Imaging of HCC—Current State of the Art
Abstract
:1. Introduction
2. Guidelines for Hepatocellular cancer (HCC) Diagnosis—Current Status
2.1. HCC Surveillance Program
2.1.1. Role of Biomarkers in Surveillance
2.1.2. Ultrasound in Surveillance
2.2. Diagnosis of HCC

2.3. Contrast Agents
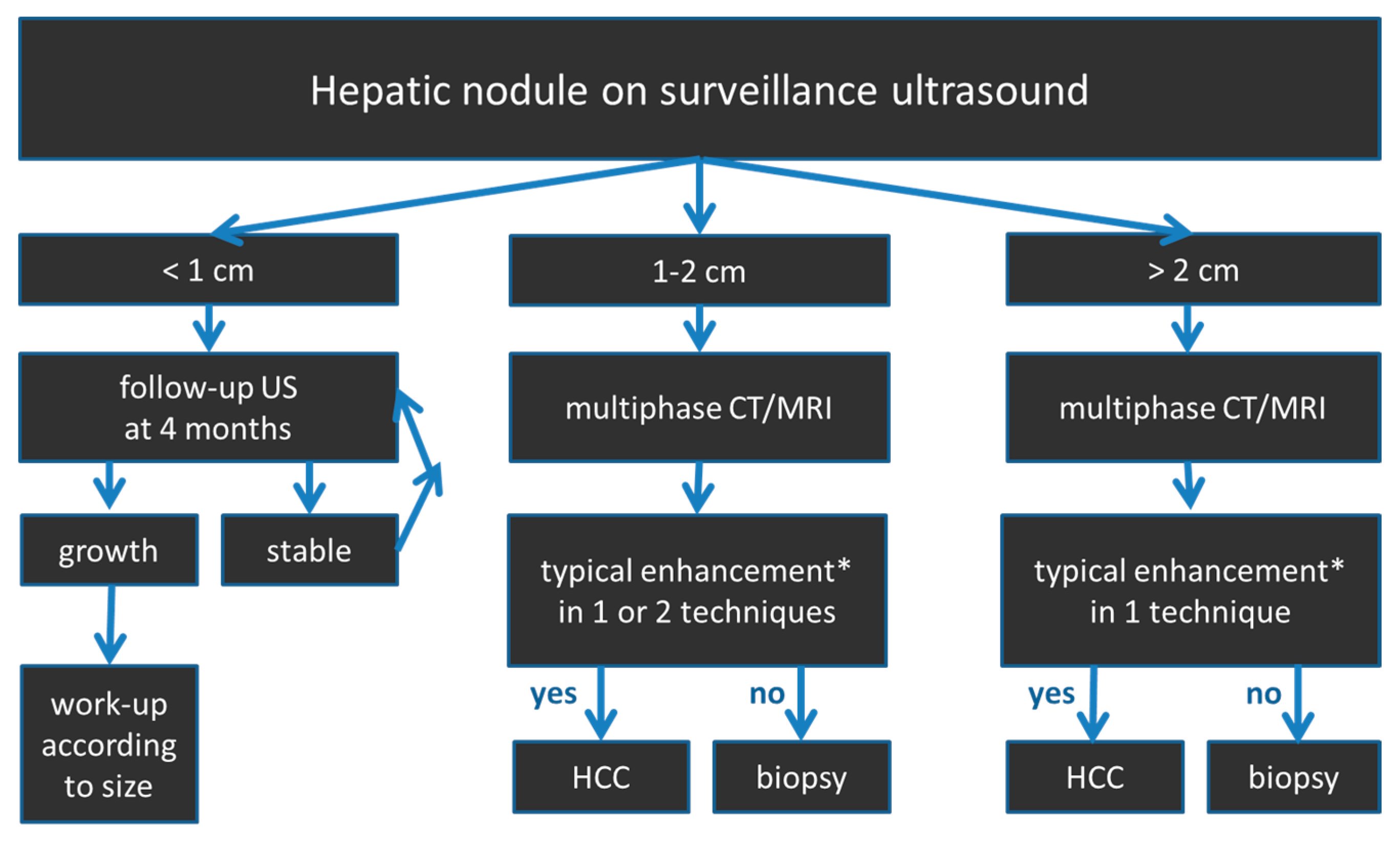
2.4. HCC Diagnostic Algorithm
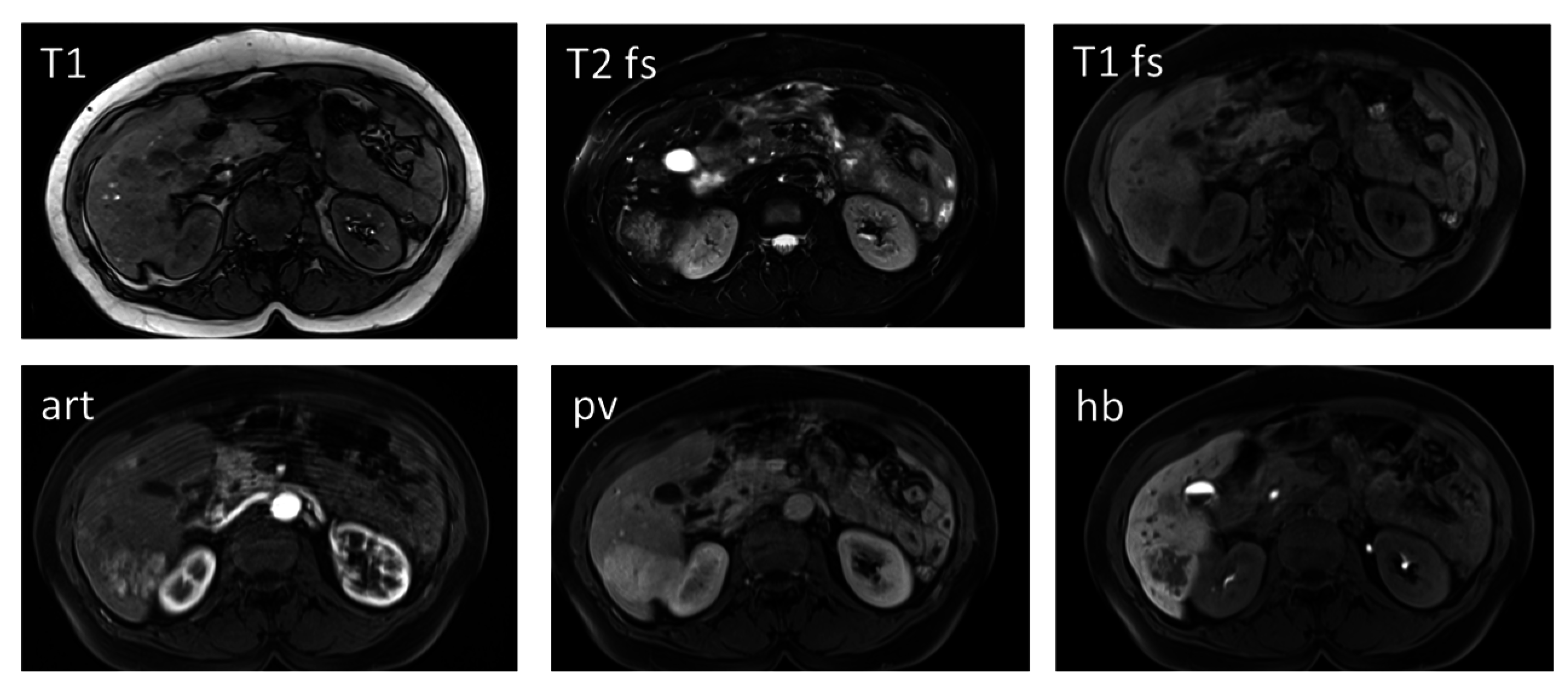
2.5. Differentiation of High-Grade Dysplastic Nodules versus HCC
2.6. Differentiating Other Hypervascular Liver Lesions from HCC
2.6.1. Benign Hypervascular Liver Lesions

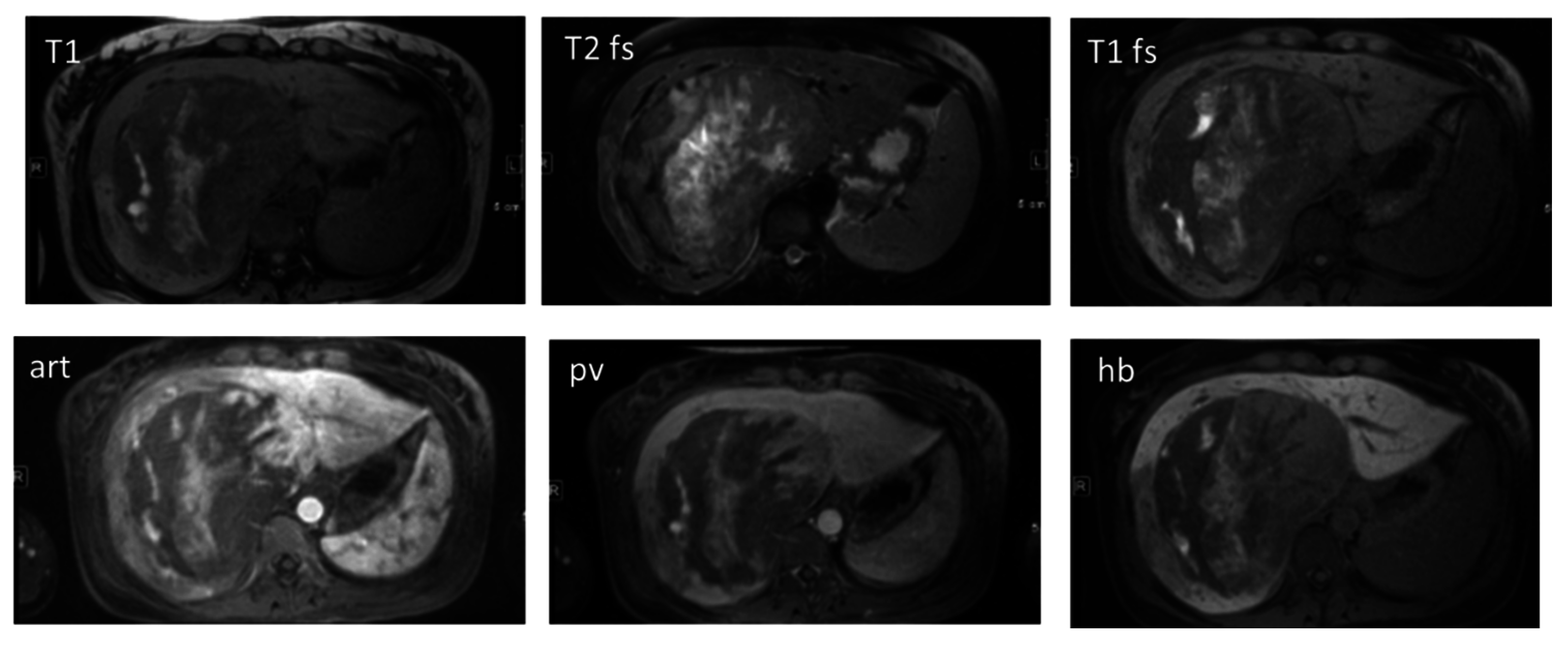
2.6.2. Malignant Hypervascular Liver Lesions
2.7. Treatment Response Evaluation in HCC Patients
2.8. Challenges of the Diagnostic (Imaging) Algorithms
2.9. Liver Imaging Reporting and Data System (LI-RADS) Categorization
3. Magnetic Resonance Imaging (MRI)
3.1. Hepatocyte Specific MRI Contrast Agents

3.2. Diffusion-Weighted MR Imaging
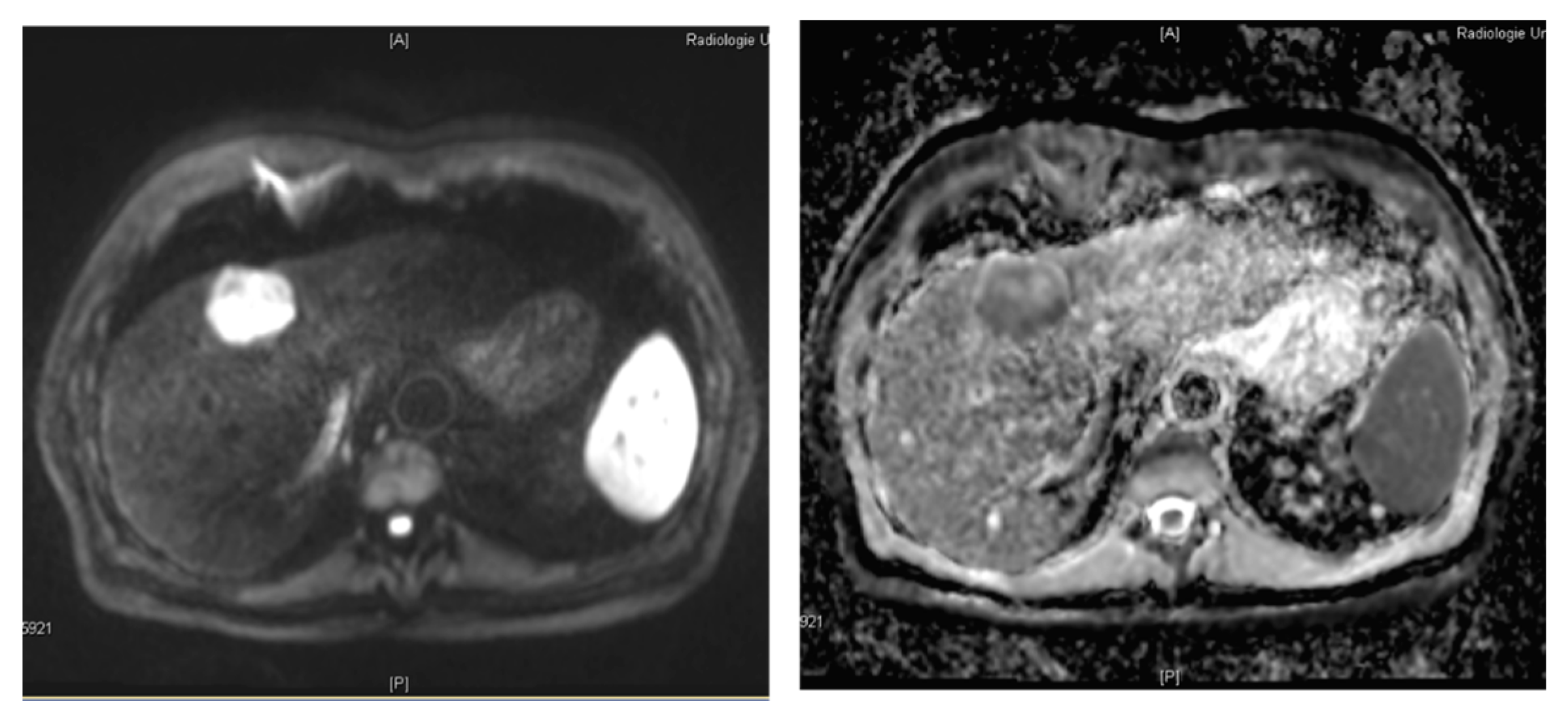
3.3. Dynamic Contrast Enhanced MRI
4. Computed Tomography (CT)
4.1. Advances in CT Imaging
4.2. Dual-Energy CT (DECT)
4.3. Volume-Perfusion CT (VPCT)
4.4. Cone Beam Computed Tomography in Peri-Procedural Imaging of HCC
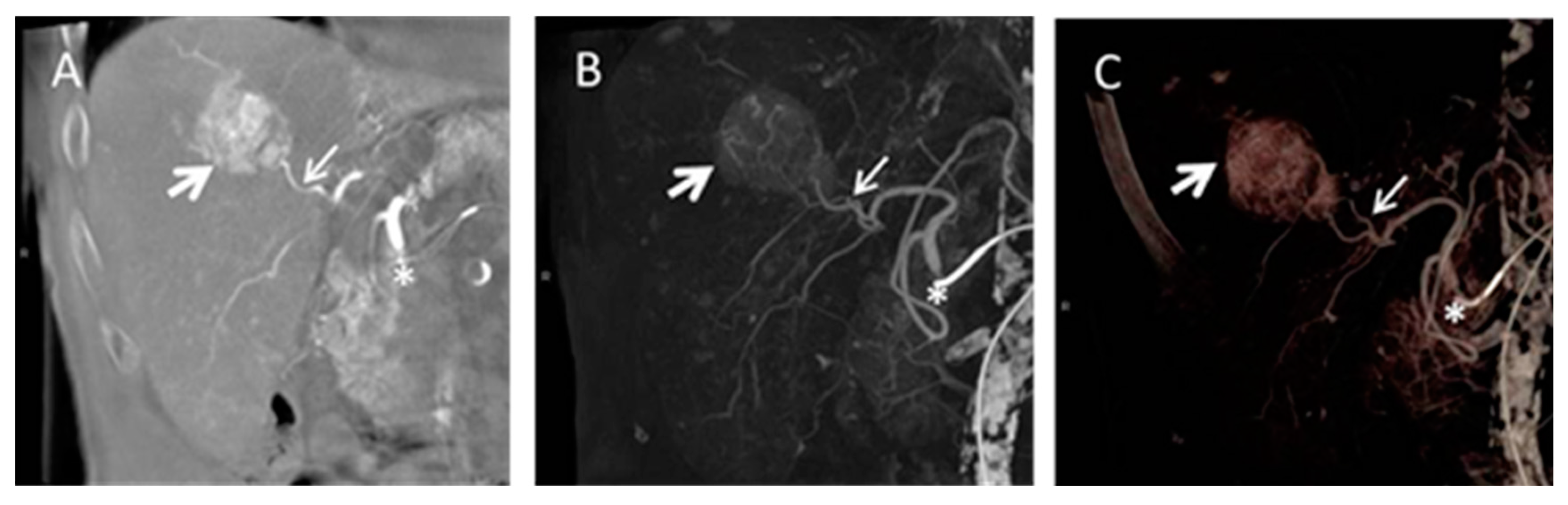
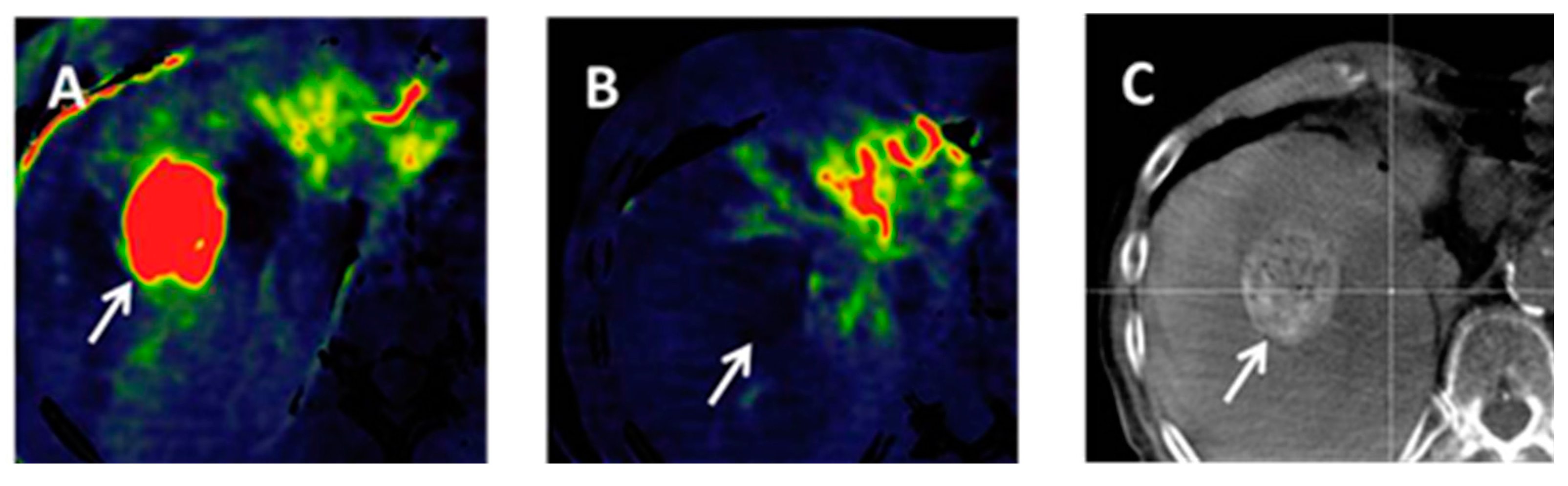
5. Future Work
6. Conclusions
Author Contributions
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar]
- Llovet, J.M.; Fuster, J.; Bruix, J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology 1999, 30, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- De Lope, C.R.; Tremosini, S.; Forner, A.; Reig, M.; Bruix, J. Management of HCC. J. Hepatol. 2012, 56, S75–S87. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Kondo, Y.; Kimura, O.; Shimosegawa, T. Significant biomarkers for the management of hepatocellular carcinoma. Clin. J. Gastroenterol. 2015, 8, 109–115. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Richardson, P.A.; Everhart, J.E. The role of diabetes in hepatocellular carcinoma: A case-control study among united states veterans. Am. J. Gastroenterol. 2001, 96, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Fontana, R.J.; Fu, S.; Conjeevaram, H.S.; Su, G.L.; Lok, A.S. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J. Hepatol. 2005, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Tobe, R.G.; Inagaki, Y.; Kokudo, N.; Hasegawa, K.; Sugawara, Y.; Tang, W. The management of hepatocellular carcinoma around the world: A comparison of guidelines from 2001 to 2011. Liver Int. 2012, 32, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Ribes, J.; Diaz, M.; Cleries, R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Bota, S.; Piscaglia, F.; Marinelli, S.; Pecorelli, A.; Terzi, E.; Bolondi, L. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma. Liver Cancer 2012, 1, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.P.; Hwang, L.Y.; Lin, C.C.; Chien, C.S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22,707 men in Taiwan. Lancet 1981, 2, 1129–1133. [Google Scholar] [CrossRef]
- Degos, F.; Christidis, C.; Ganne-Carrie, N.; Farmachidi, J.P.; Degott, C.; Guettier, C.; Trinchet, J.C.; Beaugrand, M.; Chevret, S. Hepatitis C virus related cirrhosis: Time to occurrence of hepatocellular carcinoma and death. Gut 2000, 47, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodes, J. Clinical management of hepatocellular carcinoma. Conclusions of the barcelona-2000 EASL conference. European association for the study of the liver. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Lesmana, L.A.; Tateishi, R.; Chen, P.J.; Lin, S.M.; Yoshida, H.; Kudo, M.; Lee, J.M.; Choi, B.I.; Poon, R.T.P.; et al. Asian pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010, 4, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M. The radiological diagnosis of hepatocellular carcinoma. Am. J. Gastroenterol. 2010, 105, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Lim, J.H.; Lee, W.J. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: Accuracy of ultrasonography in transplant patients. J. Ultrasound Med. 2001, 20, 99–104. [Google Scholar] [PubMed]
- Sato, T.; Tateishi, R.; Yoshida, H.; Ohki, T.; Masuzaki, R.; Imamura, J.; Goto, T.; Kanai, F.; Obi, S.; Kato, N.; et al. Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C. Hepatol. Int. 2009, 3, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Cucchetti, A.; Erroi, V.; Garuti, F.; Odaldi, F.; Trevisani, F. Surveillance for early diagnosis of hepatocellular carcinoma: How best to do it? World J. Gastroenterol. 2013, 19, 8808–8821. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Maldonado, J.; Garcia-Juarez, I.; Aguirre-Valadez, J.; Gonzalez-Aguirre, A.; Vilatoba-Chapa, M.; Armengol-Alonso, A.; Escobar-Penagos, F.; Torre, A.; Sanchez-Avila, J.F.; Carrillo-Perez, D.L. Diagnosis and treatment of hepatocellular carcinoma: An update. World J. Hepatol. 2015, 7, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Aghoram, R.; Cai, P.; Dickinson, J.A. α-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. Cochrane Database Syst. Rev. 2012, 9, CD002799. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.; Volk, M.L.; Waljee, A.; Salgia, R.; Higgins, P.; Rogers, M.A.; Marrero, J.A. Meta-analysis: Surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment. Pharmacol. Ther. 2009, 30, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bolondi, L. Screening for hepatocellular carcinoma in cirrhosis. J. Hepatol. 2003, 39, 1076–1084. [Google Scholar] [CrossRef]
