Mini-P-gp and P-gp Co-Expression in Brown Trout Erythrocytes: A Prospective Blood Biomarker of Aquatic Pollution
Abstract
:1. Introduction
2. Experimental Section
2.1. Fish and Capture
2.2. Anesthesia and Blood Samples
2.3. Ethics
2.4. Erythrocyte Primary Culture
2.5. BaP Solubilization and Cell Culture Exposure
2.6. Flow Cytometry Analysis of BaP Uptake
2.7. MDR Expression Studied by Western Blot Analysis
2.8. River Water Chemical Pollutants Analyse
2.8.1. Extraction Procedure
2.8.2. High Performance Liquid Chromatography (HPLC) Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Basal Expression of Two P-gps in Brown Trout Erythrocytes
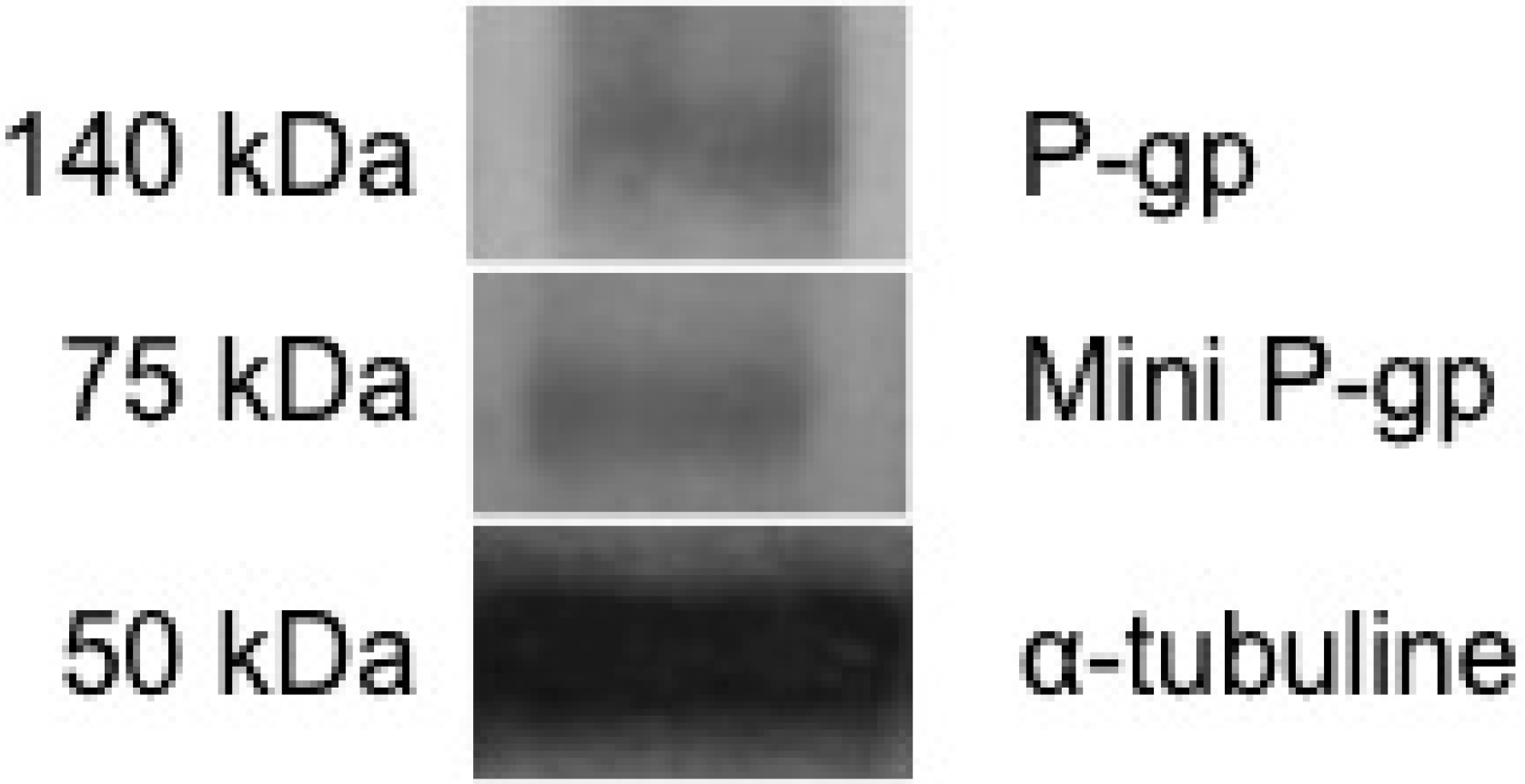
3.2. Benzo[a]Pyrene Uptake by Brown Trout Erythrocytes

3.3. Co-Expression of Mini-P-gp and P-gp in Primary Culture of Trout Erythrocytes after Exposure to BaP
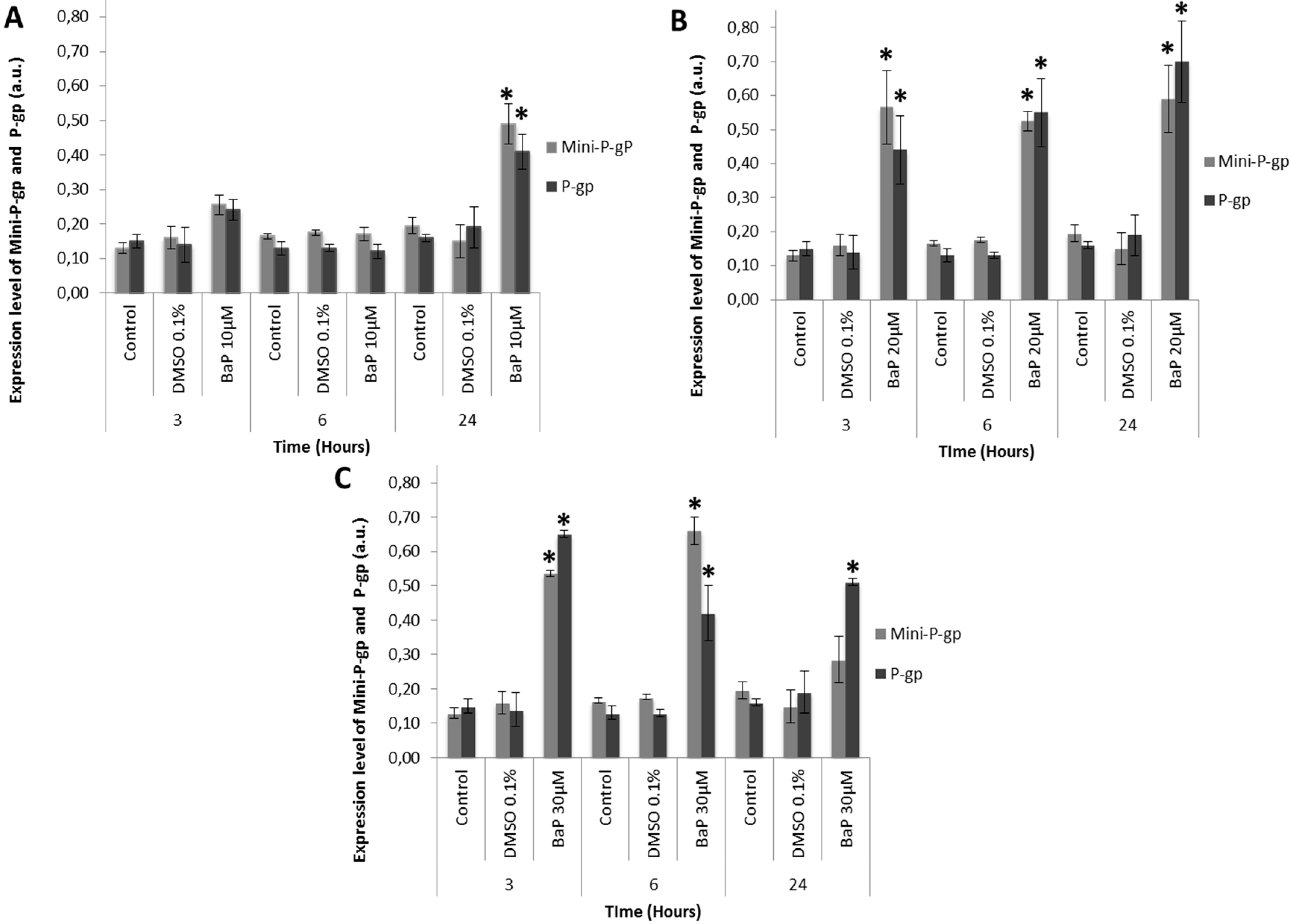
3.4. Mini-P-gp and P-gp Co-Expression Levels in Brown Trout Erythrocytes from Rivers
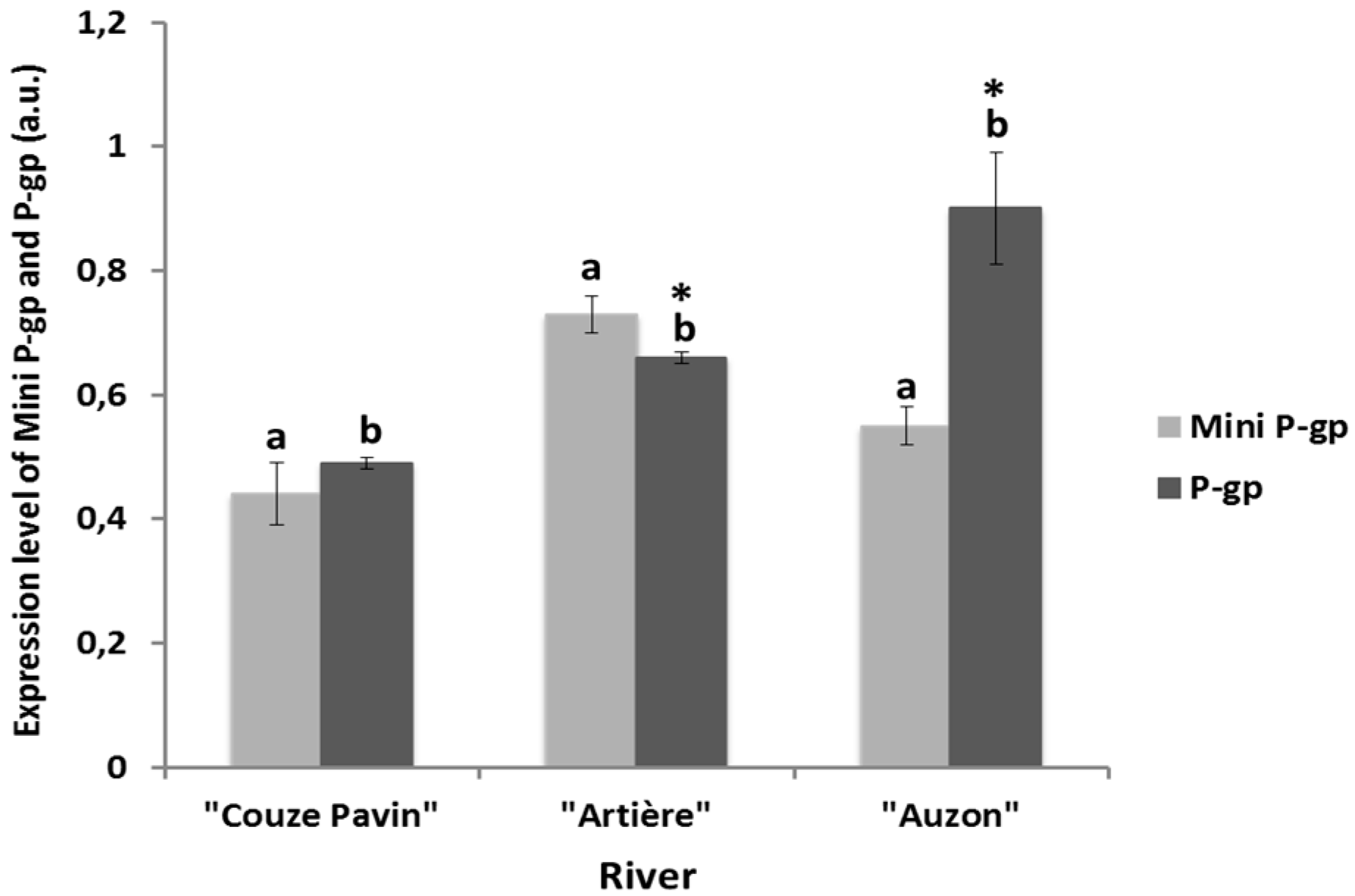
3.5. Pollution Analysis of the River Water
| River | GPS Coordinates of Sampling Sites | PAH (μg/L) | Multi-Residue Pesticide (μg/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Acenaphtene | AMPA | Oxadiazon | Atrazine | Coumatetralyl | Diuron | DCPMU | Myclobutanil | ||
| Couze Pavin | N 45°30'54.7" E 002°55'24.3" | 0.030 | - | - | - | 0.009 | - | - | - |
| Artière | N 45°45'10.7" E 003°07'13.4" | - | 0.120 | 0.021 | <LOQ | - | 0.005 | 0.008 | - |
| Auzon | N 45°42'53.0" E 003°11'09.9" | - | 0.100 | 0.017 | <LOQ | 0.022 | <LOQ | <LOQ | 0.005 |
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beaufrere, H.; Ammersbach, M.; Tully, T.N., Jr. Complete blood cell count in psittaciformes by using high-throughput image cytometry: A pilot study. J. Avian Med. Surg. 2013, 27, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Nardini, G.; Leopardi, S.; Bielli, M. Clinical hematology in reptilian species. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, A.A. Red blood system in fish: Peculiarities of its organisation and function (in Russian). Zh. Evol. Biokhim. Fiziol. 2005, 41, 217–223. [Google Scholar] [PubMed]
