Can the Pupillary Light Reflex and Pupillary Unrest Be Used as Biomarkers of Parkinson’s Disease? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- -
- Original experimental studies collecting data on human subjects.
- -
- Studies investigating a cohort of patients diagnosed with Parkinson’s disease.
- -
- Studies measuring PLR parameters or pupillary unrest.
2.2.2. Exclusion Criteria
2.3. Data Extraction
2.4. Quality and Risk of Bias Assessment
2.5. Data Analysis
3. Results
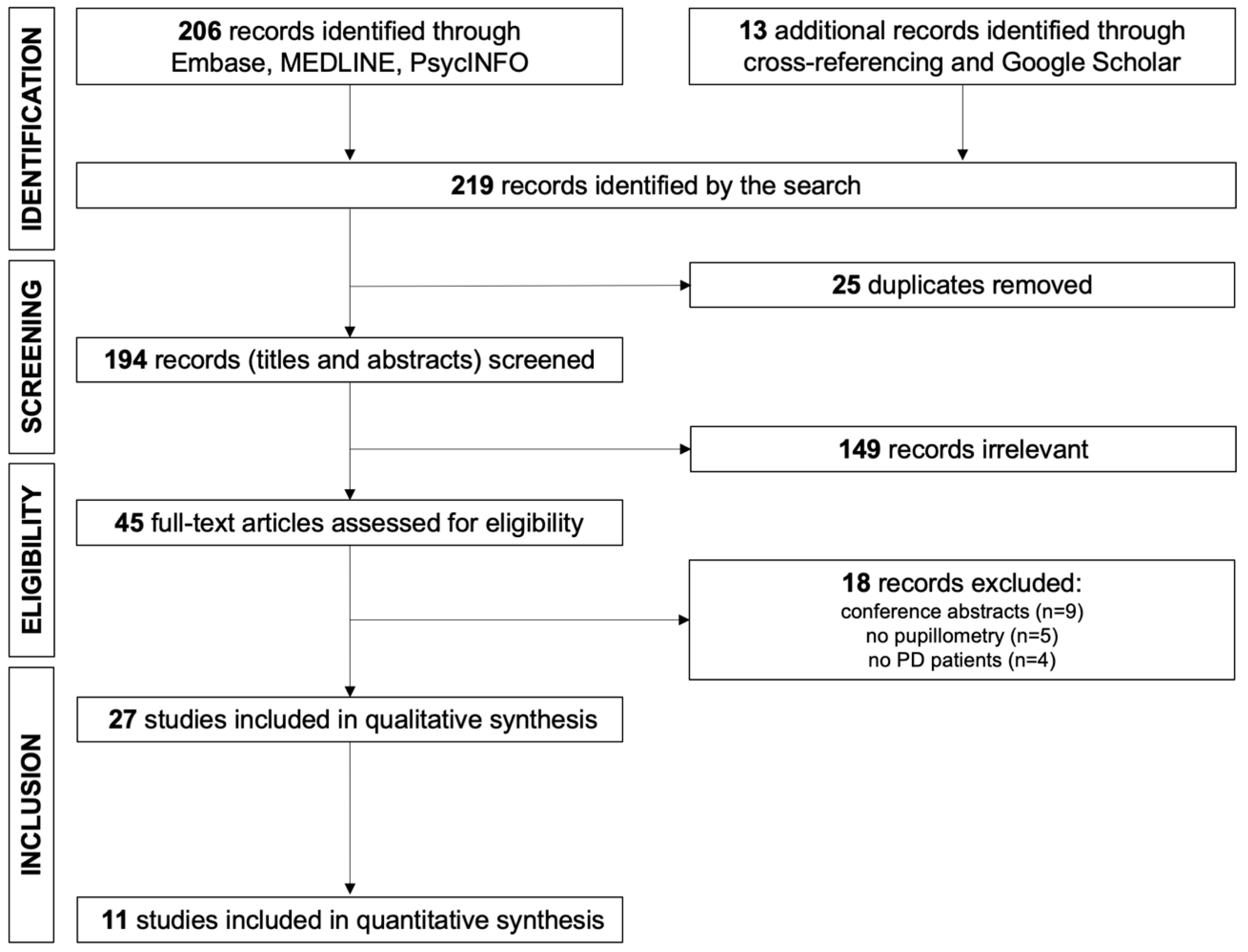
3.1. Study Selection
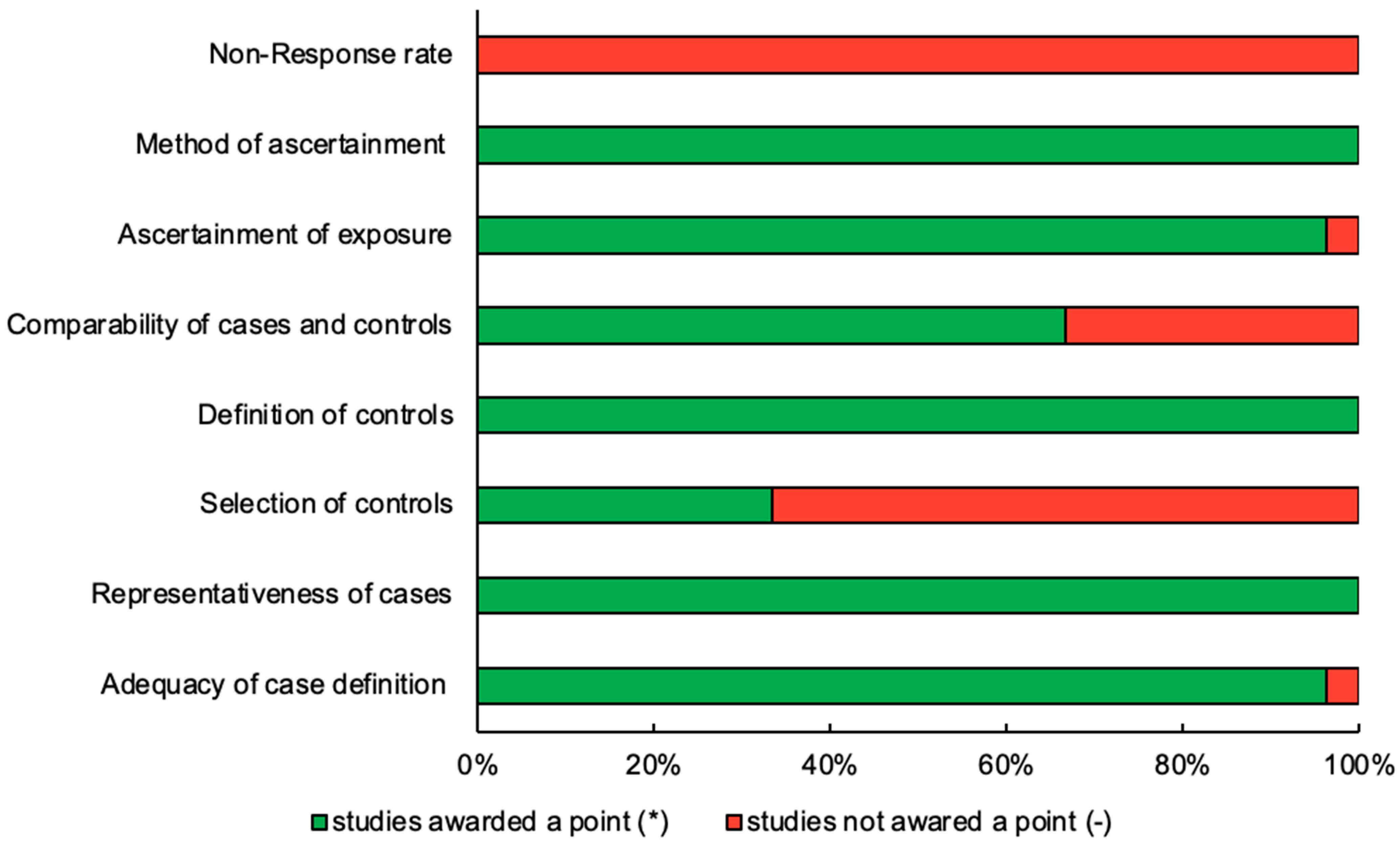
3.2. Assessment of Quality
