Endoscopic Optical Coherence Tomography for Clinical Gastroenterology
Abstract
:1. Introduction
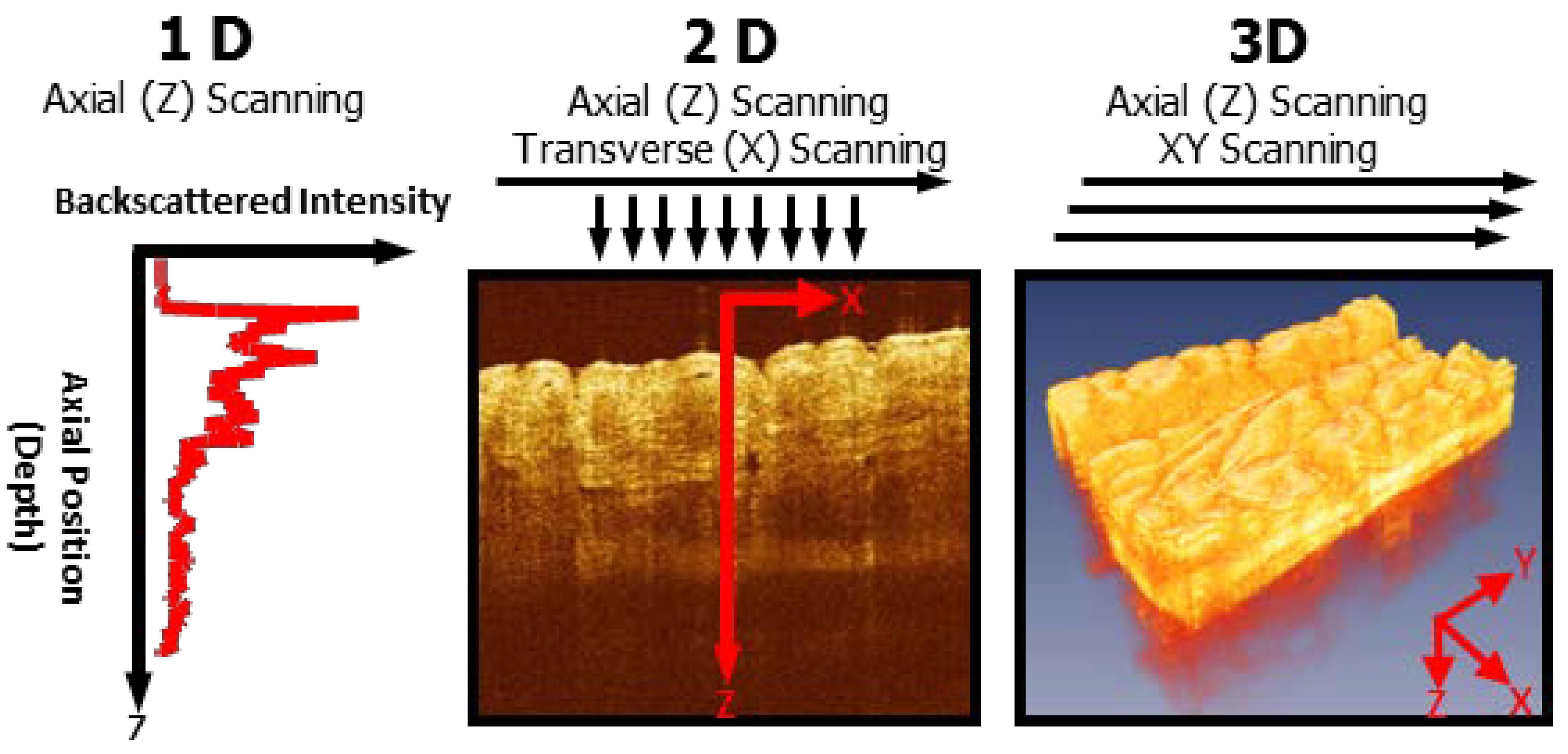
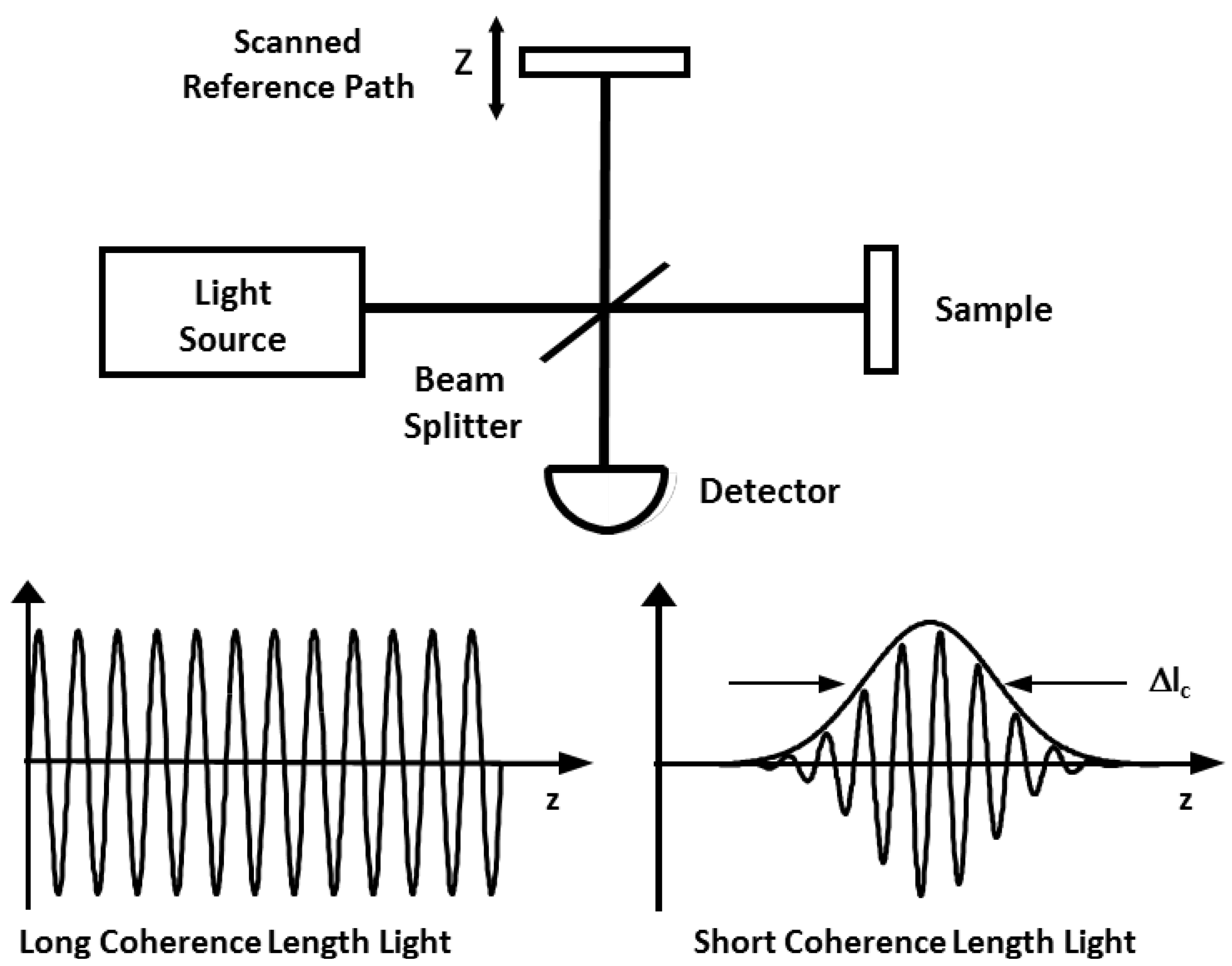
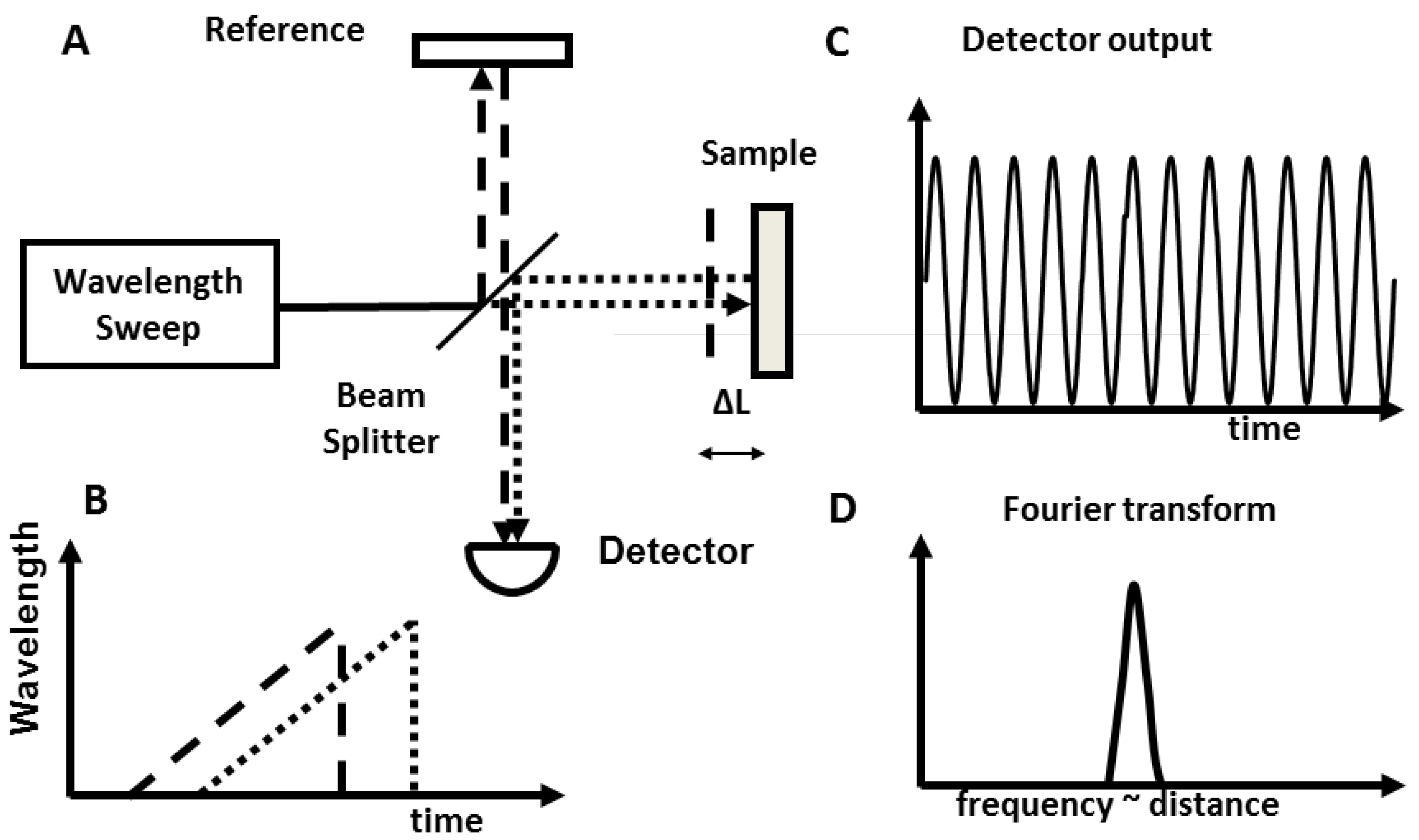
2. Utility of Endoscopic OCT in the Gastrointestinal Tract
2.1. Detection of Diseases in the GI Tract
2.2. Assessment of Endoscopic Therapies for GI Diseases
3. Development of Endoscopic OCT
3.1. Earlier Clinical Studies Using Endoscopic OCT
3.2. Important Advancement Using High Speed Fourier Domain OCT
3.3. Comparison between Different Endoscopic Imaging Technologies
| Imaging Modality | Depth | Axial Resolution | Pros | Cons |
|---|---|---|---|---|
| Ultrasound | 1–10 cm | 50 µm | Broad field Standard for staging tumors despite improvements in CT and MR | Location of neoplasia Low resolution |
| Chromoendoscopy | N/A | N/A | Enhanced endoscopic imaging contrast | Superficial imaging Large volume dye required |
| NBI | N/A | N/A | Enhanced endoscopic imaging contrast No contrast agent required | Superficial imaging |
| CLE | <250 µm | 1–5 µm | Cellular resolution Point and view | Limited imaging depth Limited field of view IV contrast required |
| LCI | 200–300 µm | N/A | Assessment of nuclear size Red flag technology No contrast agent required | Limited detection depth |
| OCT | 3 mm | 5–30 µm | No contrast agent required 3D visualization of tissue structure | Can’t image fluorescence 3D presently not real-time |
3.4. Imaging Catheter Designs Using Different Scanning Mechanism
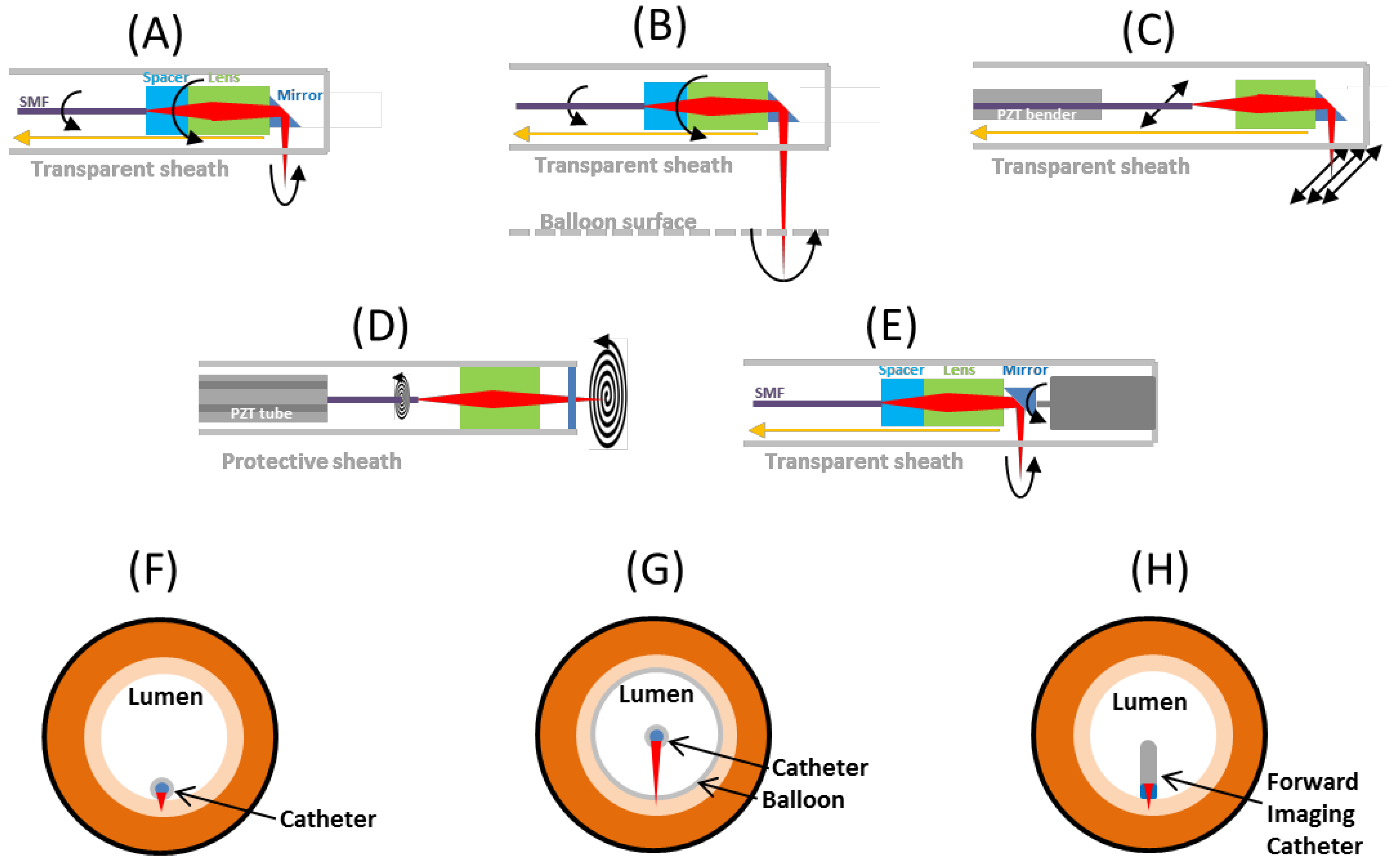
3.4.1. Proximal Rotary Side Imaging
3.4.2. Proximal Rotary Balloon Imaging
3.4.3. Raster Scan Side Imaging
3.4.4. Spiral Scan Forward Imaging
| Scanning Mechanisms | Advantage | Disadvantage |
|---|---|---|
| Proximally rotary | Simple optical design Small No aberration Moderate scanning range (1–4 cm2) Water can be used to reduce reflections/aberration | Rotary non-uniformity Pull back non-uniformity Unstable slow scan |
| Rotary with single balloon | Simple optical design Small More stable for slow scan Large scanning range (>30 cm2) Cover the whole lumen circumference Water can be used to reduce reflections/aberration | Rotary non-uniformity Deformation of tissue Probe off-centered Aberration from sheath |
| Rotary with double balloon | Simple optical design Small More stable for slow scan No tissue deformation Large scanning range (>30 cm2) Cover the whole lumen circumference Water can be used to reduce reflections/aberration | Requires balloon and inflation Rotary non-uniformity Probe off-centered Aberration from sheath Imaging location limited |
| Raster scan side (perpendicular) | Higher scanning speed Moderate scanning range No fast scan non-uniformity | Image area is long strip Larger probe Sinusoidal wave correction Sensitive to perturbation Aberration from scanning at high angle to sheath Water cannot be used |