- Phalen, L.J.; Kollner, B.; Leclair, L.A.; Hogan, N.S.; van den Heuvel, M.R. The effects of benzo[a]pyrene on leucocyte distribution and antibody response in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2014, 147, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Valton, E.; Amblard, C.; Wawrzyniak, I.; Penault-Llorca, F.; Bamdad, M. P-gp expression in brown trout erythrocytes: Evidence of a detoxification mechanism in fish erythrocytes. Sci. Rep. 2013. [Google Scholar] [CrossRef]
- Zelnickova, L.; Blahova, J.; Marsalek, P.; Hostovsky, M.; Sevcikova, M.; Dobsikova, R.; Siroka, Z.; Divisova, L.; Plhalova, L.; Svobodova, Z. Biochemical markers for the assessment of pollution of selected small streams in the czech republic. Neuro Endocrinol. Lett. 2013, 34, 109–115. [Google Scholar] [PubMed]
- Sarkadi, B.; Homolya, L.; Szakacs, G.; Varadi, A. Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Phys. Rev. 2006, 86, 1179–1236. [Google Scholar] [CrossRef]
- Gerlach, J.H.; Endicott, J.A.; Juranka, P.F.; Henderson, G.; Sarangi, F.; Deuchars, K.L.; Ling, V. Homology between p-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature 1986, 324, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Bottova, I.; Hehl, A.B.; Stefanic, S.; Fabrias, G.; Casas, J.; Schraner, E.; Pieters, J.; Sonda, S. Host cell P-glycoprotein is essential for cholesterol uptake and replication of Toxoplasma gondii. J. Biol. Chem. 2009, 284, 17438–17448. [Google Scholar] [CrossRef] [PubMed]
- Krogstad, D.J.; Gluzman, I.Y.; Herwaldt, B.L.; Schlesinger, P.H.; Wellems, T.E. Energy dependence of chloroquine accumulation and chloroquine efflux in Plasmodium falciparum. Biochem. Pharmacol. 1992, 43, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, M.; Brousseau, P.; Denizeau, F. Identification of a multidrug resistance-like system in Tetrahymena pyriformis: Evidence for a new detoxication mechanism in freshwater ciliates. FEBS Lett. 1999, 456, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, M.; Reader, S.; Grolière, C.A.; Bohatier, J.; Denizeau, F. Uptake and efflux of polycyclic aromatic hydrocarbons by “Tetrahymena pyriformis”: Evidence for a resistance mechanism. Cytometry 1997, 28, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kurelec, B. The multixenobiotic resistance mechanism in aquatic organisms. Crit. Rev. Toxicol. 