- Murakami, T.; Tsurusaki, M. Hypervascular benign and malignant liver tumors that require differentiation from hepatocellular carcinoma: Key points of imaging diagnosis. Liver Cancer 2014, 3, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Motosugi, U.; Ichikawa, T.; Sou, H.; Sano, K.; Tominaga, L.; Muhi, A.; Araki, T. Distinguishing hypervascular pseudolesions of the liver from hypervascular hepatocellular carcinomas with gadoxetic acid-enhanced MR imaging. Radiology 2010, 256, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gluud, C.; Christoffersen, P.; Eriksen, J.; Wantzin, P.; Knudsen, B.B. Influence of ethanol on development of hyperplastic nodules in alcoholic men with micronodular cirrhosis. Gastroenterology 1987, 93, 256–260. [Google Scholar] [PubMed]
- Yoneda, N.; Matsui, O.; Kitao, A.; Kita, R.; Kozaka, K.; Koda, W.; Kobayashi, S.; Gabata, T.; Ikeda, H.; Sato, Y.; et al. Hepatocyte transporter expression in FNH and FNH-like nodule: Correlation with signal intensity on gadoxetic acid enhanced magnetic resonance images. Jpn. J. Radiol. 2012, 30, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Katabathina, V.S.; Menias, C.O.; Shanbhogue, A.K.; Jagirdar, J.; Paspulati, R.M.; Prasad, S.R. Genetics and imaging of hepatocellular adenomas: 2011 update. Radiographics 2011, 31, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Bioulac-Sage, P.; Laumonier, H.; Laurent, C.; Zucman-Rossi, J.; Balabaud, C. Hepatocellular adenoma: What is new in 2008. Hepatol. Int. 2008, 2, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Kumar, A. Treatment response evaluation and follow-up in hepatocellular carcinoma. J. Clin. Exp. Hepatol. 2014, 4, S126–S129. [Google Scholar] [CrossRef] [PubMed]
- Kojiro, M.; Wanless, I.R.; Alves, V.; Badve, S.; Balabaud, C.; Bedossa, P. Pathologic diagnosis of early hepatocellular carcinoma: A report of the international consensus group for hepatocellular neoplasia. Hepatology 2009, 49, 658–664. [Google Scholar]
- Baheti, A.D.; Tirumani, S.H.; Rosenthal, M.H.; Shinagare, A.B.; Ramaiya, N.H. Diagnosis and management of intrahepatic cholangiocarcinoma: A comprehensive update for the radiologist. Clin. Radiol. 2014, 69, e463–e470. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.E.; Kim, M.J.; Park, Y.N.; Choi, J.Y.; Pyo, J.Y.; Kim, Y.C.; Cho, H.J.; Kim, K.A.; Choi, S.Y. Varying appearances of cholangiocarcinoma: Radiologic-pathologic correlation. Radiographics 2009, 29, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.M.; Ribero, D.; O’Reilly, E.M.; Kokudo, N.; Miyazaki, M.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Rimola, J.; Forner, A.; Reig, M.; Vilana, R.; de Lope, C.R.; Ayuso, C.; Bruix, J. Cholangiocarcinoma in cirrhosis: Absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology 2009, 50, 791–798. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology (ACR). Available online: http://www.acr.org/quality-safety/resources/lirads (accessed on 15 November 2015).
- Shah, A.; Tang, A.; Santillan, C.; Sirlin, C. Cirrhotic liver: What’s that nodule? The LI-RADS approach. J. Magn. Reson. Imaging 2015. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.G.; Bruix, J.; Sherman, M.; Sirlin, C.B. LI-RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI-RADS management working group and future directions. Hepatology 2015, 61, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Fowler, K.J.; Sirlin, C.B.; Costa, E.A.; Yee, J.; Yeh, B.M.; Heiken, J.P. Hepatobiliary agents and their role in LI-RADS. Abdom. Imaging 2015, 40, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.C.; Mitchell, D.G.; Weinreb, J.C.; Santillan, C.S.; Yeh, B.M.; Francois, R.; Sirlin, C.B. LI-RADS categorization of benign and likely benign findings in patients at risk of hepatocellular carcinoma: A pictorial atlas. Am. J. Roentgenol. 2014, 203, W48–W69. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, J.T.; Stark, D.D. Iron oxide-enhanced MR imaging of the liver and spleen: Review of the first 5 years. Am. J. Roentgenol. 1990, 155, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Stark, D.D.; Hahn, P.F.; Wittenberg, J.; Brady, T.J.; Ferrucci, J.T., Jr. Ferrite particles: A superparamagnetic MR contrast agent for the reticuloendothelial system. Radiology 1987, 162, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kanematsu, M.; Kondo, H.; Goshima, S.; Matsuo, M.; Hoshi, H.; Moriyama, N. Ferumoxide-enhanced MR imaging of hepatocellular carcinoma: Correlation with histologic tumor grade and tumor vascularity. J. Magn. Reson. Imaging 2004, 19, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, H.J.; Schuhmann-Giampieri, G.; Schmitt-Willich, H.; Vogler, H.; Frenzel, T.; Gries, H. A new lipophilic gadolinium chelate as a tissue-specific contrast medium for MRI. Magn. Reson. Med. 1991, 22, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Cereser, L.; Furlan, A.; Bagatto, D.; Girometti, R.; Como, G.; Avellini, C.; Orsaria, M.; Zuiani, C.; Bazzocchi, M. Comparison of portal venous and delayed phases of gadolinium-enhanced magnetic resonance imaging study of cirrhotic liver for the detection of contrast washout of hypervascular hepatocellular carcinoma. J. Comput. Assist. Tomogr. 