| Spiral/Lissajou forward scan | Small Simple design No fast scan non-uniformity Simultaneous 2D scan | Limited imaging range Scanning pattern correction Sensitive to perturbation Slower scanning speed |
| Micromotor | Highest scanning speed No fast scan non-uniformity Large scanning range Could incorporate with other design | Proximal pullback required Field of view blocked by electric cables |
4. Recent Achievements of Endoscopic OCT in Clinical Gastroenterology
4.1. Laser Marking and OCT Guided Biopsy

4.2. Cancer Classification
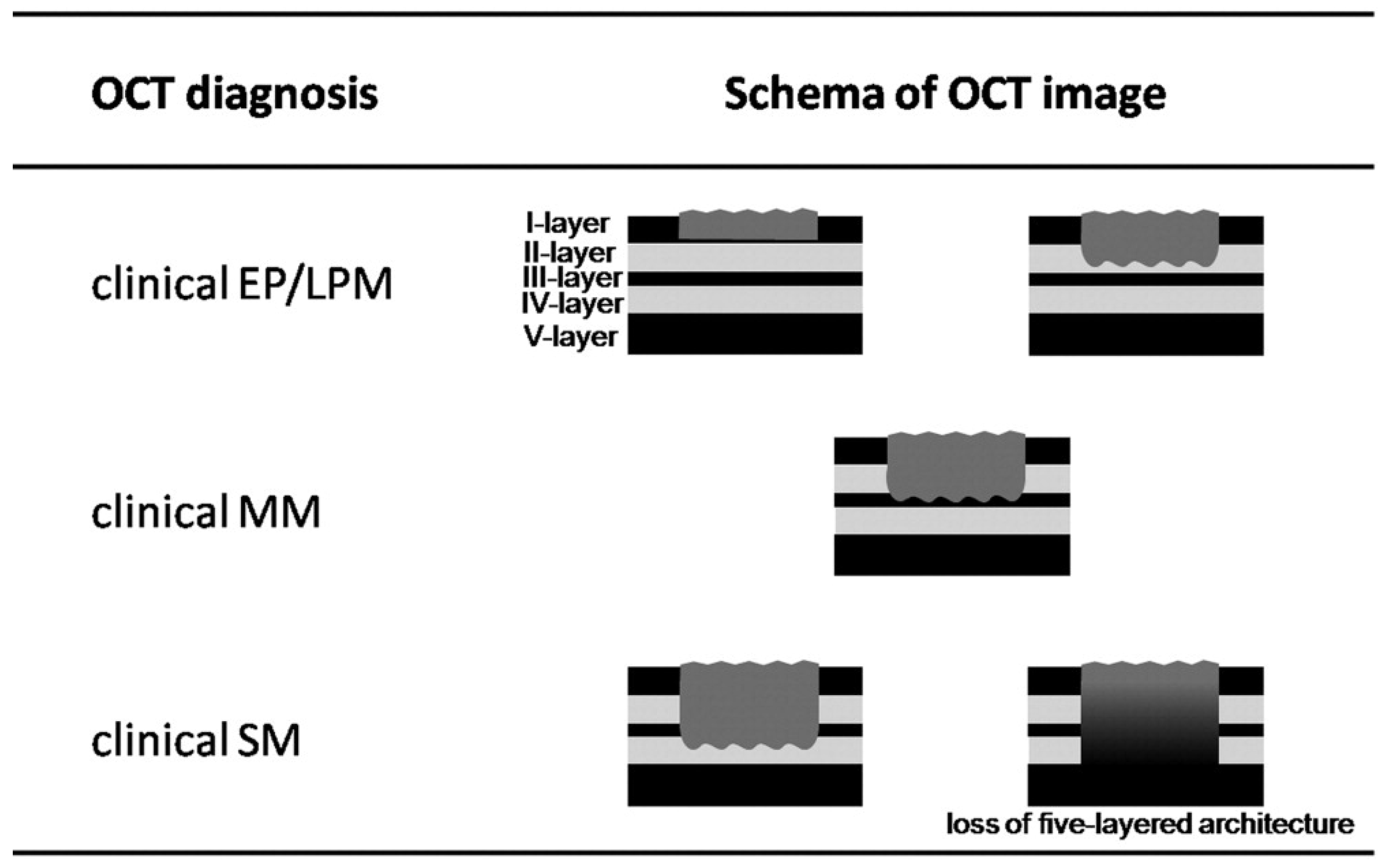
4.3. Investigation of Subsquamous Intestinal Metaplasia
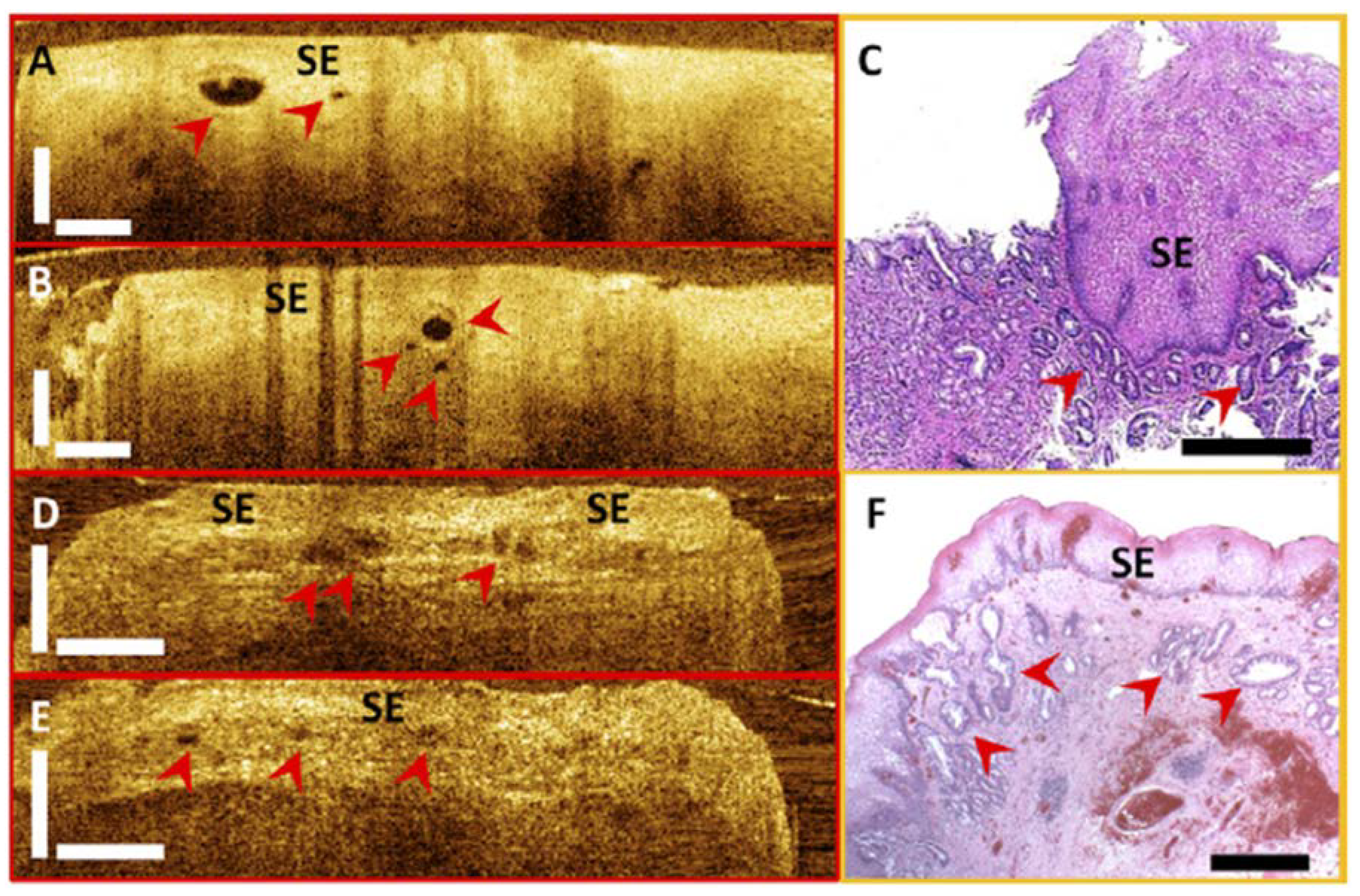
4.4. RFA Treatment Response Evaluation
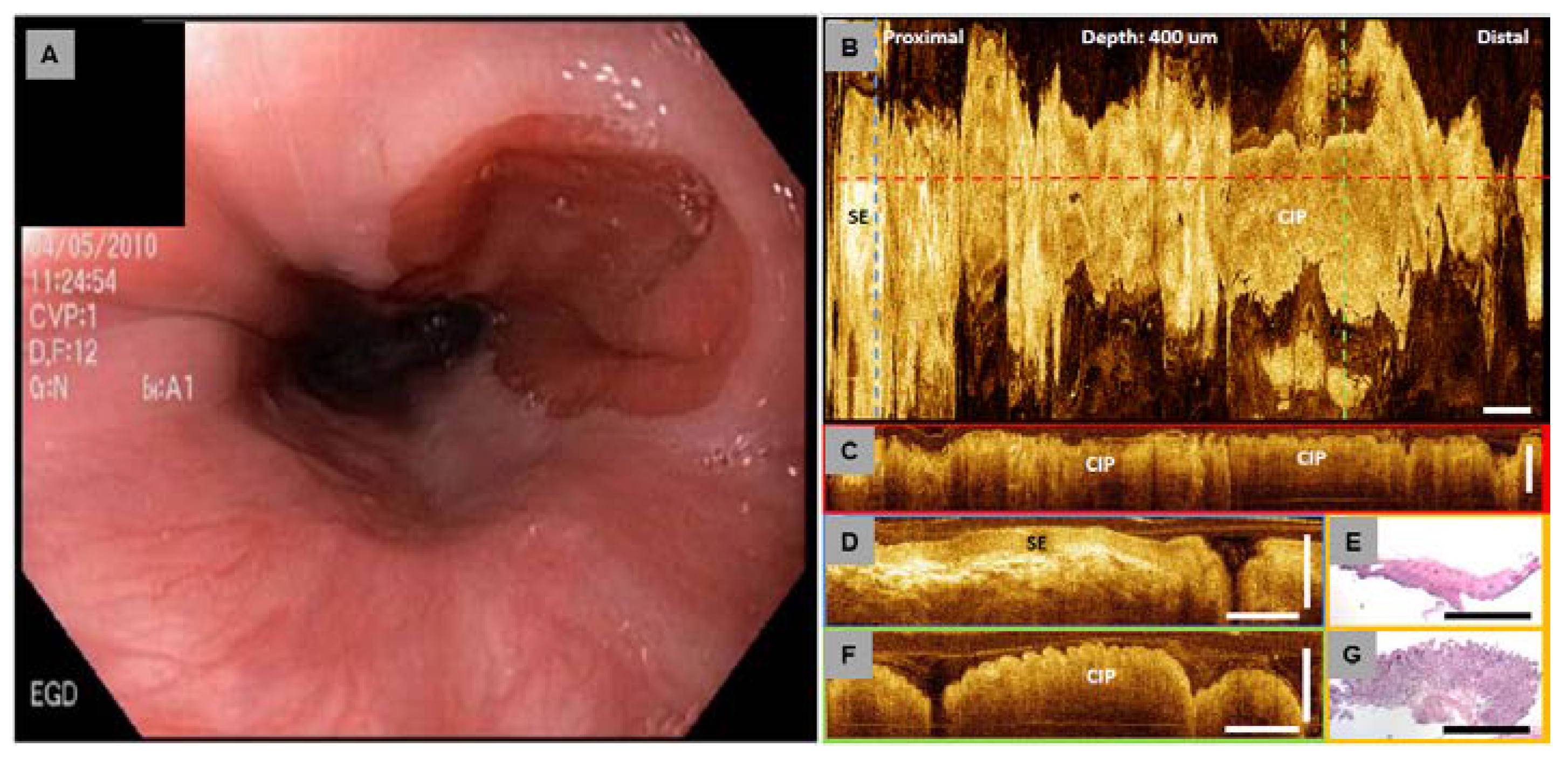
4.5. Investigation of Cervical Inlet Patch
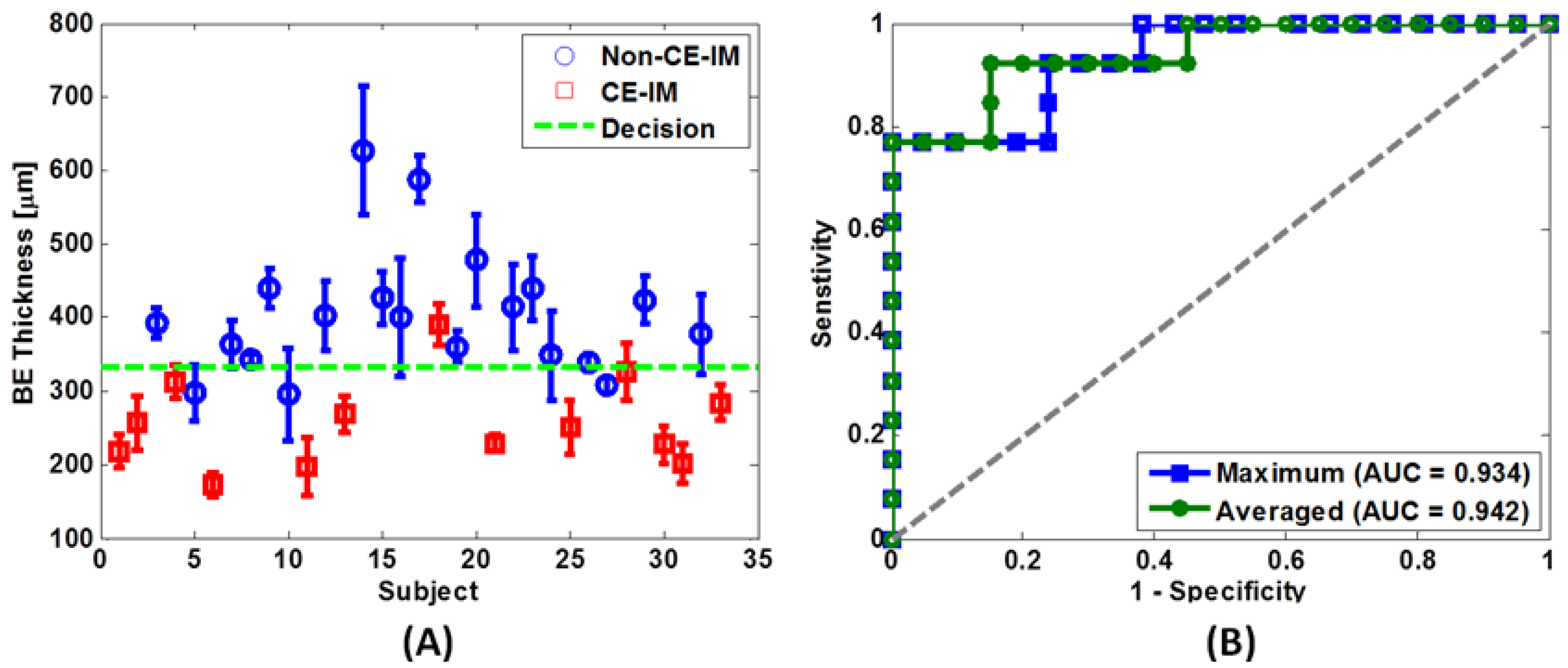
4.6. Diagnostic Accuracy Using Endoscopic OCT Imaging
5. Future Applications
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.A.; Brezinski, M.E. Optical coherence tomography: An emerging technology for biomedical imaging and optical biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef]
- Fujimoto, J.G. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat. Biotechnol. 2003, 21, 1361–1367. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Brezinski, M.E.; Tearney, G.J.; Boppart, S.A.; Bouma, B.; Hee, M.R.; Southern, J.F.; Swanson, E.A. Optical biopsy and imaging using optical coherence tomography. Nat. Med. 1995, 1, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, M.E.; Tearney, G.J.; Bouma, B.E.; Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Southern, J.F.; Fujimoto, J.G. Optical coherence tomography for optical biopsy. Properties and demonstration of vascular pathology. Circulation 1996, 93, 1206–1213. [Google Scholar] [CrossRef]
- Tearney, G.J.; Brezinski, M.E.; Bouma, B.E.; Boppart, S.A.; Pitvis, C.; Southern, J.F.; Fujimoto, J.G. In vivo endoscopic optical biopsy with optical coherence tomography. Science 1997, 276, 2037–2039. [Google Scholar] [CrossRef]
- Takada, K.; Yokohama, I.; Chida, K.; Noda, J. New measurement system for fault location in optical waveguide devices based on an interferometric technique. Appl. Opt. 1987, 26, 1603–1608. [Google Scholar] [CrossRef]
- Gilgen, H.H.; Novak, R.P.; Salathe, R.P.; Hodel, W.; Beaud, P. Submillimeter optical reflectometry. IEEE J. Lightwave Technol. 1989, 7, 1225–1233. [Google Scholar] [CrossRef]
- Youngquist, R.; Carr, S.; Davies, D. Optical coherence-domain reflectometry: A new optical evaluation technique. Opt. Lett. 1987, 12, 158–160. [Google Scholar] [CrossRef]
- Swanson, E.A.; Huang, D.; Hee, M.R.; Fujimoto, J.G.; Lin, C.P.; Puliafito, C.A. High-speed optical coherence domain reflectometry. Opt. Lett. 1992, 17, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.A.; Izatt, J.A.; Hee, M.R.; Huang, D.; Lin, C.P.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. In vivo retinal imaging by optical coherence tomography. Opt. Lett. 1993, 18, 1864–1866. [Google Scholar] [CrossRef]