1992, 22, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Loncar, J.; Popovic, M.; Zaja, R.; Smital, T. Gene expression analysis of the abc efflux transporters in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zaja, R.; Klobucar, R.S.; Smital, T. Detection and functional characterization of Pgp1 (ABCB1) and MRP3 (ABCC3) efflux transporters in the PLHC-1 fish hepatoma cell line. Aquat. Toxicol. 2007, 81, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Vache, C.; Camares, O.; Cardoso-Ferreira, M.C.; Dastugue, B.; Creveaux, I.; Vaury, C.; Bamdad, M. A potential genomic biomarker for the detection of polycyclic aromatic hydrocarbon pollutants: Multidrug resistance gene 49 in Drosophila melanogaster. Environ. Toxicol. Chem. 2007, 26, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Vache, C.; Camares, O.; De Graeve, F.; Dastugue, B.; Meiniel, A.; Vaury, C.; Pellier, S.; Leoz-Garziandia, E.; Bamdad, M. Drosophila melanogaster p-glycoprotein: A membrane detoxification system toward polycyclic aromatic hydrocarbon pollutants. Environ. Toxicol. Chem. 2006, 25, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Dudler, R.; Sidler, M. Arabidopsis mdr genes: Molecular cloning and protein chemical aspects. Methods Enzymol. 1998, 292, 162–173. [Google Scholar] [PubMed]
- Geisler, M.; Blakeslee, J.J.; Bouchard, R.; Lee, O.R.; Vincenzetti, V.; Bandyopadhyay, A.; Titapiwatanakun, B.; Peer, W.A.; Bailly, A.; Richards, E.L.; et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005, 44, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. BBA Biomembr. 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Drach, J.; Zhao, S.; Drach, D.; Korbling, M.; Engel, H.; Andreeff, M. Expression of MDR1 by normal bone marrow cells and its implication for leukemic hematopoiesis. Leuk. Lymphoma 1995, 16, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Stavrovskaya, A.; Turkina, A.; Sedyakhina, N.; Stromskaya, T.; Zabotina, T.; Khoroshko, N.; Baryshnikov, A. Prognostic value of P-glycoprotein and leukocyte differentiation antigens in chronic myeloid leukemia. Leuk. Lymphoma 1998, 28, 469–482. [Google Scholar] [PubMed]