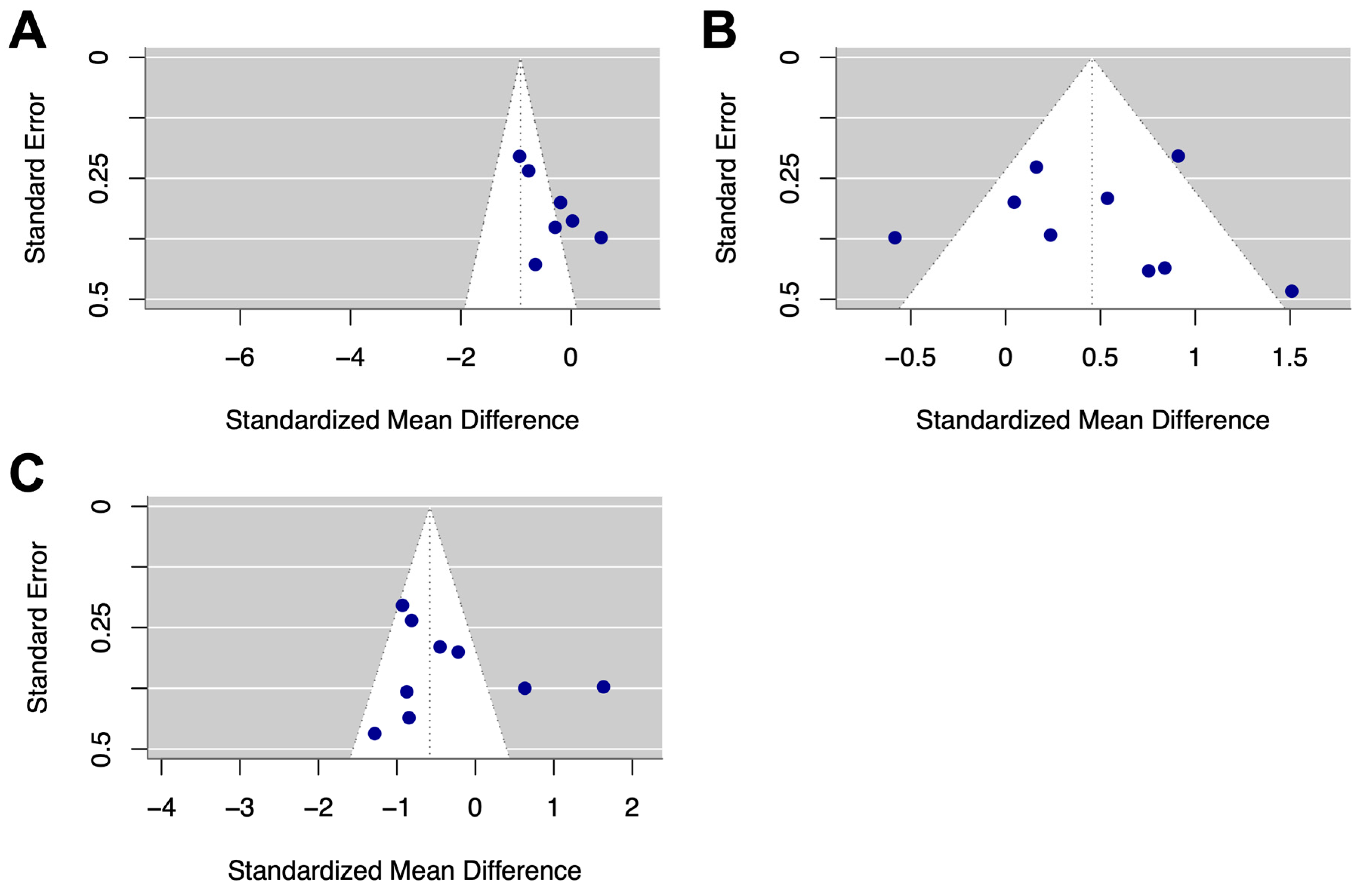
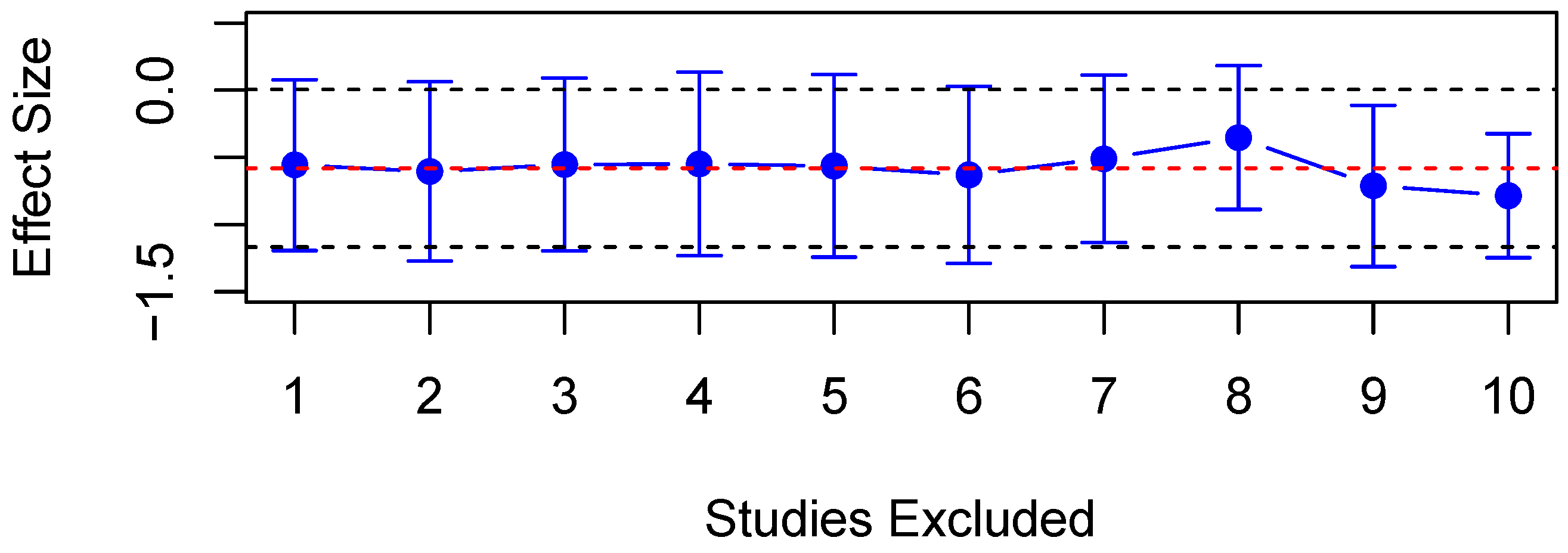
3.3. Evaluation for Publication Bias