- Fercher, A.F.; Hitzenberger, C.K.; Kamp, G.; Elzaiat, S.Y. Measurement of intraocular distances by backscattering spectral interferometry. Opt. Commun. 1995, 117, 43–48. [Google Scholar] [CrossRef]
- Choma, M.A.; Sarunic, M.V.; Yang, C.H.; Izatt, J.A. Sensitivity advantage of swept source and fourier domain optical coherence tomography. Opt. Exp. 2003, 11, 2183–2189. [Google Scholar] [CrossRef]
- de Boer, J.F.; Cense, B.; Park, B.H.; Pierce, M.C.; Tearney, G.J.; Bouma, B.E. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt. Lett. 2003, 28, 2067–2069. [Google Scholar] [CrossRef]
- Leitgeb, R.; Hitzenberger, C.K.; Fercher, A.F. Performance of fourier domain vs. Time domain optical coherence tomography. Opt. Exp. 2003, 11, 889–894. [Google Scholar] [CrossRef]
- Cense, B.; Nassif, N.; Chen, T.C.; Pierce, M.C.; Yun, S.; Park, B.H.; Bouma, B.; Tearney, G.; de Boer, J.F. Ultrahigh-resolution high-speed retinal imaging using spectral-domain optical coherence tomography. Opt. Exp. 2004, 12, 2435–2447. [Google Scholar] [CrossRef]
- Nassif, N.; Cense, B.; Park, B.H.; Yun, S.H.; Chen, T.C.; Bouma, B.E.; Tearney, G.J.; de Boer, J.F. In vivo human retinal imaging by ultrahigh-speed spectral domain optical coherence tomography. Opt. Lett. 2004, 29, 480–482. [Google Scholar] [CrossRef]
- Zhang, Y.; Cense, B.; Rha, J.; Jonnal, R.S.; Gao, W.; Zawadzki, R.J.; Werner, J.S.; Jones, S.; Olivier, S.; Miller, D.T. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Opt. Exp. 2006, 14, 4380–4394. [Google Scholar] [CrossRef]
- Potsaid, B.; Gorczynska, I.; Srinivasan, V.J.; Chen, Y.L.; Jiang, J.; Cable, A.; Fujimoto, J.G. Ultrahigh speed spectral/fourier domain oct ophthalmic imaging at 70,000 to 312,500 axial scans per second. Opt. Exp. 2008, 16, 15149–15169. [Google Scholar] [CrossRef]
- LaRocca, F.; Chiu, S.J.; McNabb, R.P.; Kuo, A.N.; Izatt, J.A.; Farsiu, S. Robust automatic segmentation of corneal layer boundaries in sdoct images using graph theory and dynamic programming. Biomed. Opt. Exp. 2011, 2, 1524–1538. [Google Scholar] [CrossRef]
- Wojtkowski, M.; Srinivasan, V.; Fujimoto, J.G.; Ko, T.; Schuman, J.S.; Kowalczyk, A.; Duker, J.S. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 2005, 112, 1734–1746. [Google Scholar] [CrossRef]
- Srinivasan, V.J.; Wojtkowski, M.; Witkin, A.J.; Duker, J.S.; Ko, T.H.; Carvalho, M.; Schuman, J.S.; Kowalczyk, A.; Fujimoto, J.G. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 2006, 113, 2054–2065. [Google Scholar] [CrossRef]
- Chinn, S.R.; Swanson, E.A.; Fujimoto, J.G. Optical coherence tomography using a frequency-tunable optical source. Opt. Lett. 1997, 22, 340–342. [Google Scholar] [CrossRef]