- Pilarski, L.M.; Belch, A.R. Intrinsic expression of the multidrug transporter, P-glycoprotein 170, in multiple myeloma: Implications for treatment. Leuk. Lymphoma 1995, 17, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Fiche de données toxicologiques et environnementales des substances chimiques. (in French). Available online: http://www.ineris.fr/rapports-d%C3%A9tude/toxicologie-et-environnement/fiches-de-donn%C3%A9es-toxicologiques-et-environnementales-d (accessed on 4 January 2015).
- Menzie, C.A.; Potocki, B.B.; Santodonato, J. Exposure to carcinogenic pahs in the environment. Environ. Sci. Technol. 1992, 26, 1278–1284. [Google Scholar] [CrossRef]
- Sturm, A.; Ziemann, C.; Hirsch-Ernst, K.I.; Segner, H. Expression and functional activity of P-glycoprotein in cultured hepatocytes from Oncorhynchus mykiss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1119–R1126. [Google Scholar] [PubMed]
- Kawai, K.; Kusano, I.; Ido, M.; Sakurai, M.; Shiraishi, T.; Yatani, R. Identification of a P-glycoprotein-related protein (mini-P-glycoprotein) which is overexpressed in multidrug resistant cells. Biochem. Biophys. Res. Commun. 1994, 198, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Oshimi, K. NK cell lymphoma. Int. J. Hematol. 2002, 76, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Prevodnik, A.; Lilja, K.; Bollner, T. Benzo[a]pyrene up-regulates the expression of the proliferating cell nuclear antigen (PCNA) and multixenobiotic resistance polyglycoprotein (P-gp) in baltic sea blue mussels (Mytilus edulis L.). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, N.; Toyama, K.; Michihara, A.; Akasaki, K.; Tsuji, H.; Furuno, K. Effect of benzo[a]pyrene on P-glycoprotein-mediated transport in Caco-2 cell monolayer. Toxicology 2006, 223, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, N.; Toyama, K.; Okamoto, T.; Kadowaki, M.; Terao, K.; Furuno, K. Effects of benzo(e)pyrene and benzo(a)pyrene on P-glycoprotein-mediated transport in Caco-2 cell monolayer: A comparative approach. Toxicol. Vitro 2007, 21, 827–834. [Google Scholar] [CrossRef]
