665 | 0.051 |
| 36–40 | 0.120 | 0.649 | 0.078 |
| 41–45 | 0.196 | 0.719 | 0.141 |
| 46–50 | 0.256 | 0.879 | 0.225 |
| 51–55 | 0.379 | 1.010 | 0.383 |
| 56–60 | 0.415 | 1.033 | 0.429 |
| 61–65 | 0.461 | 1.021 | 0.471 |
| 66–70 | 0.513 | 0.988 | 0.507 |
| 71–75 | 0.517 | 0.963 | 0.498 |
| 76–80 | 0.532 | 0.900 | 0.479 |
| 81+ | 0.512 | 0.829 | 0.425 |
| Type of Population Under Risk | All Ages | ||||
|---|---|---|---|---|---|
| Population under Risk | N | Adj Rate | 95% CI | ||
| Standard Population ^ | 11,083,781 | 1403 | 10.73 | 10.16 | 11.32 |
| Standard Population with Modified Age Group * | 11,083,781 | 1403 | 10.73 | 10.17 | 11.32 |
| Modified Population based on KCHD-Assumption 1 ~ | 9,630,865 | 1403 | 15.47 | 14.65 | 16.32 |
| Modified Population based on KCHD-Assumption 2 ~ | 9,414,282 | 1403 | 16.88 | 15.98 | 17.82 |
| Modified Population based on KCHD-Assumption 3 ~ | 9,847,449 | 1403 | 14.34 | 13.58 | 15.12 |
| Modified Population based on BRFS † | 9,009,436 | 1387 | 17.72 | 16.78 | 18.69 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldwin, L.A.; Chen, Q.; Tucker, T.C.; White, C.G.; Ore, R.N.; Huang, B. Ovarian Cancer Incidence Corrected for Oophorectomy. Diagnostics 2017, 7, 19. https://doi.org/10.3390/diagnostics7020019
Baldwin LA, Chen Q, Tucker TC, White CG, Ore RN, Huang B. Ovarian Cancer Incidence Corrected for Oophorectomy. Diagnostics. 2017; 7(2):19. https://doi.org/10.3390/diagnostics7020019
Chicago/Turabian StyleBaldwin, Lauren A., Quan Chen, Thomas C. Tucker, Connie G. White, Robert N. Ore, and Bin Huang. 2017. "Ovarian Cancer Incidence Corrected for Oophorectomy" Diagnostics 7, no. 2: 19. https://doi.org/10.3390/diagnostics7020019
APA StyleBaldwin, L. A., Chen, Q., Tucker, T. C., White, C. G., Ore, R. N., & Huang, B. (2017). Ovarian Cancer Incidence Corrected for Oophorectomy. Diagnostics, 7(2), 19. https://doi.org/10.3390/diagnostics7020019