- Golubovic, B.; Bouma, B.E.; Tearney, G.J.; Fujimoto, J.G. Optical frequency-domain reflectometry using rapid wavelength tuning of a Cr4+:Forsterite laser. Opt. Lett. 1997, 22, 1704–1706. [Google Scholar] [CrossRef]
- Yun, S.H.; Tearney, G.J.; de Boer, J.F.; Iftimia, N.; Bouma, B.E. High-speed optical frequency-domain imaging. Opt. Exp. 2003, 11, 2953–2963. [Google Scholar] [CrossRef]
- Oh, W.Y.; Yun, S.H.; Vakoc, B.J.; Tearney, G.J.; Bouma, B.E. Ultrahigh-speed optical frequency domain imaging and application to laser ablation monitoring. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Huber, R.; Adler, D.C.; Fujimoto, J.G. Buffered fourier domain mode locking: Unidirectional swept laser sources for optical coherence tomography imaging at 370,000 lines/s. Opt. Lett. 2006, 31, 2975–2977. [Google Scholar] [CrossRef]
- Adler, D.C.; Huber, R.; Fujimoto, J.G. Phase-sensitive optical coherence tomography at up to 370,000 lines per second using buffered fourier domain mode-locked lasers. Opt. Lett. 2007, 32, 626–628. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Potsaid, B.; Kraus, M.F.; Zhou, C.; Tao, Y.K.; Hornegger, J.; Fujimoto, J.G. Piezoelectric-transducer-based miniature catheter for ultrahigh-speed endoscopic optical coherence tomography. Biomed. Opt. Exp. 2011, 2, 2438–2448. [Google Scholar] [CrossRef]
- Klein, T.; Wieser, W.; Eigenwillig, C.M.; Biedermann, B.R.; Huber, R. Megahertz OCT for ultrawide-field retinal imaging with a 1050 nm fourier domain mode-locked laser. Opt. Exp. 2011, 19, 3044–3062. [Google Scholar] [CrossRef]
- Wieser, W.; Biedermann, B.R.; Klein, T.; Eigenwillig, C.M.; Huber, R. Multi-megahertz OCT: High quality 3D imaging at 20 million A-scans and 4.5 GVoxels per second. Opt. Exp. 2011, 18, 14685–14704. [Google Scholar]
- Passy, R.; Gisin, N.; Vonderweid, J.P.; Gilgen, H.H. Experimental and theoretical investigations of coherent ofdr with semiconductor-laser sources. J. Lightwave Technol. 1994, 12, 1622–1630. [Google Scholar] [CrossRef]
- Choma, M.A.; Ellerbee, A.K.; Yang, C.H.; Creazzo, T.L.; Izatt, J.A. Spectral-domain phase microscopy. Opt. Lett. 2005, 30, 1162–1164. [Google Scholar] [CrossRef]
- Moon, S.J.; Berger, A.S.; Tolentino, M.J.; Misch, D.M. Intravitreal bevacizumab for macular edema from idiopathic juxtafoveal retinal telanglectasis. Ophthalmic Surg. Lasers Imag. 2007, 38, 164–166. [Google Scholar]
- Robles, F.E.; Chowdhury, S.; Wax, A. Assessing hemoglobin concentration using spectroscopic optical coherence tomography for feasibility of tissue diagnostics. Biomed. Opt. Exp. 2010, 1, 310–317. [Google Scholar] [CrossRef]
- Ko, T.H.; Adler, D.C.; Fujimoto, J.G.; Mamedov, D.; Prokhorov, V.; Shidlovski, V.; Yakubovich, S. Ultrahigh resolution optical coherence tomography imaging with a broadband superluminescent diode light source. Opt. Exp. 2004, 12, 2112–2119. [Google Scholar] [CrossRef]
- Cancer Facts and Figures 2013; American Cancer Society: Atlanta, GA, USA, 2013.