2010, 34, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Hamm, B.; Staks, T.; Muhler, A.; Bollow, M.; Taupitz, M.; Frenzel, T.; Wolf, K.J.; Weinmann, H.J.; Lange, L. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: Safety, pharmacokinetics, and MR imaging. Radiology 1995, 195, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Rummeny, E.J.; Shamsi, K.; Balzer, T.; Daldrup, H.E.; Tombach, B.; Hesse, T.; Berns, T.; Peters, P.E. Phase II clinical evaluation of Gd-EOB-DTPA: Dose, safety aspects, and pulse sequence. Radiology 1996, 199, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, A.; Lorusso, V.; Pirovano, G.; Taroni, P.; Kirchin, M.; Davies, A. Multihance clinical pharmacology: Biodistribution and MR enhancement of the liver. Acad. Radiol. 1998, 5, S86–S89. [Google Scholar] [CrossRef]
- Cruite, I.; Schroeder, M.; Merkle, E.M.; Sirlin, C.B. Gadoxetate disodium-enhanced MRI of the liver: Part 2, protocol optimization and lesion appearance in the cirrhotic liver. Am. J. Roentgenol. 2010, 195, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Blakeborough, A.; Breuer, J.; Grazioli, L.; Gschwend, S.; Hammerstingl, R.; Heinz-Peer, G.; Kittner, T.; Laghi, A.; Leen, E.; et al. Enhancement of liver parenchyma after injection of hepatocyte-specific MRI contrast media: A comparison of gadoxetic acid and gadobenate dimeglumine. J. Magn. Reson. Imaging 2010, 31, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, S.H.; Jeon, Y.H.; Lee, J.; Kim, M.J.; Choi, D.; Lee, W.J.; Kim, H.; Koo, J.H.; Lim, H.K. Gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI versus gadobenate dimeglumine (Gd-BOPTA)-enhanced MRI for preoperatively detecting hepatocellular carcinoma: An initial experience. Korean J. Radiol. 2010, 11, 433–440. [Google Scholar] [PubMed]
- Okada, M.; Imai, Y.; Kim, T.; Kogita, S.; Takamura, M.; Kumano, S.; Onishi, H.; Hori, M.; Fukuda, K.; Hayashi, N.; et al. Comparison of enhancement patterns of histologically confirmed hepatocellular carcinoma between gadoxetate- and ferucarbotran-enhanced magnetic resonance imaging. J. Magn. Reson. Imaging 2010, 32, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, C.S.; Han, Y.M.; Kwak, H.S.; Jin, G.Y.; Hwang, S.B.; Chung, G.H.; Lee, S.Y.; Yu, H.C. Detection of hepatocellular carcinoma: Gadoxetic acid-enhanced 3-dimensional magnetic resonance imaging versus multi-detector row computed tomography. J. Comput. Assist. Tomogr. 2009, 33, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Marin, D.; Guerrisi, A.; Baski, M.; Galati, F.; Rossi, M.; Brozzetti, S.; Masciangelo, R.; Passariello, R.; Catalano, C. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 2010, 256, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, A.; Haraida, S.; Kraus, A.; Zech, C.J.; Scheidler, J.; Breuer, J.; Helmberger, T.K.; Reiser, M.F. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: Correlation with histopathologic findings and spiral CT—Initial observations. Radiology 2005, 234, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, A.; Lee, J.M.; Murakami, T.; Huppertz, A.; Kudo, M.; Grazioli, L. Consensus report of the 2nd international forum for liver MRI. Eur. Radiol. 2009, 19, S975–S989. [Google Scholar] [CrossRef] [PubMed]
- Ringe, K.I.; Husarik, D.B.; Sirlin, C.B.; Merkle, E.M. Gadoxetate disodium-enhanced MRI of the liver: Part 1, protocol optimization and lesion appearance in the noncirrhotic liver. Am. J. Roentgenol. 2010, 195, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Neri, E.; Bali, M.A.; Ba-Ssalamah, A.; Boraschi, P.; Brancatelli, G.; Alves, F.C.; Grazioli, L.; Helmberger, T.; Lee, J.M.; Manfredi, R.; et al. Esgar consensus statement on liver MR imaging and clinical use of liver-specific contrast agents. Eur. Radiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Han, N.Y.; Park, B.J.; Sung, D.J.; Kim, M.J.; Cho, S.B.; Lee, C.H.; Jang, Y.J.; Kim, S.Y.; Kim, D.S.; Um, S.H.; et al. Chemotherapy-induced focal hepatopathy in patients with gastrointestinal malignancy: Gadoxetic acid—Enhanced and diffusion-weighted MR imaging with clinical-pathologic correlation. Radiology 2014, 271, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.M.; Collins, D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S. Diffusion-weighted MRI of hepatocellular carcinoma in cirrhosis. Clin. Radiol. 2014, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.B. Mechanisms of human hepatocarcinogenesis. Curr. Mol. Med. 2003, 3, 573–588. [Google Scholar] [PubMed]
- Chen, J.; Wu, M.; Liu, R.; Li, S.; Gao, R.; Song, B. Preoperative evaluation of the histological grade of hepatocellular carcinoma with diffusion-weighted imaging: A meta-analysis. PLoS ONE 2015, 10, e0117661. [Google Scholar] [CrossRef] [PubMed]