3.4. Pupillary Light Reflex in Parkinson’s Disease
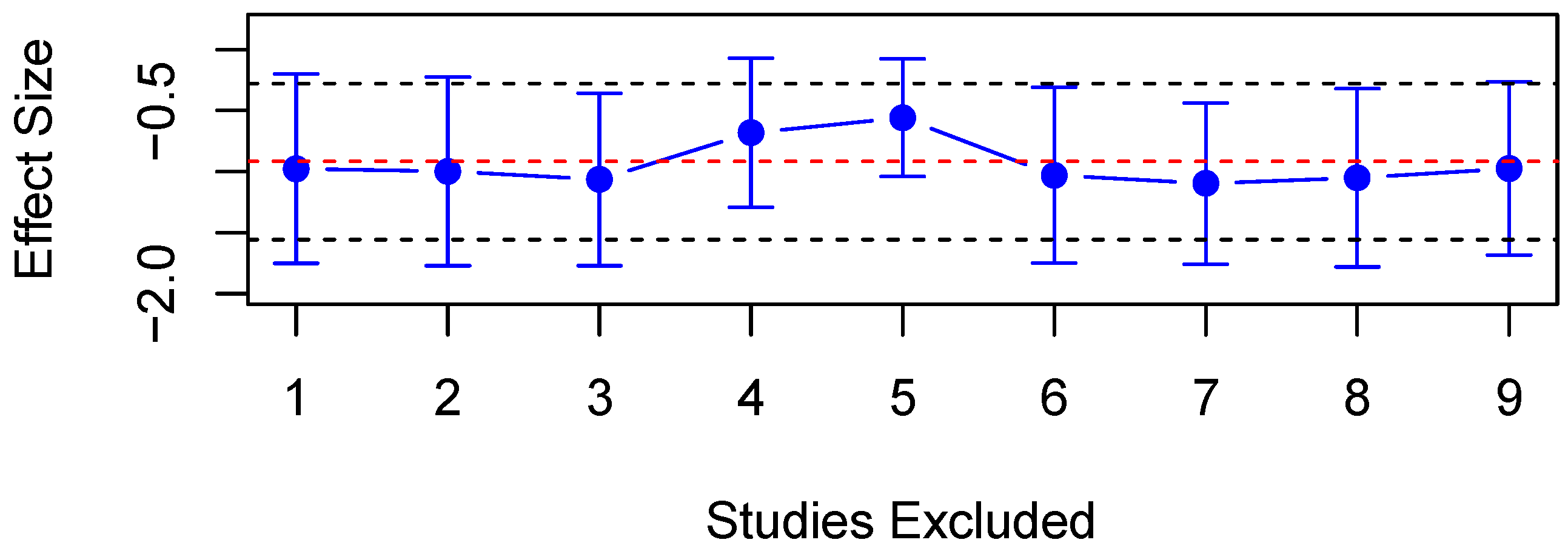
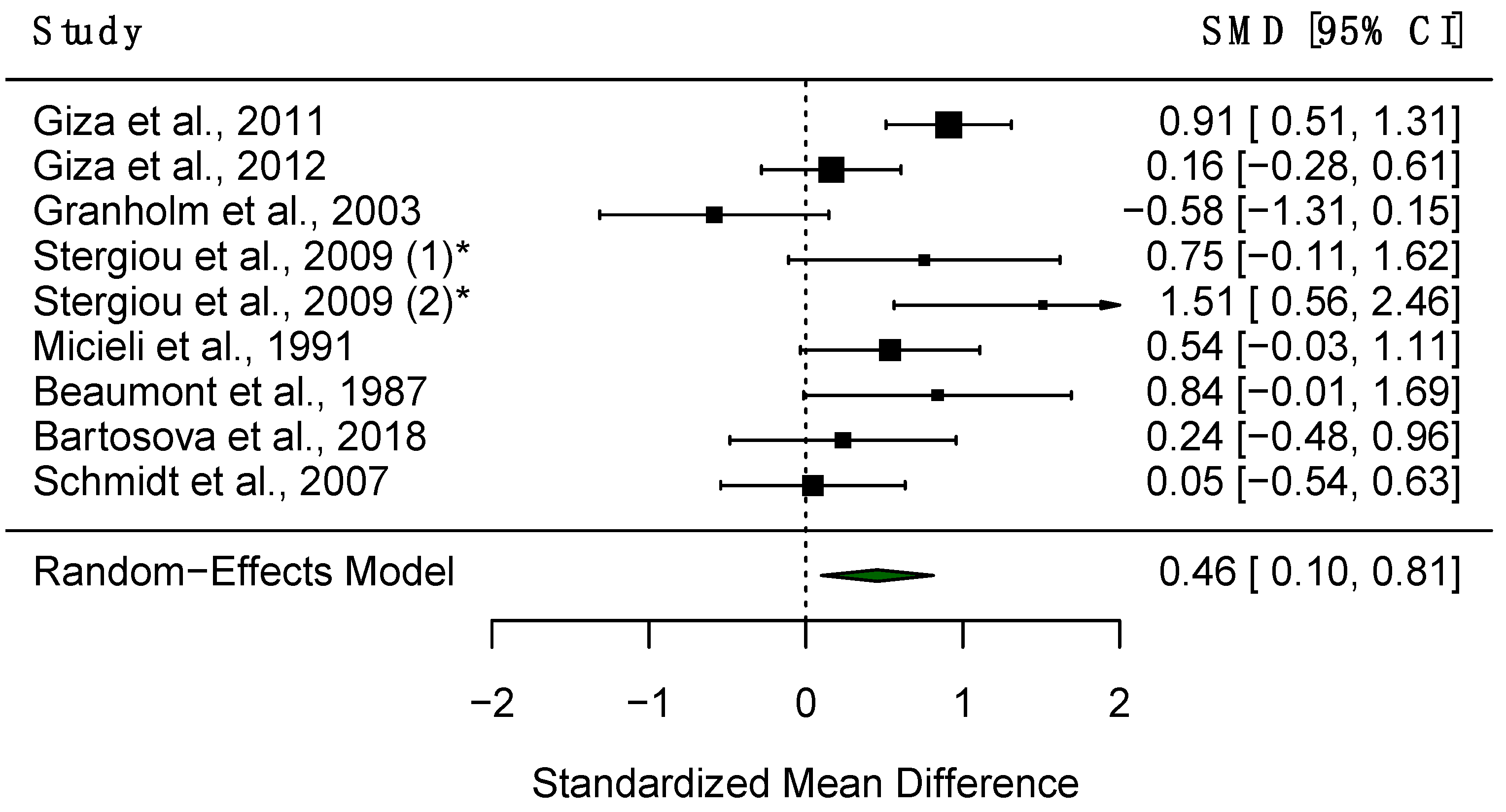
3.4.1. Maximum Contrition Velocity (VMax)