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef]
- Shaheen, N.J. Advances in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology 2005, 128, 1554–1566. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.S. Esophageal adenocarcinoma: A review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis 2001, 22, 1119–1129. [Google Scholar] [CrossRef]
- Cameron, A.J.; Carpenter, H.A. Barrett’s esophagus, high-grade dysplasia, and early adenocarcinoma: A pathological study. Am. J. Gastroenterol. 1997, 92, 586–591. [Google Scholar] [PubMed]
- Overholt, B.F.; Panjehpour, M.; Haydek, J.M. Photodynamic therapy for Barrett’s esophagus: Follow-up in 100 patients. Gastrointest. Endoscop. 1999, 49, 1–7. [Google Scholar] [CrossRef]
- Rex, D.K. Maximizing detection of adenomas and cancers during colonoscopy. Am. J. Gastroenterol. 2006, 101, 2866–2877. [Google Scholar] [CrossRef]
- Kiesslich, R.; von Bergh, M.; Hahn, M.; Hermann, G.; Jung, M. Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy 2001, 33, 1001–1006. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Harpaz, N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology 2004, 126, 1634–1648. [Google Scholar] [CrossRef]
- Loftus, E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Sampliner, R.E. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am. J. Gastroenterol. 2008, 103, 788–797. [Google Scholar] [CrossRef]
- Rex, D.K.; Johnson, D.A.; Anderson, J.C.; Schoenfeld, P.S.; Burke, C.A.; Inadomi, J.M. American college of gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am. J. Gastroenterol. 2009, 104, 739–750. [Google Scholar] [CrossRef]
- Inoue, H.; Endo, M. Endoscopic esophageal mucosal resection using a transparent tube. Surg. Endoscopy 1990, 4, 198–201. [Google Scholar] [CrossRef]
- Oyama, T.; Tomori, A.; Hotta, K.; Morita, S.; Kominato, K.; Tanaka, M.; Miyata, Y. Endoscopic submucosal dissection of early esophageal cancer. Clin. Gastroenterol. Hepatol. 2005, 3, S67–S70. [Google Scholar] [CrossRef]
- Van Laethem, J.L.; Peny, M.O.; Salmon, I.; Cremer, M.; Deviere, J. Intramucosal adenocarcinoma arising under squamous re-epithelialisation of Barrett’s oesophagus. Gut 2000, 46, 574–577. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Sharma, P.; Overholt, B.F.; Wolfsen, H.C.; Sampliner, R.E.; Wang, K.K.; Galanko, J.A.; Bronner, M.P.; Goldblum, J.R.; Bennett, A.E.; et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N. E. J. Med. 2009, 360, 2277–2288. [Google Scholar] [CrossRef]
- Johnston, M.H.; Eastone, J.A.; Horwhat, J.D.; Cartledge, J.; Mathews, J.S.; Foggy, J.R. Cryoablation of Barrett’s esophagus: A pilot study. Gastrointest. Endoscop. 2005, 62, 842–848. [Google Scholar] [CrossRef]
- Ganz, R.A.; Overholt, B.F.; Sharma, V.K.; Fleischer, D.E.; Shaheen, N.J.; Lightdale, C.J.; Freeman, S.R.; Pruitt, R.E.; Urayama, S.M.; Gress, F.; et al. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: A U.S. Multicenter registry. Gastrointest. Endoscop. 2008, 68, 35–40. [Google Scholar] [CrossRef]
- Sharma, V.K.; Wang, K.K.; Overholt, B.F.; Lightdale, C.J.; Fennerty, M.B.; Dean, P.J.; Pleskow, D.K.; Chuttani, R.; Reymunde, A.; Santiago, N.; et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patients (with video). Gastrointest. Endoscop. 2007, 65, 185–195. [Google Scholar] [CrossRef]
- Fleischer, D.E.; Overholt, B.F.; Sharma, V.K.; Reymunde, A.; Kimmey, M.B.; Chuttani, R.; Chang, K.J.; Lightdale, C.J.; Santiago, N.; Pleskow, D.K.; et al. Endoscopic ablation of Barrett’s esophagus: A multicenter study with 2.5-year follow-up. Gastrointest. Endoscop. 2008, 68, 867–876. [Google Scholar] [CrossRef]
- Pouw, R.; Gondrie, J.; Sondermeijer, C.; ten Kate, F.; van Gulik, T.; Krishnadath, K.; Fockens, P.; Weusten, B.; Bergman, J. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J. Gastrointest. Surg. 2008, 12, 1627–1637. [Google Scholar] [CrossRef]
- Greenwald, B.D.; Dumot, J.A.; Horwhat, J.D.; Lightdale, C.J.; Abrams, J.A. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis. Esophagus 2010, 23, 13–19. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Greenwald, B.D.; Peery, A.F.; Dumot, J.A.; Nishioka, N.S.; Wolfsen, H.C.; Burdick, J.S.; Abrams, J.A.; Wang, K.K.; Mallat, D.; et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest. Endoscop. 2010, 71, 680–685. [Google Scholar] [CrossRef]
- Odze, R.D.; Lauwers, G.Y. Histopathology of Barrett’s esophagus after ablation and endoscopic mucosal resection therapy. Endoscopy 2008, 40, 1008–1015. [Google Scholar] [CrossRef]
- Bergman, J.J.G.H.M. Radiofrequency ablation—Great for some or justified for many? N. E. J. Med. 2009, 360, 2353–2355. [Google Scholar] [CrossRef]
- Vaccaro, B.; Gonzalez, S.; Poneros, J.; Stevens, P.; Capiak, K.; Lightdale, C.; Abrams, J. Detection of intestinal metaplasia after successful eradication of Barrett’s esophagus with radiofrequency ablation. Dig. Dis. Sci. 2011, 56, 1996–2000. [Google Scholar] [CrossRef]
- Fleischer, D.E.; Overholt, B.F.; Sharma, V.K.; Reymunde, A.; Kimmey, M.B.; Chuttani, R.; Chang, K.J.; Muthasamy, R.; Lightdale, C.J.; Santiago, N.; et al. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy 2010, 42, 781–789. [Google Scholar] [CrossRef]
- Byrne, J.P.; Armstrong, G.R.; Attwood, S.E. Restoration of the normal squamous lining in Barrett’s esophagus by argon beam plasma coagulation. Am. J. Gastroenterol. 1998, 93, 1810–1815. [Google Scholar] [CrossRef]
- Kelty, C.J.; Ackroyd, R.; Brown, N.J.; Stephenson, T.J.; Stoddard, C.J.; Reed, M.W. Endoscopic ablation of Barrett’s oesophagus: A randomized-controlled trial of photodynamic therapy vs. Argon plasma coagulation. Aliment. Pharmacol. Ther. 2004, 20, 1289–1296. [Google Scholar] [CrossRef]
- Ragunath, K.; Krasner, N.; Raman, V.S.; Haqqani, M.T.; Phillips, C.J.; Cheung, I. Endoscopic ablation of dysplastic Barrett’s oesophagus comparing argon plasma coagulation and photodynamic therapy: A randomized prospective trial assessing efficacy and cost-effectiveness. Scand. J. Gastroenterol. 2005, 40, 750–758. [Google Scholar] [CrossRef]
- Spechler, S.J. Screening and surveillance for complications related to gastroesophageal reflux disease. Am. J. Med. 2001, 111 (Suppl 8A), 130S–136S. [Google Scholar] [CrossRef]
- Mashimo, H.; Wagh, M.S.; Goyal, R.K. Surveillance and screening for barrett esophagus and adenocarcinoma. J. Clin. Gastroenterol. 2005, 39, S33–S41. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Brezinski, M.E.; Boppart, S.A.; Bouma, B.E.; Weissman, N.; Southern, J.F.; Swanson, E.A.; Fujimoto, J.G. Catheter-based optical imaging of a human coronary artery. Circulation 1996, 94, 3013. [Google Scholar] [CrossRef]
- Bouma, B.E.; Tearney, G.J.; Compton, C.C.; Nishioka, N.S. High-resolution imaging of the human esophagus and stomach in vivo using optical coherence tomography. Gastrointest. Endoscop. 2000, 51, 467–474. [Google Scholar] [CrossRef]
- Tearney, G.J.; Brezinski, M.E.; Southern, J.F.; Bouma, B.E.; Boppart, S.A.; Fujimoto, J.G. Optical biopsy in human urologic tissue using optical coherence tomography. J. Urol. 1997, 157, 1915–1919. [Google Scholar] [CrossRef]
- Escobar, P.F.; Belinson, J.L.; White, A.; Shakhova, N.M.; Feldchtein, F.I.; Kareta, M.V.; Gladkova, N.D. Diagnostic efficacy of optical coherence tomography in the management of preinvasive and invasive cancer of uterine cervix and vulva. Int. J. Gynecol. Cancer 2004, 14, 470–474. [Google Scholar] [CrossRef]
- Yaqoob, Z.; Wu, J.G.; McDowell, E.J.; Heng, X.; Yang, C.H. Methods and application areas of endoscopic optical coherence tomography. J. Biomed. Opt. 2006, 11. [Google Scholar] [CrossRef]
- Sergeev, A.M.; Gelikonov, V.M.; Gelikonov, G.V.; Feldchtein, F.I.; Kuranov, R.V.; Gladkova, N.D.; Shakhova, N.M.; Suopova, L.B.; Shakhov, A.V.; Kuznetzova, I.A.; et al. In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa. Opt. Exp. 1997, 1, 432–440. [Google Scholar] [CrossRef]
- Sivak, M.V., Jr.; Kobayashi, K.; Izatt, J.A.; Rollins, A.M.; Ung-Runyawee, R.; Chak, A.; Wong, R.C.; Isenberg, G.A.; Willis, J. High-resolution endoscopic imaging of the gi tract using optical coherence tomography. Gastrointest. Endoscop. 2000, 51, 474–479. [Google Scholar] [CrossRef]
- Jäckle, S.; Gladkova, N.; Feldchtein, F.; Terentieva, A.; Brand, B.; Gelikonov, G.; Gelikonov, V.; Sergeev, A.; Fritscher-Ravens, A.; Freund, J.; et al. In vivo endoscopic optical coherence tomography of esophagitis, Barrett’s esophagus, and adenocarcinoma of the esophagus. Endoscopy 2000, 32, 750–755. [Google Scholar] [CrossRef]
- Jäckle, S.; Gladkova, N.; Feldchtein, F.; Terentieva, A.; Brand, B.; Gelikonov, G.; Gelikonov, V.; Sergeev, A.; Fritscher-Ravens, A.; Freund, J.; et al. In vivo endoscopic optical coherence tomography of the human gastrointestinal tract—Toward optical biopsy. Endoscopy 2000, 32, 743–749. [Google Scholar] [CrossRef]