- Di Pietropaolo, M.; Briani, C.; Federici, G.F.; Marignani, M.; Begini, P.; Delle Fave, G.; Iannicelli, E. Comparison of diffusion-weighted imaging and gadoxetic acid-enhanced MR images in the evaluation of hepatocellular carcinoma and hypovascular hepatocellular nodules. Clin. Imaging 2015, 39, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Rao, S.X.; Chen, C.; Li, R.; Zeng, M.S. Assessing liver function in patients with hbv-related HCC: A comparison of T1 mapping on Gd-EOB-DTPA-enhanced MR imaging with DWI. Eur. Radiol. 2015, 25, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, Y.K.; Jeong, W.K.; Choi, D.; Rhim, H.; Lee, W.J. Nonhypervascular hypointense nodules at gadoxetic acid-enhanced MR imaging in chronic liver disease: Diffusion-weighted imaging for characterization. Radiology 2015, 276, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, S.H.; Kang, T.W.; Song, K.D.; Choi, D.; Park, C.K. Value of gadoxetic acid-enhanced and diffusion-weighted MR imaging in evaluation of hepatocellular carcinomas with atypical enhancement pattern on contrast-enhanced multiphasic MDCT in patients with chronic liver disease. Eur. J. Radiol. 2015, 84, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, Y.K.; Park, H.J.; Park, M.J.; Lee, W.J.; Choi, D. Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma. Magn. Reson. Imaging 2014, 32, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Song, K.D.; Kim, S.H.; Lim, H.K.; Jung, S.H.; Sohn, I.; Kim, H.S. Subcentimeter hypervascular nodule with typical imaging findings of hepatocellular carcinoma in patients with history of hepatocellular carcinoma: Natural course on serial gadoxetic acid-enhanced MRI and diffusion-weighted imaging. Eur. Radiol. 2015, 25, 2789–2796. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, S.H.; Lee, S.J.; Rhim, H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: Characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. Am. J. Roentgenol. 2011, 196, W758–W765. [Google Scholar] [CrossRef] [PubMed]
- Muhi, A.; Ichikawa, T.; Motosugi, U.; Sano, K.; Fatima, Z.; Matsuda, M.; Fujii, H.; Enomoto, N.; Araki, T. Diffusion-weighted imaging of hepatocellular carcinoma for predicting early recurrence and survival after hepatectomy. Hepatol. Int. 2013, 7, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Parikh, T.; Drew, S.J.; Lee, V.S.; Wong, S.; Hecht, E.M.; Babb, J.S.; Taouli, B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: Comparison with standard breath-hold T2-weighted imaging. Radiology 2008, 246, 812–822. [Google Scholar]
- Vandecaveye, V.; de Keyzer, F.; Verslype, C.; Op de Beeck, K.; Komuta, M.; Topal, B.; Roebben, I.; Bielen, D.; Roskams, T.; Nevens, F.; et al. Diffusion-weighted MRI provides additional value to conventional dynamic contrast-enhanced MRI for detection of hepatocellular carcinoma. Eur. Radiol. 2009, 19, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Muhi, A.; Ichikawa, T.; Motosugi, U.; Sano, K.; Matsuda, M.; Kitamura, T.; Nakazawa, T.; Araki, T. High-b-value diffusion-weighted MR imaging of hepatocellular lesions: Estimation of grade of malignancy of hepatocellular carcinoma. J. Magn. Reson. Imaging 2009, 30, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Lee, J.M.; Yoon, J.H.; Joo, I.; Han, J.K.; Choi, B.I. Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: Correlation with enhancement degree and histologic grade. Radiology 2014, 270, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Goshima, S.; Kanematsu, M.; Noda, Y.; Kondo, H.; Watanabe, H.; Bae, K.T. Diffusion kurtosis imaging to assess response to treatment in hypervascular hepatocellular carcinoma. Am. J. Roentgenol. 2015, 204, W543–W549. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Sigmund, E.E.; Winnick, A.; Niver, B.E.; Spieler, B.; Morgan, G.R.; Hajdu, C.H. Assessment of hepatocellular carcinoma using apparent diffusion coefficient and diffusion kurtosis indices: Preliminary experience in fresh liver explants. Magn. Reson. Imaging 2012, 30, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Rempp, H.; Schraml, C.; Schwenzer, N.; Grozinger, G.; Blumenstock, G.; Rothgang, E.; Pereira, P.L.; Claussen, C.D.; Clasen, S. Diffusion-weighted imaging during MR-guided radiofrequency ablation of hepatic malignancies: Analysis of immediate pre- and post-ablative diffusion characteristics. Acta Radiol. 2015, 56, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Kamel, I.R.; Bluemke, D.A.; Ramsey, D.; Abusedera, M.; Torbenson, M.; Eng, J.; Szarf, G.; Geschwind, J.F. Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. Am. J. Roentgenol. 2003, 181, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Kamel, I.R.; Reyes, D.K.; Liapi, E.; Bluemke, D.A.; Geschwind, J.F. Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2007, 18, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rhee, T.K.; Naik, N.K.; Deng, J.; Atassi, B.; Mulcahy, M.F.; Kulik, L.M.; Ryu, R.K.; Miller, F.H.; Larson, A.C.; Salem, R.; et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: Comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J. Vasc. Interv. Radiol. 2008, 19, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Harman, M.; Cinar, C.; Bozkaya, H.; Parildar, M.; Elmas, N. Evaluation of treatment response of chemoembolization in hepatocellular carcinoma with diffusion-weighted imaging on 3.0-T MR imaging. J. Vasc. Interv. Radiol. 2012, 23, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Schraml, C.; Schwenzer, N.F.; Martirosian, P.; Bitzer, M.; Lauer, U.; Claussen, C.D.; Horger, M. Diffusion-weighted MRI of advanced hepatocellular carcinoma during sorafenib treatment: Initial results. Am. J. Roentgenol. 2009, 193, W301–W307. [Google Scholar] [CrossRef] [PubMed]