- Agarwala, S.; Chen, W.; Cook, T.J. Effect of chlorpyrifos on efflux transporter gene expression and function in Caco-2 cells. Toxicol. Vitro 2004, 18, 403–409. [Google Scholar] [CrossRef]
- Bard, S.M. Multixenobiotic resistance as a cellular defense mechanism in aquatic organisms. Aquat. Toxicol. 2000, 48, 357–389. [Google Scholar] [CrossRef] [PubMed]
- Einicker-Lamas, M.; Morales, M.M.; Miranda, K.; Garcia-Abreu, J.; Oliveira, A.J.F.; Silva, F.L.S.; Oliveira, M.M. P-glycoprotein-like protein contributes to cadmium resistance in Euglena gracilis. J. Comp. Physiol. B 2003, 173, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Habibollahi, P.; Ghahremani, M.H.; Azizi, E.; Ostad, S.N. Multi drug resistance-1 (MDR1) expression in response to chronic diazinon exposure: An in vitro study on Caco-2 cells. Bull. Environ. Contam. Toxicol. 2011, 86, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; van Bambeke, F. ABC multidrug transporters: Target for modulation of drug pharmacokinetics and drug-drug interactions. Curr. Drug Targets 2011, 12, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; DiStasio, S.; Zhao, J.; Cardozo, C.P.; Wolff, M.S.; Caplan, A.J. Differential effects of the organochlorine pesticide DDT and its metabolite p,p'-DDE on p-glycoprotein activity and expression. Toxicol. Appl. Pharmacol. 2005, 203, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Urayama, K.Y.; Wiencke, J.K.; Buffler, P.A.; Chokkalingam, A.P.; Metayer, C.; Wiemels, J.L. MDR1 gene variants, indoor insecticide exposure, and the risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1172–1177. [Google Scholar] [CrossRef]
- Pampanin, D.M.; Viarengo, A.; Garrigues, P.; Andersen, O.K. Background for the beep stavanger workshops: Biological effects on marine organisms in two common, large, laboratory experiments and in a field study: Comparison of the value (sensitivity, specificity, etc.) of core and new biomarkers. Aquat. Toxicol. 