- Li, X.D.; Boppart, S.A.; Van Dam, J.; Mashimo, H.; Mutinga, M.; Drexler, W.; Klein, M.; Pitris, C.; Krinsky, M.L.; Brezinski, M.E.; et al. Optical coherence tomography: Advanced technology for the endoscopic imaging of Barrett’s esophagus. Endoscopy 2000, 32, 921–930. [Google Scholar] [CrossRef]
- Zuccaro, G.; Gladkova, N.; Vargo, J.; Feldchtein, F.; Zagaynova, E.; Conwell, D.; Falk, G.; Goldblum, J.; Dumot, J.; Ponsky, J.; et al. Optical coherence tomography of the esophagus and proximal stomach in health and disease. Am. J. Gastroenterol. 2001, 96, 2633–2639. [Google Scholar] [CrossRef]
- Adler, D.C.; Zhou, C.; Tsai, T.H.; Lee, H.C.; Becker, L.; Schmitt, J.M.; Huang, Q.; Fujimoto, J.G.; Mashimo, H. Three-dimensional optical coherence tomography of Barrett’s esophagus and buried glands beneath neosquamous epithelium following radiofrequency ablation. Endoscopy 2009, 41, 773–776. [Google Scholar] [CrossRef]
- Suter, M.J.; Jillella, P.A.; Vakoc, B.J.; Halpern, E.F.; Mino-Kenudson, M.; Lauwers, G.Y.; Bouma, B.E.; Nishioka, N.S.; Tearney, G.J. Image-guided biopsy in the esophagus through comprehensive optical frequency domain imaging and laser marking: A study in living swine. Gastrointest. Endoscop. 2010, 71, 346–353. [Google Scholar] [CrossRef]
- Das, A.; Sivak, M.V., Jr.; Chak, A.; Wong, R.C.; Westphal, V.; Rollins, A.M.; Willis, J.; Isenberg, G.; Izatt, J.A. High-resolution endoscopic imaging of the gi tract: A comparative study of optical coherence tomography versus high-frequency catheter probe eus. Gastrointest. Endoscop. 2001, 54, 219–224. [Google Scholar] [CrossRef]
- Shen, B.; Zuccaro, G., Jr.; Gramlich, T.L.; Gladkova, N.; Trolli, P.; Kareta, M.; Delaney, C.P.; Connor, J.T.; Lashner, B.A.; Bevins, C.L.; et al. In vivo colonoscopic optical coherence tomography for transmural inflammation in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2004, 2, 1080–1087. [Google Scholar] [CrossRef]
- Pfau, P.R.; Sivak, M.V., Jr.; Chak, A.; Kinnard, M.; Wong, R.C.; Isenberg, G.A.; Izatt, J.A.; Rollins, A.; Westphal, V. Criteria for the diagnosis of dysplasia by endoscopic optical coherence tomography. Gastrointest. Endoscop. 2003, 58, 196–202. [Google Scholar] [CrossRef]
- Zhou, C.; Adler, D.C.; Becker, L.; Chen, Y.; Tsai, T.-H.; Figueiredo, M.; Schmitt, J.M.; Fujimoto, J.G.; Mashimo, H. Effective treatment of chronic radiation proctitis using radiofrequency ablation. Ther. Adv. Gastroenterol. 2009, 2, 149–156. [Google Scholar] [CrossRef]
- Adler, D.C.; Zhou, C.; Tsai, T.H.; Schmitt, J.; Huang, Q.; Mashimo, H.; Fujimoto, J.G. Three-dimensional endomicroscopy of the human colon using optical coherence tomography. Opt. Exp. 2009, 17, 784–796. [Google Scholar] [CrossRef]
- Seitz, U.; Freund, J.; Jaeckle, S.; Feldchtein, F.; Bohnacker, S.; Thonke, F.; Gladkova, N.; Brand, B.; Schröder, S.; Soehendra, N. First in vivo optical coherence tomography in the human bile duct. Endoscopy 2001, 33, 1018–1021. [Google Scholar] [CrossRef]
- Poneros, J.M.; Tearney, G.J.; Shiskov, M.; Kelsey, P.B.; Lauwers, G.Y.; Nishioka, N.S.; Bouma, B.E. Optical coherence tomography of the biliary tree during ercp. Gastrointest. Endoscop. 2002, 55, 84–88. [Google Scholar] [CrossRef]
- Poneros, J.M.; Brand, S.; Bouma, B.E.; Tearney, G.J.; Compton, C.C.; Nishioka, N.S. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology 2001, 120, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Bouma, B.E.; Bressner, J.; Shishkov, M.; Lauwers, G.Y.; Mino-Kenudson, M.; Nishioka, N.S.; Tearney, G.J. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointest. Endoscop. 2007, 65, 50–56. [Google Scholar] [CrossRef]
- Evans, J.A.; Poneros, J.M.; Bouma, B.E.; Bressner, J.; Halpern, E.F.; Shishkov, M.; Lauwers, G.Y.; Mino-Kenudson, M.; Nishioka, N.S.; Tearney, G.J. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 2006, 4, 38–43. [Google Scholar] [CrossRef]
- Isenberg, G.; Sivak, M.V.; Chak, A.; Wong, R.C.K.; Willis, J.E.; Wolf, B.; Rowland, D.Y.; Das, A.; Rollins, A. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett’s esophagus: A prospective, double-blinded study. Gastrointest. Endoscop. 2005, 62, 825–831. [Google Scholar] [CrossRef]
- Qi, X.; Sivak, M.V.; Isenberg, G.; Willis, J.E.; Rollins, A.M. Computer-aided diagnosis of dysplasia in Barrett’s esophagus using endoscopic optical coherence tomography. J. Biomed. Opt. 2006, 11. [Google Scholar] [CrossRef]
- Tumlinson, A.R.; Barton, J.K.; Povazay, B.; Sattman, H.; Unterhuber, A.; Leitgeb, R.A.; Drexler, W. Endoscope-tip interferometer for ultrahigh resolution frequency domain optical coherence tomography in mouse colon. Opt. Exp. 2006, 14, 1878–1887. [Google Scholar] [CrossRef]
- Yun, S.H.; Tearney, G.J.; Vakoc, B.J.; Shishkov, M.; Oh, W.Y.; Desjardins, A.E.; Suter, M.J.; Chan, R.C.; Evans, J.A.; Jang, I.K.; et al. Comprehensive volumetric optical microscopy in vivo. Nat. Med. 2006, 12, 1429–1433. [Google Scholar] [PubMed]
- Vakoc, B.J.; Shishko, M.; Yun, S.H.; Oh, W.Y.; Suter, M.J.; Desjardins, A.E.; Evans, J.A.; Nishioka, N.S.; Tearney, G.J.; Bouma, B.E. Comprehensive esophageal microscopy by using optical frequency-domain imaging (with video). Gastrointest. Endoscop. 2007, 65, 898–905. [Google Scholar] [CrossRef]
- Adler, D.C.; Chen, Y.; Huber, R.; Schmitt, J.; Connolly, J.; Fujimoto, J.G. Three-dimensional endomicroscopy using optical coherence tomography. Nat. Photon. 2007, 1, 709–716. [Google Scholar] [CrossRef]
- Suter, M.J.; Vakoc, B.J.; Yachimski, P.S.; Shishkov, M.; Lauwers, G.Y.; Mino-Kenudson, M.; Bouma, B.E.; Nishioka, N.S.; Tearney, G.J. Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest. Endoscop. 2008, 68, 745–753. [Google Scholar] [CrossRef]
- Canto, M.I.F.; Setrakian, S.; Willis, J.E.; Chak, A.; Petras, R.E.; Sivak, M.V. Methylene blue staining of dysplastic and nondysplastic Barrett’s esophagus: An in vivo and ex vivo study. Endoscopy 2001, 33, 391–400. [Google Scholar] [CrossRef]
- Sharma, P.; Topalovski, M.; Mayo, M.S.; Weston, A.P. Methylene blue chromoendoscopy for detection of short-segment Barrett’s esophagus. Gastrointest. Endoscop. 2001, 54, 289–293. [Google Scholar] [CrossRef]
- Endo, T.; Awakawa, T.; Takahashi, H.; Arimura, Y.; Itoh, F.; Yamashita, K.; Sasaki, S.; Yamamoto, H.; Tang, X.F.; Imai, K. Classification of Barrett’s epithelium by magnifying endoscopy. Gastrointest. Endoscop. 2002, 55, 641–647. [Google Scholar] [CrossRef]
- Pohl, J.; May, A.; Robenstein, T.; Pech, O.; Nguyen-Tat, M.; Fissler-Eckhoff, A.; Ell, C. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy 2007, 39, 594–598. [Google Scholar] [CrossRef]
- Olliver, J.R.; Wild, C.P.; Sahay, P.; Dexter, S.; Hardie, L.J. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet 2003, 362, 373–374. [Google Scholar] [CrossRef]
- Panossian, A.M.; Raimondo, M.; Wolfsen, H.C. State of the art in the endoscopic imaging and ablation of Barrett’s esophagus. Digest. Liver Dis. 2011, 43, 365–373. [Google Scholar] [CrossRef]
- Sharma, P.; Bansal, A.; Mathur, S.; Wani, S.; Cherian, R.; McGregor, D.; Higbee, A.; Hall, S.; Weston, A. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest. Endoscop. 2006, 64, 167–175. [Google Scholar] [CrossRef]
- Singh, R.; Anagnostopoulos, G.K.; Yao, K.; Karageorgiou, H.; Fortun, P.J.; Shonde, A.; Garsed, K.; Kaye, P.V.; Hawkey, C.J.; Ragunath, K. Narrow-band imaging with magnification in Barrett’s esophagus: Validation of a simplified grading system of mucosal morphology patterns against histology. Endoscopy 2008, 40, 457–463. [Google Scholar] [CrossRef]
- Silva, F.B.; Dinis-Ribeiro, M.; Vieth, M.; Rabenstein, T.; Goda, K.; Kiesslich, R.; Haringsma, J.; Edebo, A.; Toth, E.; Soares, J. Endoscopic assessment and grading of Barrett’s esophagus using magnification endoscopy and narrow-band imaging: Accuracy and interobserver agreement of different classification systems (with videos). Gastrointest. Endoscop. 2011, 73, 7–14. [Google Scholar] [CrossRef]
- Giacchino, M.; Bansal, A.; Kim, R.E.; Singh, V.; Hall, S.B.; Singh, M.; Rastogi, A.; Moloney, B.; Wani, S.B.; Gaddam, S.; et al. Clinical utility and interobserver agreement of autofluorescence imaging and magnification narrow-band imaging for the evaluation of Barrett’s esophagus: A prospective tandem study. Gastrointest. Endoscop. 2013, 77, 711–718. [Google Scholar] [CrossRef]
- Kudo, S.E.; Tamura, S.; Nakajima, T.; Yamano, H.O.; Kusaka, H.; Watanabe, H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest. Endoscop. 1996, 44, 8–14. [Google Scholar] [CrossRef]
- The paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon—November 30 to December 1, 2002. Gastrointest. Endoscop. 2003, 58, S3–S43. [CrossRef]
- Axon, A.; Diebold, M.D.; Fujino, M.; Fujita, R.; Genta, R.M.; Gonvers, J.J.; Guelrud, M.; Inoue, H.; Jung, M.; Kashida, H.; et al. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005, 37, 570–578. [Google Scholar] [CrossRef]
- Wilson, T. Confocal Microscopy; Academic Press: London, UK, 1990. [Google Scholar]