- Goshima, S.; Kanematsu, M.; Kondo, H.; Yokoyama, R.; Tsuge, Y.; Shiratori, Y.; Onozuka, M.; Moriyama, N. Evaluating local hepatocellular carcinoma recurrence post-transcatheter arterial chemoembolization: Is diffusion-weighted MRI reliable as an indicator? J. Magn. Reson. Imaging 2008, 27, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Chung, J.J.; Kim, J.H.; Kim, K.W. Limited value of diffusion-weighted MR imaging for differentiating bland from malignant portal venous thrombi. Radiology 2010, 256, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.S.; Pialat, J.B.; Wiart, M.; Duboeuf, F.; Mabrut, J.Y.; Bancel, B.; Rode, A.; Ducerf, C.; Baulieux, J.; Berthezene, Y. Characterization of hepatocellular carcinoma and colorectal liver metastasis by means of perfusion MRI. J. Magn. Reson. Imaging 2008, 28, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Yang, T.S.; Huo, T.I.; Hsieh, R.K.; Yu, C.W.; Hwang, W.S.; Hsieh, T.Y.; Huang, W.T.; Chao, Y.; Meng, R.; et al. Vandetanib in patients with inoperable hepatocellular carcinoma: A phase II, randomized, double-blind, placebo-controlled study. J. Hepatol. 2012, 56, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Shen, Y.C.; Yu, C.W.; Hsu, C.; Hu, F.C.; Hsu, C.H.; Chen, B.B.; Wei, S.Y.; Cheng, A.L.; Shih, T.T. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J. Hepatol. 2011, 55, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.T.; Tsang, Y.M.; Liu, T.W.; Shih, T.T. Dynamic contrast-enhanced MRI analysis of perfusion changes in advanced hepatocellular carcinoma treated with an antiangiogenic agent: A preliminary study. Am. J. Roentgenol. 2004, 183, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Akisik, F.M.; Sandrasegaran, K.; Aisen, A.M.; Lin, C.; Lall, C. Abdominal MR imaging at 3.0 T. Radiographics 2007, 27, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Materne, R.; Smith, A.M.; Peeters, F.; Dehoux, J.P.; Keyeux, A.; Horsmans, Y.; Van Beers, B.E. Assessment of hepatic perfusion parameters with dynamic MRI. Magn. Reson. Med. 2002, 47, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Notohamiprodjo, M.; Reiser, M.F.; Sourbron, S.P. Diffusion and perfusion of the kidney. Eur. J. Radiol. 2010, 76, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Essig, M.; Nguyen, T.B.; Shiroishi, M.S.; Saake, M.; Provenzale, J.M.; Enterline, D.S.; Anzalone, N.; Dorfler, A.; Rovira, A.; Wintermark, M.; et al. Perfusion MRI: The five most frequently asked clinical questions. Am. J. Roentgenol. 2013, 201, W495–W510. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.S.; Bisdas, S.; Koh, D.M.; Thng, C.H. Fundamentals of tracer kinetics for dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging 2011, 34, 1262–1276. [Google Scholar] [CrossRef] [PubMed]
- Sourbron, S.P.; Michaely, H.J.; Reiser, M.F.; Schoenberg, S.O. MRI-measurement of perfusion and glomerular filtration in the human kidney with a separable compartment model. Invest. Radiol. 2008, 43, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.S.; Helck, A.D.; Ingrisch, M.; Staehler, M.; Stief, C.; Sommer, W.H.; Braunagel, M.; Kazmierczak, P.M.; Reiser, M.F.; Nikolaou, K.; et al. Dynamic contrast-enhanced magnetic resonance imaging assessment of kidney function and renal masses: Single slice versus whole organ/tumor. Invest. Radiol. 2014, 49, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.; Nordell, A.; Vargas, R.; Douglas, L.; Jonas, E.; Blomqvist, L. Assessment of hepatic extraction fraction and input relative blood flow using dynamic hepatocyte-specific contrast-enhanced MRI. J. Magn. Reson. Imaging 2009, 29, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Yopp, A.C.; Schwartz, L.H.; Kemeny, N.; Gultekin, D.H.; Gonen, M.; Bamboat, Z.; Shia, J.; Haviland, D.; D’Angelica, M.I.; Fong, Y.; et al. Antiangiogenic therapy for primary liver cancer: Correlation of changes in dynamic contrast-enhanced magnetic resonance imaging with tissue hypoxia markers and clinical response. Ann. Surg. Oncol. 2011, 18, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Sahani, D.V.; Duda, D.G.; di Tomaso, E.; Ancukiewicz, M.; Catalano, O.A.; Sindhwani, V.; Blaszkowsky, L.S.; Yoon, S.S.; Lahdenranta, J.; et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J. Clin. Oncol. 2009, 27, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, S.M.; Lodge, M.A.; Taylor, N.J.; Rustin, G.J.; Bentzen, S.; Stirling, J.J.; Padhani, A.R. Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumors: Comparison of quantitative and semi-quantitative analysis. NMR Biomed. 