2006, 78, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Andru, J.; Cosson, J.F.; Caliman, J.P.; Benoit, E. Coumatetralyl resistance of rattus tanezumi infesting oil palm plantations in indonesia. Ecotoxicology 2013, 22, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Gschwind, L.; Rollason, V.; Daali, Y.; Bonnabry, P.; Dayer, P.; Desmeules, J.A. Role of p-glycoprotein in the uptake/efflux transport of oral vitamin K antagonists and rivaroxaban through the Caco-2 cell model. Basic Clin. Pharmacol. Toxicol. 2013, 113, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Stollberger, C.; Finsterer, J. Reservations against new oral anticoagulants after stroke and cerebral bleeding. J. Neurol. Sci. 2013, 330, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Costa, J.; Reis-Henriques, M.A. ABC transporters in fish species: A review. Front. Physiol. 2014. [Google Scholar] [CrossRef]
- Raaijmakers, M.H. ATP-binding-cassette transporters in hematopoietic stem cells and their utility as therapeutical targets in acute and chronic myeloid leukemia. Leukemia 2007, 21, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valton, E.; Amblard, C.; Desmolles, F.; Combourieu, B.; Penault-Llorca, F.; Bamdad, M. Mini-P-gp and P-gp Co-Expression in Brown Trout Erythrocytes: A Prospective Blood Biomarker of Aquatic Pollution. Diagnostics 2015, 5, 10-26. https://doi.org/10.3390/diagnostics5010010
Valton E, Amblard C, Desmolles F, Combourieu B, Penault-Llorca F, Bamdad M. Mini-P-gp and P-gp Co-Expression in Brown Trout Erythrocytes: A Prospective Blood Biomarker of Aquatic Pollution. Diagnostics. 2015; 5(1):10-26. https://doi.org/10.3390/diagnostics5010010
Chicago/Turabian StyleValton, Emeline, Christian Amblard, François Desmolles, Bruno Combourieu, Frédérique Penault-Llorca, and Mahchid Bamdad. 2015. "Mini-P-gp and P-gp Co-Expression in Brown Trout Erythrocytes: A Prospective Blood Biomarker of Aquatic Pollution" Diagnostics 5, no. 1: 10-26. https://doi.org/10.3390/diagnostics5010010
APA StyleValton, E., Amblard, C., Desmolles, F., Combourieu, B., Penault-Llorca, F., & Bamdad, M. (2015). Mini-P-gp and P-gp Co-Expression in Brown Trout Erythrocytes: A Prospective Blood Biomarker of Aquatic Pollution. Diagnostics, 5(1), 10-26. https://doi.org/10.3390/diagnostics5010010