- Sandison, D.R.; Webb, W.W. Background rejection and signal-to-noise optimization in confocal and alternative fluorescence microscopes. Appl. Opt. 1994, 33, 603–615. [Google Scholar] [CrossRef]
- Kino, G.S.; Corle, T.R. Confocal Scanning Optical Microscopy and Related Imaging Systems; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Pawley, J. Handbook of Biological Confocal Microscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Polglase, A.L.; McLaren, W.J.; Delaney, P.M. Pentax confocal endomicroscope: A novel imaging device for in vivo histology of the upper and lower gastrointestinal tract. Expert Rev. Med. Devices 2006, 3, 549–556. [Google Scholar] [CrossRef]
- Dunbar, K.B.; Canto, M.I. Confocal endomicroscopy. Tech. Gastrointest. Endoscop. 2010, 12, 90–99. [Google Scholar] [CrossRef]
- Hoffman, A.; Goetz, M.; Vieth, M.; Galle, P.R.; Neurath, M.F.; Kiesslich, R. Confocal laser endomicroscopy: Technical status and current indications. Endoscopy 2006, 38, 1275–1283. [Google Scholar] [CrossRef]
- Canto, M.I. Endomicroscopy of Barrett’s esophagus. Gastroenterol. Clin. North Am. 2010, 39, 759–769. [Google Scholar] [CrossRef]
- Goetz, M.; Wang, T.D. Molecular imaging in gastrointestinal endoscopy. Gastroenterology 2010, 138, U828–U854. [Google Scholar] [CrossRef]
- Miller, S.J.; Joshi, B.P.; Feng, Y.; Gaustad, A.; Fearon, E.R.; Wang, T.D. In vivo fluorescence-based endoscopic detection of colon dysplasia in the mouse using a novel peptide probe. PLoS One 2011, 6, e17384. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.B.; Joshi, B.P.; Lu, S.; Piraka, C.; Khondee, S.; Elmunzer, B.J.; Kwon, R.S.; Beer, D.G.; Appelman, H.D.; Turgeon, D.K.; et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: First-in-human results. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef]
- Liu, Z.; Miller, S.J.; Joshi, B.P.; Wang, T.D. In vivo targeting of colonic dysplasia on fluorescence endoscopy with near-infrared octapeptide. Gut 2013, 62, 395–403. [Google Scholar] [CrossRef]
- Wax, A.; Yang, C.H.; Muller, M.G.; Nines, R.; Boone, C.W.; Steele, V.E.; Stoner, G.D.; Dasari, R.R.; Feld, M.S. In situ detection of neoplastic transformation and chemopreventive effects in rat esophagus epithelium using angle-resolved low-coherence interferometry. Cancer Res. 2003, 63, 3556–3559. [Google Scholar] [PubMed]
- Wax, A.; Pyhtila, J.W.; Graf, R.N.; Nines, R.; Boone, C.W.; Dasari, R.R.; Feld, M.S.; Steele, V.E.; Stoner, G.D. Prospective grading of neoplastic change in rat esophagus epithelium using angle-resolved low-coherence interferometry. J. Biomed. Opt. 2005, 10. [Google Scholar] [CrossRef]
- Terry, N.G.; Zhu, Y.; Rinehart, M.T.; Brown, W.J.; Gebhart, S.C.; Bright, S.; Carretta, E.; Ziefle, C.G.; Panjehpour, M.; Galanko, J.; et al. Detection of dysplasia in Barrett’s esophagus with in vivo depth-resolved nuclear morphology measurements. Gastroenterology 2011, 140, 42–50. [Google Scholar] [CrossRef]
- Qiu, L.; Pleskow, D.K.; Chuttani, R.; Vitkin, E.; Leyden, J.; Ozden, N.; Itani, S.; Guo, L.; Sacks, A.; Goldsmith, J.D.; et al. Multispectral scanning during endoscopy guides biopsy of dysplasia in Barrett’s esophagus. Nat. Med. 2010, 16, U603–U140. [Google Scholar] [CrossRef]
- Tearney, G.J.; Boppart, S.A.; Bouma, B.E.; Brezinski, M.E.; Weissman, N.J.; Southern, J.F.; Fujimoto, J.G. Scanning single-mode fiber optic catheter-endoscope for optical coherence tomography. Opt. Lett. 1996, 21, 543–545. [Google Scholar] [CrossRef]
- Fu, H.L.; Leng, Y.X.; Cobb, M.J.; Hsu, K.; Hwang, J.H.; Li, X.D. Flexible miniature compound lens design for high-resolution optical coherence tomography balloon imaging catheter. J. Biomed. Opt. 2008, 13. [Google Scholar] [CrossRef]
- Xi, J.F.; Huo, L.; Wu, Y.C.; Cobb, M.J.; Hwang, J.H.; Li, X.D. High-resolution oct balloon imaging catheter with astigmatism correction. Opt. Lett. 2009, 34, 1943–1945. [Google Scholar] [CrossRef]
- Kang, W.; Wang, H.; Pan, Y.S.; Jenkins, M.W.; Isenberg, G.A.; Chak, A.; Atkinson, M.; Agrawal, D.; Hu, Z.L.; Rollins, A.M. Endoscopically guided spectral-domain OCT with double-balloon catheters. Opt. Exp. 2010, 18, 17364–17372. [Google Scholar] [CrossRef]
- Seibel, E.J.; Smithwick, Q.Y.J. Unique features of optical scanning, single fiber endoscopy. Lasers Surg. Med. 2002, 30, 177–183. [Google Scholar] [CrossRef]
- Liu, X.M.; Cobb, M.J.; Chen, Y.C.; Kimmey, M.B.; Li, X.D. Rapid-scanning forward-imaging miniature endoscope for real-time optical coherence tomography. Opt. Lett. 2004, 29, 1763–1765. [Google Scholar] [CrossRef]
- Aguirre, A.D.; Sawinski, J.; Huang, S.W.; Zhou, C.; Denk, W.; Fujimoto, J.G. High speed optical coherence microscopy with autofocus adjustment and a miniaturized endoscopic imaging probe. Opt. Exp. 2010, 18, 4222–4239. [Google Scholar] [CrossRef]
- Pan, Y.T.; Xie, H.K.; Fedder, G.K. Endoscopic optical coherence tomography based on a microelectromechanical mirror. Opt. Lett. 2001, 26, 1966–1968. [Google Scholar] [CrossRef]
- Xi, J.; Chen, Y.; Zhang, Y.; Murari, K.; Li, M.-J.; Li, X. Integrated multimodal endomicroscopy platform for simultaneous en face optical coherence and two-photon fluorescence imaging. Opt. Lett. 2012, 37, 362–364. [Google Scholar] [CrossRef]
- Moon, S.; Lee, S.-W.; Rubinstein, M.; Wong, B.J.F.; Chen, Z. Semi-resonant operation of a fiber-cantilever piezotube scanner for stable optical coherence tomography endoscope imaging. Opt. Exp. 2010, 18, 21183–21197. [Google Scholar] [CrossRef]
- Herz, P.R.; Chen, Y.; Aguirre, A.D.; Schneider, K.; Hsiung, P.; Fujimoto, J.G.; Madden, K.; Schmitt, J.; Goodnow, J.; Petersen, C. Micromotor endoscope catheter for in vivo, ultrahigh-resolution optical coherence tomography. Opt. Lett. 2004, 29, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Mukai, D.S.; Brenner, M.; Chen, Z.P. In vivo endoscopic optical coherence tomography by use of a rotational microelectromechanical system probe. Opt. Lett. 2004, 29, 1236–1238. [Google Scholar] [CrossRef]
- Lee, S.-W.; Heidary, A.E.; Yoon, D.; Mukai, D.; Ramalingam, T.; Mahon, S.; Yin, J.; Jing, J.; Liu, G.; Chen, Z.; et al. Quantification of airway thickness changes in smoke-inhalation injury using in-vivo 3-d endoscopic frequency-domain optical coherence tomography. Biomed. Opt. Exp. 2011, 2, 243–254. [Google Scholar] [CrossRef]
- Liang, S.; Saidi, A.; Jing, J.; Liu, G.; Li, J.; Zhang, J.; Sun, C.; Narula, J.; Chen, Z. Intravascular atherosclerotic imaging with combined fluorescence and optical coherence tomography probe based on a double-clad fiber combiner. J. Biomed. Opt. 2012, 17. [Google Scholar] [CrossRef]
- Li, J.; de Groot, M.; Helderman, F.; Mo, J.; Daniels, J.M.A.; Grünberg, K.; Sutedja, T.G.; de Boer, J.F. High speed miniature motorized endoscopic probe for optical frequency domain imaging. Opt. Exp. 2012, 20, 24132–24138. [Google Scholar] [CrossRef]
- Wang, T.; Wieser, W.; Springeling, G.; Beurskens, R.; Lancee, C.T.; Pfeiffer, T.; van der Steen, A.F.W.; Huber, R.; Soest, G.V. Intravascular optical coherence tomography imaging at 3200 frames per second. Opt. Lett. 2013, 38, 1715–1717. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Potsaid, B.; Tao, Y.K.; Jayaraman, V.; Jiang, J.; Heim, P.J.S.; Kraus, M.F.; Zhou, C.; Hornegger, J.; Mashimo, H.; et al. Ultrahigh speed endoscopic optical coherence tomography using micromotor imaging catheter and vcsel technology. Biomed. Opt. Exp. 2013, 4, 1119–1132. [Google Scholar] [CrossRef]
- Adler, D.C.; Zhou, C.; Tsai, T.H.; Lee, H.C.; Becker, L.; Schmitt, J.M.; Huang, Q.; Fujimoto, J.G.; Mashimo, H. Biased assessment of 3D optical coherence tomography in a single post-radiofrequency ablation patient without histological correlation reply. Endoscopy 2010, 42, 180–181. [Google Scholar] [CrossRef]
- Diseases, T.J.S.F.E. Esophagheal Cancer Treatement Guidelines; Kanehara: Tokyo, Japan, 2007. [Google Scholar]
- Murata, Y.; Suzuki, S.; Ohta, M.; Mitsunaga, A.; Hayashi, K.; Yoshida, K.; Ide, H. Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest. Endoscop. 1996, 44, 23–28. [Google Scholar] [CrossRef]
- Yokosawa, S.; Koike, T.; Kitagawa, Y.; Hatta, W.; Uno, K.; Abe, Y.; Iijima, K.; Imatani, A.; Ohara, S.; Shimosegawa, T. Identification of the layered morphology of the esophageal wall by optical coherence tomography. World J. Gastroenterol. 2009, 15, 4402–4409. [Google Scholar] [CrossRef]
- Hatta, W.; Uno, K.; Koike, T.; Yokosawa, S.; Iijima, K.; Imatani, A.; Shimosegawa, T. Optical coherence tomography for the staging of tumor infiltration in superficial esophageal squamous cell carcinoma. Gastrointest. Endoscop. 2010, 71, 899–906. [Google Scholar] [CrossRef]
- Chen, Y.; Aguirre, A.D.; Hsiung, P.L.; Desai, S.; Herz, P.R.; Pedrosa, M.; Huang, Q.; Figueiredo, M.; Huang, S.W.; Koski, A.; et al. Ultrahigh resolution optical coherence tomography of Barrett’s esophagus: Preliminary descriptive clinical study correlating images with histology. Endoscopy 2007, 39, 599–605. [Google Scholar] [CrossRef]