2002, 15, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Murakami, T.; Takamura, M.; Kim, T.; Hori, M.; Narumi, Y.; Nakamura, H.; Kudo, M. Multi-detector row helical CT angiography of hepatic vessels: Depiction with dual-arterial phase acquisition during single breath hold. Radiology 2002, 222, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Kadoya, M.; Kameyama, T.; Yoshikawa, J.; Takashima, T.; Nakanuma, Y.; Unoura, M.; Kobayashi, K.; Izumi, R.; Ida, M.; et al. Benign and malignant nodules in cirrhotic livers: Distinction based on blood supply. Radiology 1991, 178, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.F.; Heuschmid, M.; Claussen, C.D. Multidetector helical CT of the liver for tumor detection and characterization. Eur. Radiol. 2002, 12, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, R.; Laghi, A.; Catalano, C.; Rossi, P.; Mangiapane, F.; Murakami, T.; Hori, M.; Piacentini, F.; Nofroni, I.; Passariello, R. Hepatocellular carcinoma: Role of unenhanced and delayed phase multi-detector row helical CT in patients with cirrhosis. Radiology 2005, 234, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Choi, J.Y.; Lim, J.S.; Kim, J.Y.; Kim, J.H.; Oh, Y.T.; Yoo, E.H.; Chung, J.J.; Kim, K.W. Optimal scan window for detection of hypervascular hepatocellular carcinomas during MDCT examination. Am. J. Roentgenol. 2006, 187, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.J.; Kim, M.J.; Yoo, H.S.; Lee, J.T. Nodular hepatocellular carcinomas: Detection with arterial-, portal-, and delayed-phase images at spiral CT. Radiology 1997, 202, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Choi, D.; Kim, S.H.; Lee, S.J.; Lee, W.J.; Lim, H.K.; Kim, S. Detection of hepatocellular carcinoma: Value of adding delayed phase imaging to dual-phase helical CT. Am. J. Roentgenol. 2002, 179, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kudo, M.; Komuta, M.; Hayaishi, S.; Ueda, T.; Takita, M.; Kitai, S.; Hatanaka, K.; Yada, N.; Hagiwara, S.; et al. Assessment of Gd-EOB-DTPA-enhanced MRI for HCC and dysplastic nodules and comparison of detection sensitivity versus MDCT. J. Gastroenterol. 2012, 47, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, G. Imaging of dysplastic nodules and small hepatocellular carcinomas: Experience with explanted livers. Intervirology 2004, 47, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Imaging diagnosis of hepatocellular carcinoma and premalignant/borderline lesions. Semin. Liver Dis. 1999, 19, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, K.; Muramatsu, Y.; Furukawa, H.; Wakao, F.; Moriyama, N.; Takayama, T.; Yamasaki, S.; Sakamoto, M.; Hirohashi, S. Early hepatocellular carcinoma: Appearance at CT during arterial portography and CT arteriography with pathologic correlation. Radiology 1995, 194, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, K.; Muramatsu, Y.; Mizuguchi, Y.; Ojima, H. CT imaging of early hepatocellular carcinoma and the natural outcome of hypoattenuating nodular lesions in chronic liver disease. Oncology 2007, 72, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kojiro, M.; Roskams, T. Early hepatocellular carcinoma and dysplastic nodules. Semin. Liver Dis. 2005, 25, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, O.; Sugihara, S.; Kage, M.; Kojiro, M. Pathomorphologic characteristics of small hepatocellular carcinoma: A special reference to small hepatocellular carcinoma with indistinct margins. Hepatology 1995, 22, 101–105. [Google Scholar] [CrossRef]
- Peterson, M.S.; Baron, R.L.; Marsh, J.W., Jr.; Oliver, J.H., 3rd; Confer, S.R.; Hunt, L.E. Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: Epidemiology and CT-based tumor detection rate in 430 cases with surgical pathologic correlation. Radiology 2000, 217, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, L.; Bao, J.; Zhang, J.; Li, C.; Xia, Y.; Huang, X.; Wang, J. Comparison of MRI with liver-specific contrast agents and multidetector row CT for the detection of hepatocellular carcinoma: A meta-analysis of 15 direct comparative studies. Gut 2013, 62, 1520–1521. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, M.; Sofue, K.; Isoda, H.; Okada, M.; Kitajima, K.; Murakami, T. Comparison of gadoxetic acid-enhanced magnetic resonance imaging and contrast-enhanced computed tomography with histopathological examinations for the identification of hepatocellular carcinoma: A multicenter phase III study. J. Gastroenterol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Anzidei, M.; Di Martino, M.; Sacconi, B.; Saba, L.; Boni, F.; Zaccagna, F.; Geiger, D.; Kirchin, M.A.; Napoli, A.; Bezzi, M.; et al. Evaluation of image quality, radiation dose and diagnostic performance of dual-energy CT datasets in patients with hepatocellular carcinoma. Clin. Radiol. 2015, 70, 996–973. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.; Sauter, A.; Spira, D.; Gatidis, S.; Ketelsen, D.; Heuschmid, M.; Claussen, C.D.; Thomas, C. Tin-filter enhanced dual-energy-CT: Image quality and accuracy of CT numbers in virtual noncontrast imaging. Acad. Radiol. 2013, 20, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qin, L.; Pan, D.; Huang, Y.; Yan, L.; Wang, G.; Liu, Y.; Liang, C.; Liu, Z. Liver diffusion-weighted MR imaging: Reproducibility comparison of adc measurements obtained with multiple breath-hold, free-breathing, respiratory-triggered, and navigator-triggered techniques. Radiology 2014, 271, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Schlemmer, H.P.; Schmidt, B.; Hoh, K.; Xu, K.; Ganten, T.M.; Ganten, M.K. Quantitative therapy response assessment by volumetric iodine-uptake measurement: Initial experience in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur. J. Radiol. 2013, 82, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Donati, O.F.; Chong, D.; Nanz, D.; Boss, A.; Froehlich, J.M.; Andres, E.; Seifert, B.; Thoeny, H.C. Diffusion-weighted MR imaging of upper abdominal organs: Field strength and intervendor variability of apparent diffusion coefficients. Radiology 2014, 270, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Matsui, O.; Kobayashi, S.; Sanada, J.; Kouda, W.; Ryu, Y.; Kozaka, K.; Kitao, A.; Nakamura, K.; Gabata, T. Hepatocelluar nodules in liver cirrhosis: Hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom. Imaging 2011, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Tateishi, R.; Akahane, M.; Mikami, S.; Sato, M.; Uchino, K.; Arano, T.; Enooku, K.; Kondo, Y.; Yamashiki, N.; et al. CT with hepatic arterioportography as a pretreatment examination for hepatocellular carcinoma patients: A randomized controlled trial. Am. J. Gastroenterol. 2013, 108, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Oi, H.; Hori, M.; Kim, T.; Takahashi, S.; Tomoda, K.; Narumi, Y.; Nakamura, H. Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. Am. J. Roentgenol. 1997, 169, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Tajima, T.; Taguchi, K.; Kuroiwa, T.; Yoshimitsu, K.; Irie, H.; Aibe, H.; Shinozaki, K.; Asayama, Y.; Shimada, M.; et al. Recent developments in imaging diagnostics for HCC: CT arteriography and CT arterioportography evaluation of vascular changes in premalignant and malignant hepatic nodules. J. Hepatobiliary Pancreat. Surg. 2000, 7, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kwak, H.S.; Han, Y.M.; Kim, C.S. Usefulness of combining sequentially acquired gadobenate dimeglumine-enhanced magnetic resonance imaging and resovist-enhanced magnetic resonance imaging for the detection of hepatocellular carcinoma: Comparison with computed tomography hepatic arteriography and computed tomography arterioportography using 16-slice multidetector computed tomography. J. Comput. Assist. Tomogr. 2007, 31, 702–711. [Google Scholar] [PubMed]
- Cenic, A.; Nabavi, D.G.; Craen, R.A.; Gelb, A.W.; Lee, T.Y. Dynamic CT measurement of cerebral blood flow: A validation study. AJNR Am. J. Neuroradiol. 1999, 20, 63–73. [Google Scholar] [PubMed]
- Cuenod, C.; Leconte, I.; Siauve, N.; Resten, A.; Dromain, C.; Poulet, B.; Frouin, F.; Clement, O.; Frija, G. Early changes in liver perfusion caused by occult metastases in rats: Detection with quantitative CT. Radiology 2001, 218, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Kartalis, N.; Grigoriadis, A.; Loizou, L.; Stal, P.; Leidner, B.; Aspelin, P.; Brismar, T.B. Perfusion computed tomography for detection of hepatocellular carcinoma in patients with liver cirrhosis. Eur. Radiol. 2015, 25, 3123–3132. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Bonaffini, P.A.; Capraro, C.; Leni, D.; Corso, R.; Sironi, S. Viable residual tumor tissue after radiofrequency ablation treatment in hepatocellular carcinoma: Evaluation with CT perfusion. Abdom. Imaging 2013, 38, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.; Horger, T.; Oelker, A.; Kloth, C.; Nikolaou, K.; Schulze, M.; Horger, M. Characterization of hepatocellular carcinoma (HCC) lesions using a novel CT-based volume perfusion (VPCT) technique. Eur. J. Radiol. 2015, 84, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Kambadakone, A.; Kulkarni, N.M.; Zhu, A.X.; Sahani, D.V. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST). Investig. Radiol. 2012, 47, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Bonaffini, P.A.; Ratti, L.; Antolini, L.; Corso, R.; Fazio, F.; Sironi, S. Hepatocellular carcinoma treated with transarterial chemoembolization: Dynamic perfusion-CT in the assessment of residual tumor. World J. Gastroenterol. 2010, 16, 5993–6000. [Google Scholar] [PubMed]
- Okada, M.; Kim, T.; Murakami, T. Hepatocellular nodules in liver cirrhosis: State of the art CT evaluation (perfusion CT/volume helical shuttle scan/dual-energy CT, etc.). Abdom. Imaging 2011, 36, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Sironi, S.; Pozzi, M.; Antolini, L.; Invernizzi, F.; Ratti, L.; Leone, E.B.; Fazio, F. Perfusion CT in cirrhotic patients with early stage hepatocellular carcinoma: Assessment of tumor-related vascularization. Eur. J. Radiol. 2010, 73, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Bapst, B.; Lagadec, M.; Breguet, R.; Vilgrain, V.; Ronot, M. Cone beam computed tomography (CBCT) in the field of interventional oncology of the liver. Cardiovasc. Interv. Radiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Huppert, P.E.; Firlbeck, G.; Meissner, O.A.; Wietholtz, H. C-arm-CT bei der chemoembolisation von lebertumoren. Radiology 2009, 49, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Lucatelli, P.; Corona, M.; Argirò, R.; Anzidei, M.; Vallati, G.; Fanelli, F.; Bezzi, M.; Catalano, C. Impact of 3D rotational angiography on liver embolization procedures: Review of technique and applications. Cardiovasc. Interv. Radiol. 2015, 38, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoven, A.; Prince, J.; de Keizer, B.; Vonken, E.-J.A.; Bruijnen, R.G.; Verkooijen, H.; Lam, M.E.H.; van den Bosch, M.A.J. Use of C-arm cone beam CT during hepatic radioembolization: Protocol optimization for extrahepatic shunting and parenchymal enhancement. Cardiovasc. Interv. Radiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, P.M.; Kane, P.A.; Ryder, S.D.; Ramage, J.K.; Gane, E.; Tan, K.C.; Portmann, B.; Karani, J.; Williams, R. Accuracy of radiology in detection of hepatocellular carcinoma before liver transplantation. Gastroenterology 1994, 107, 1425–1429. [Google Scholar] [CrossRef]