- Yang, V.X.D.; Tang, S.-J.; Gordon, M.L.; Qi, B.; Gardiner, G.; Cirocco, M.; Kortan, P.; Haber, G.B.; Kandel, G.; Vitkin, I.A.; et al. Endoscopic doppler optical coherence tomography in the human gi tract: Initial experience. Gastrointest. Endoscop. 2005, 61, 879–890. [Google Scholar] [CrossRef]
- Mashimo, H. Subsquamous intestinal metaplasia after ablation of Barrett’s esophagus: Frequency and importance. Curr. Opin. Gastroenterol. 2013, 29, 454–459. [Google Scholar] [CrossRef]
- Zhou, C.; Tsai, T.H.; Lee, H.C.; Kirtane, T.; Figueiredo, M.; Tao, Y.K.K.; Ahsen, O.O.; Adler, D.C.; Schmitt, J.M.; Huang, Q.; et al. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest. Endoscop. 2012, 76, 32–40. [Google Scholar] [CrossRef]
- Cobb, M.J.; Hwang, J.H.; Upton, M.P.; Chen, Y.C.; Oelschlager, B.K.; Wood, D.E.; Kimmey, M.B.; Li, X.D. Imaging of subsquamous Barrett’s epithelium with ultrahigh-resolution optical coherence tomography: A histologic correlation study. Gastrointest. Endoscop. 2010, 71, 223–230. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Overholt, B.F.; Sampliner, R.E.; Wolfsen, H.C.; Wang, K.K.; Fleischer, D.E.; Sharma, V.K.; Eisen, G.M.; Fennerty, M.B.; Hunter, J.G.; et al. Durability of radiofrequency ablation in barrett's esophagus with dysplasia. Gastroenterology 2011, 141, 460–468. [Google Scholar] [CrossRef]
- Dunkin, B.; Martinez, J.; Bejarano, P.; Smith, C.; Chang, K.; Livingstone, A.; Melvin, W. Thin-layer ablation of human esophageal epithelium using a bipolar radiofrequency balloon device. Surg. Endoscopy 2006, 20, 125–130. [Google Scholar] [CrossRef]
- Trunzo, J.; McGee, M.; Poulose, B.; Willis, J.; Ermlich, B.; Laughinghouse, M.; Champagne, B.; Delaney, C.; Marks, J. A feasibility and dosimetric evaluation of endoscopic radiofrequency ablation for human colonic and rectal epithelium in a treat and resect trial. Surg. Endoscopy 2011, 25, 491–496. [Google Scholar] [CrossRef]
- Tsai, T.H.; Zhou, C.; Tao, Y.K.; Lee, H.C.; Ahsen, O.O.; Figueiredo, M.; Kirtane, T.; Adler, D.C.; Schmitt, J.M.; Huang, Q.; et al. Structural markers observed with endoscopic 3-dimensional optical coherence tomography correlating with Barrett’s esophagus radiofrequency ablation treatment response. Gastrointest. Endoscop. 2012, 76, 1104–1112. [Google Scholar] [CrossRef]
- Tang, P.; McKinley, M.J.; Sporrer, M.; Kahn, E. Inlet patch: Prevalence, histologic type, and association with esophagitis, barrett esophagus, and antritis. Arch. Pathol. Lab. Med. 2004, 128, 444–447. [Google Scholar] [PubMed]
- Borhan-Manesh, F.; Farnum, J.B. Incidence of heterotopic gastric mucosa in the upper oesophagus. Gut 1991, 32, 968–972. [Google Scholar] [CrossRef]
- Feurle, G.E.; Helmstaedter, V.; Buehring, A.; Bettendorf, U.; Eckardt, V.F. Distinct immunohistochemical findings in columnar epithelium of esophageal inlet patch and of Barrett’s esophagus. Dig. Dis. Sci. 1990, 35, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, İ.; Üsküdar, O.; Köklü, S.; Başar, Ö.; Gültuna, S.; Ünverdi, S.; Öztürk, Z.A.; Şengül, D.; Arikök, A.T.; Yüksel, O.; et al. Inlet patch: Associations with endoscopic findings in the upper gastrointestinal system. Scand. J. Gastroenterol. 2008, 43, 910–914. [Google Scholar] [CrossRef]
- Avidan, B.; Sonnenberg, A.; Chejfec, G.; Schnell, T.G.; Sontag, S.J. Is there a link between cervical inlet patch and Barrett’s esophagus? Gastrointest. Endoscop. 2001, 53, 717–721. [Google Scholar] [CrossRef]
- Malhi-Chowla, N.; Ringley, R.K.; Wolfsen, H.C. Gastric metaplasia of the proximal esophagus associated with esophageal adenocarcinoma and Barrett’s esophagus: What is the connection? Inlet patch revisited. Digest. Dis. 2000, 18, 183–185. [Google Scholar] [CrossRef]
- Mion, F.; Lambert, R.; Partensky, C.; Cherkaoui, M.; Berger, F. High-grade dysplasia in an adenoma of the upper esophagus developing on heterotopic gastric mucosa. Endoscopy 1996, 28, 633–635. [Google Scholar] [CrossRef]
- Sperling, R.M.; Grendell, J.H. Adenocarcinoma arising in an inlet patch of the esophagus. Am. J. Gastroenterol. 1995, 90, 150–152. [Google Scholar] [PubMed]
- von Rahden, B.H.A.; Stein, H.J.; Becker, K.; Liebermann-Meffert, D.; Siewert, J.R. Heterotopic gastric mucosa of the esophagus: Literature-review and proposal of a clinicopathologic classification. Am. J. Gastroenterol. 2004, 99, 543–551. [Google Scholar] [CrossRef]
- Berkelhammer, C.; Bhagavan, M.; Templeton, A.; Raines, R.; Walloch, J. Gastric inlet patch containing submucosally infiltrating adenocarcinoma. J. Clin. Gastroenterol. 1997, 25, 678–681. [Google Scholar] [CrossRef]
- Zhou, C.; Kirtane, T.; Tsai, T.H.; Lee, H.C.; Adler, D.C.; Schmitt, J.; Huang, Q.; Fujimoto, J.G.; Mashimo, H. Three-dimensional endoscopic optical coherence tomography imaging of cervical inlet patch. Gastrointest. Endoscop. 2012, 75, 675–677. [Google Scholar] [CrossRef]
- Zhou, C.; Kirtane, T.; Tsai, T.H.; Lee, H.C.; Adler, D.C.; Schmitt, J.M.; Huang, Q.; Fujimoto, J.G.; Mashimo, H. Cervical inlet patch-optical coherence tomography imaging and clinical significance. World J. Gastroenterol. 2012, 18, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dent, J.; Armstrong, D.; Bergman, J.J.G.H.M.; Gossner, L.; Hoshihara, Y.; Jankowski, J.A.; Junghard, O.; Lundell, L.; Tytgat, G.N.J.; et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: The prague C & M criteria. Gastroenterology 2006, 131, 1392–1399. [Google Scholar] [CrossRef]
- Hanna, S.; Rastogi, A.; Weston, A.P.; Totta, F.; Schmitz, R.; Mathur, S.; McGregor, D.; Cherian, R.; Sharma, P. Detection of Barrett’s esophagus after endoscopic healing of erosive esophagitis. Am. J. Gastroenterol. 2006, 101, 1416–1420. [Google Scholar] [CrossRef]
- Ishimura, N.; Amano, Y.; Appelman, H.D.; Penagini, R.; Tenca, A.; Falk, G.W.; Wong, R.K.; Gerson, L.B.; Ramirez, F.C.; Horwhat, J.D.; et al. Barrett’s esophagus: Endoscopic diagnosis. Ann. N. Y. Acad. Sci. 2011, 1232, 53–75. [Google Scholar] [CrossRef]
- Sauk, J.; Coron, E.; Kava, L.; Suter, M.; Gora, M.; Gallagher, K.; Rosenberg, M.; Ananthakrishnan, A.; Nishioka, N.; Lauwers, G.; et al. Interobserver agreement for the detection of Barrett’s esophagus with optical frequency domain imaging. Dig. Dis. Sci. 2013, 58, 2261–2265. [Google Scholar] [CrossRef]
- Zhou, C.; Adler, D.C.; Becker, L.; Chen, Y.; Tsai, T.H.; Lee, H.C.; Figueiredo, M.; Schmitt, J.M.; Huang, Q.; Fujimoto, J.G.; et al. Radiofrequency ablation with BARRx Halo90 effectively treated chronic radiation proctitis as confirmed with endoscopic three-dimensional optical coherence tomography. Gastroenterology 2009, 136, A644–A644. [Google Scholar]
- Tsai, T.H.; Zhou, C.; Lee, H.C.; Tao, Y.K.; Ahsen, O.O.; Figueiredo, M.; Adler, D.C.; Schmitt, J.M.; Huang, Q.; Fujimoto, J.G.; et al. Comparison of tissue architectural changes between radiofrequency ablation and cryospray ablation in Barrett’s esophagus using endoscopic three-dimensional optical coherence tomography. Gastroenterol. Res. Pract. 2012. [Google Scholar] [CrossRef]
- Gora, M.; Yoo, H.; Suter, M.J.; Gallagher, K.A.; Bouma, B.E.; Nishioka, N.S.; Tearney, G.J. Optical frequency domain imaging system and catheters for volumetric imaging of the human esophagus. Photon. Lett. Pol. 2011, 3, 144–146. [Google Scholar]
- Gora, M.J.; Sauk, J.S.; Carruth, R.W.; Gallagher, K.A.; Suter, M.J.; Nishioka, N.S.; Kava, L.E.; Rosenberg, M.; Bouma, B.E.; Tearney, G.J. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat. Med. 2013, 19, 238–240. [Google Scholar] [CrossRef]
- Ratto, F.; Matteini, P.; Centi, S.; Rossi, F.; Pini, R. Gold nanorods as new nanochromophores for photothermal therapies. J. Biophoton. 2011, 4, 64–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zhang, Y.; Hu, Z.; Hu, D.; Pan, Y.; Ou, S.; Liu, G.; Yin, X.; Zhao, J.; et al. Panning and identification of a colon tumor binding peptide from a phage display peptide library. J. Biomol. Screen. 2007, 12, 429–435. [Google Scholar] [CrossRef]
- Zhou, C.; Tsai, T.H.; Adler, D.C.; Lee, H.C.; Cohen, D.W.; Mondelblatt, A.; Wang, Y.H.; Connolly, J.L.; Fujimoto, J.G. Photothermal optical coherence tomography in ex vivo human breast tissues using gold nanoshells. Opt. Lett. 2010, 35, 700–702. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, T.-H.; Fujimoto, J.G.; Mashimo, H. Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics 2014, 4, 57-93. https://doi.org/10.3390/diagnostics4020057
Tsai T-H, Fujimoto JG, Mashimo H. Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics. 2014; 4(2):57-93. https://doi.org/10.3390/diagnostics4020057
Chicago/Turabian StyleTsai, Tsung-Han, James G. Fujimoto, and Hiroshi Mashimo. 2014. "Endoscopic Optical Coherence Tomography for Clinical Gastroenterology" Diagnostics 4, no. 2: 57-93. https://doi.org/10.3390/diagnostics4020057
APA StyleTsai, T.-H., Fujimoto, J. G., & Mashimo, H. (2014). Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics, 4(2), 57-93. https://doi.org/10.3390/diagnostics4020057