- Santis, M.D.; Romagnoli, R.; Cristani, A.; Cioni, G.; Casolo, A.; Vici, F.F.; Ventura, E. MRI of small hepatocellular carcinoma: Comparison with US, CT, DSA, and lipiodol-CT. J. Comput. Assist. Tomogr. 1992, 16, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Sumida, M.; Ohto, M.; Ebara, M.; Kimura, K.; Okuda, K.; Hirooka, N. Accuracy of angiography in the diagnosis of small hepatocellular carcinoma. Am. J. Roentgenol. 1986, 147, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Tacher, V.; Lin, M.; Duran, R.; Yarmohammadi, H.; Lee, H.; Chapiro, J.; Chao, M.; Wang, Z.; Frangakis, C.; Sohn, J.H.; et al. Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach. Radiology 2015, 142951. [Google Scholar] [CrossRef] [PubMed]
- Kakeda, S.; Korogi, Y.; Ohnari, N.; Moriya, J.; Oda, N.; Nishino, K.; Miyamoto, W. Usefulness of cone-beam volume CT with flat panel detectors in conjunction with catheter angiography for transcatheter arterial embolization. J. Vasc. Interv. Radiol. 2007, 18, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Tognolini, A.; Louie, J.D.; Hwang, G.L.; Hofmann, L.V.; Sze, D.Y.; Kothary, N. Utility of C-arm CT in patients with hepatocellular carcinoma undergoing transhepatic arterial chemoembolization. J. Vasc. Interv. Radiol. 2010, 21, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Loffroy, R.; Lin, M.; Rao, P.; Bhagat, N.; Noordhoek, N.; Radaelli, A.; Blijd, J.; Geschwind, J.-F. Comparing the detectability of hepatocellular carcinoma by C-arm dual-phase cone-beam computed tomography during hepatic arteriography with conventional contrast-enhanced magnetic resonance imaging. Cardiovasc. Interv. Radiol. 2012, 35, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.F.; Lin, C.J.; Chen, W.S.; Hung, S.C.; Chiu, C.F.; Wu, T.H.; Guo, W.Y. Radiation doses of cerebral blood volume measurements using C-arm CT: A phantom study. Am. J. Neuro Radiol. 2014, 35, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Fiorella, D.; Turk, A.; Chaudry, I.; Turner, R.; Dunkin, J.; Roque, C.; Sarmiento, M.; Deuerling-Zheng, Y.; Denice, C.M.; Baumeister, M.; et al. A prospective, multicenter pilot study investigating the utility of flat detector derived parenchymal blood volume maps to estimate cerebral blood volume in stroke patients. J. Neuroint. Surg. 2014, 6, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Peynircioğlu, B.; Hızal, M.; Barbaros, Ç.B.; Deuerling-Zheng, Y.; Von Roden, M.; Hazırolan, T.; Akata, D.; Özmen, M.; Balkancı, F. Quantitative liver tumor blood volume measurements by a C-arm CT post-processing software before and after hepatic arterial embolization therapy: Comparison with MDCT perfusion. Diagn. Interv. Radiol. 2015, 21, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Schaefer, P.; Lehnert, T.; Nour-Eldin, N.-E.; Ackermann, H.; Mbalisike, E.; Hammerstingl, R.; Eichler, K.; Zangos, S.; Naguib, N.N. Intraprocedural blood volume measurement using C-arm CT as a predictor for treatment response of malignant liver tumors undergoing repetitive transarterial chemoembolization (TACE). Eur. Radiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-G.; Zhang, X.-B.; Han, J.-F.; Beilner, J.; Deuerling-Zheng, Y.; Chi, J.-C.; Wang, J.; Qian, L.-J.; Zhou, Y.; Xu, J.-R. Hepatic blood volume imaging with the use of flat-detector CT perfusion in the angiography suite: Comparison with results of conventional multislice CT perfusion. J. Vasc. Interv. Radiol. 2014, 25, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, A.H.; Weinreb, J.C. The role of imaging in hepatocellular carcinoma: The present and future. J. Clin. Gastroenterol. 2013, 47, S7–S10. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schraml, C.; Kaufmann, S.; Rempp, H.; Syha, R.; Ketelsen, D.; Notohamiprodjo, M.; Nikolaou, K. Imaging of HCC—Current State of the Art. Diagnostics 2015, 5, 513-545. https://doi.org/10.3390/diagnostics5040513
Schraml C, Kaufmann S, Rempp H, Syha R, Ketelsen D, Notohamiprodjo M, Nikolaou K. Imaging of HCC—Current State of the Art. Diagnostics. 2015; 5(4):513-545. https://doi.org/10.3390/diagnostics5040513
Chicago/Turabian StyleSchraml, Christina, Sascha Kaufmann, Hansjoerg Rempp, Roland Syha, Dominik Ketelsen, Mike Notohamiprodjo, and Konstantin Nikolaou. 2015. "Imaging of HCC—Current State of the Art" Diagnostics 5, no. 4: 513-545. https://doi.org/10.3390/diagnostics5040513
APA StyleSchraml, C., Kaufmann, S., Rempp, H., Syha, R., Ketelsen, D., Notohamiprodjo, M., & Nikolaou, K. (2015). Imaging of HCC—Current State of the Art. Diagnostics, 5(4), 513-545. https://doi.org/10.3390/diagnostics5040513