3.4.2. Constriction Latency (CL)
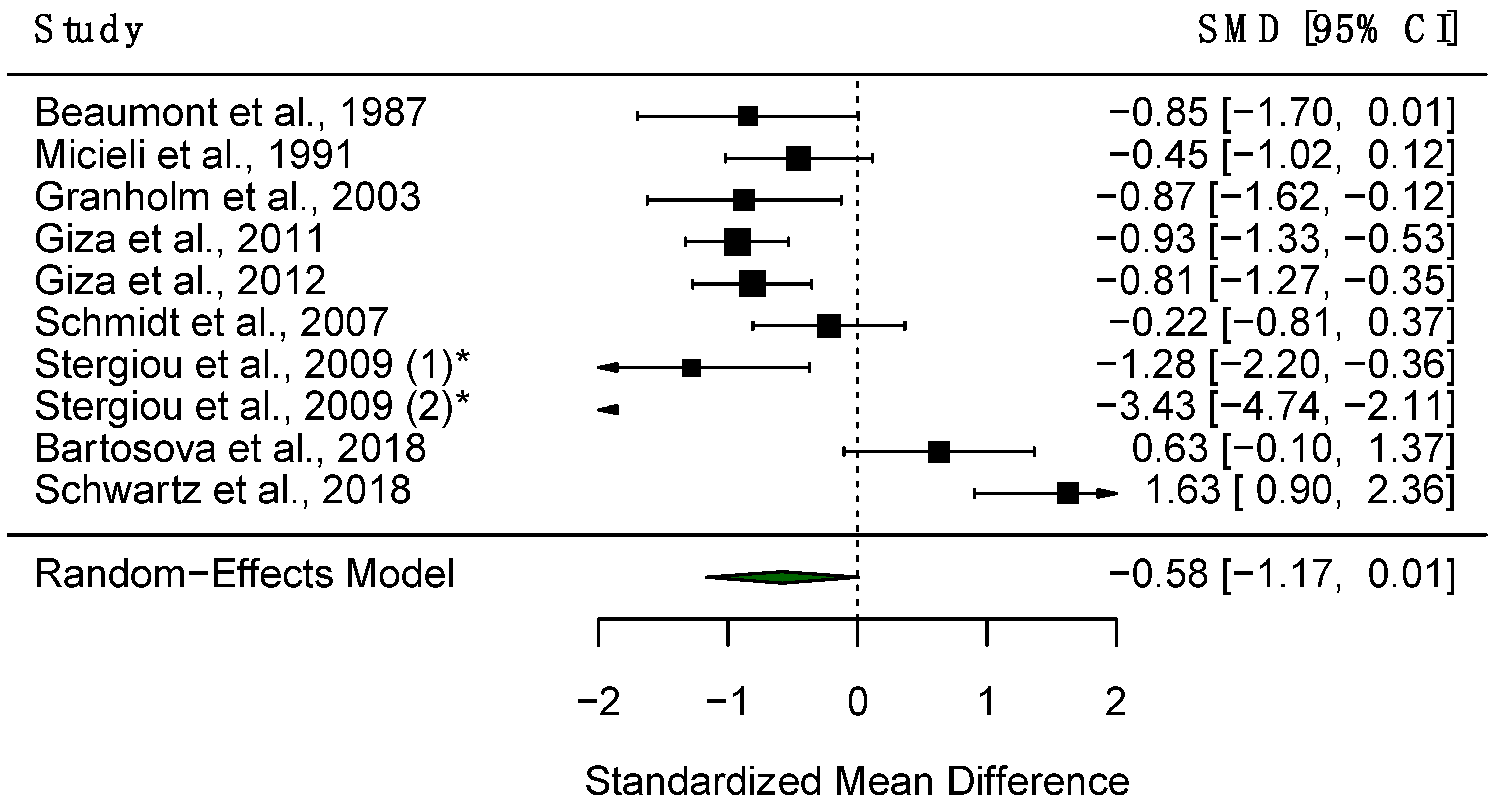
3.4.3. Constriction Amplitude
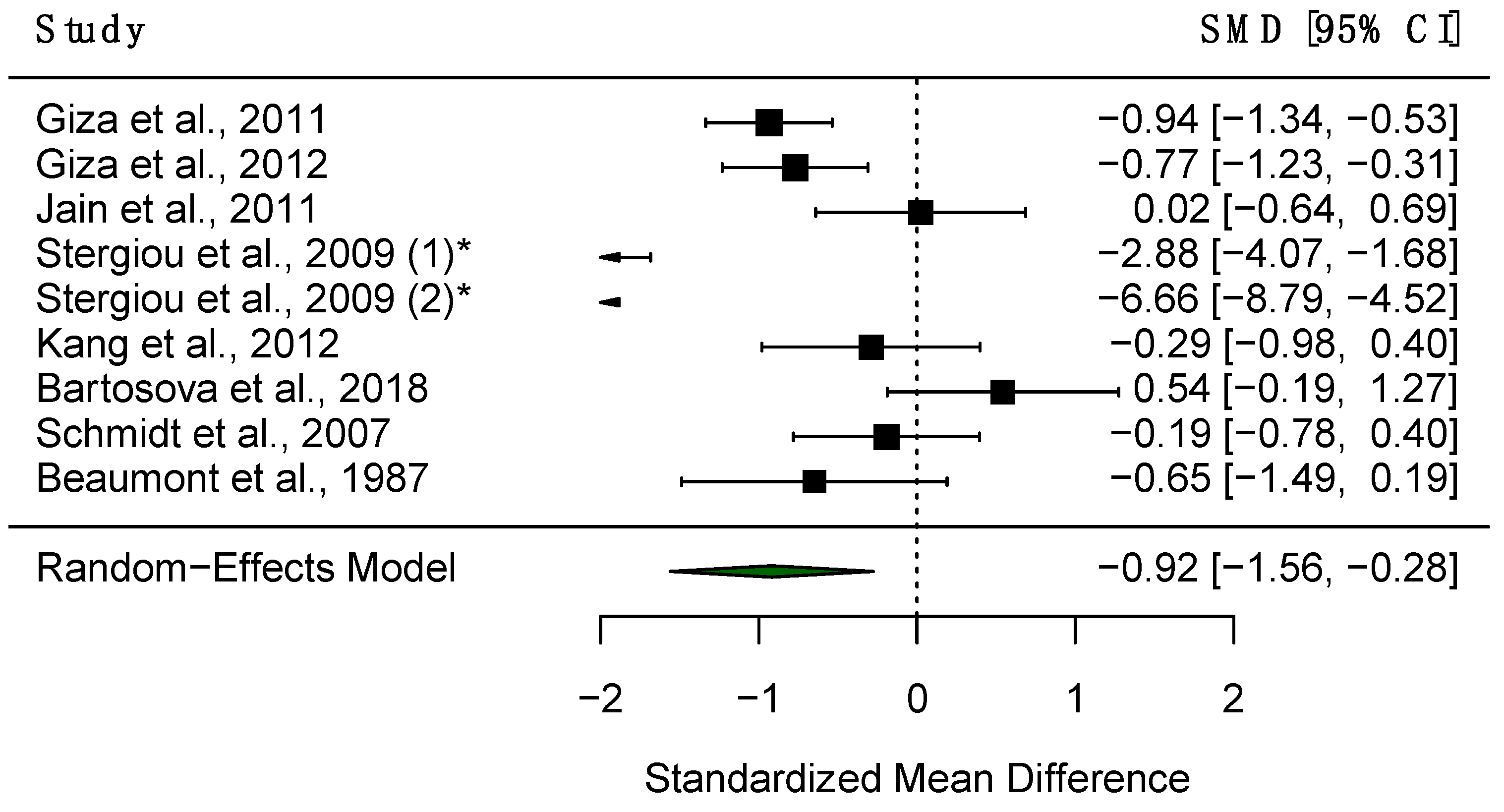
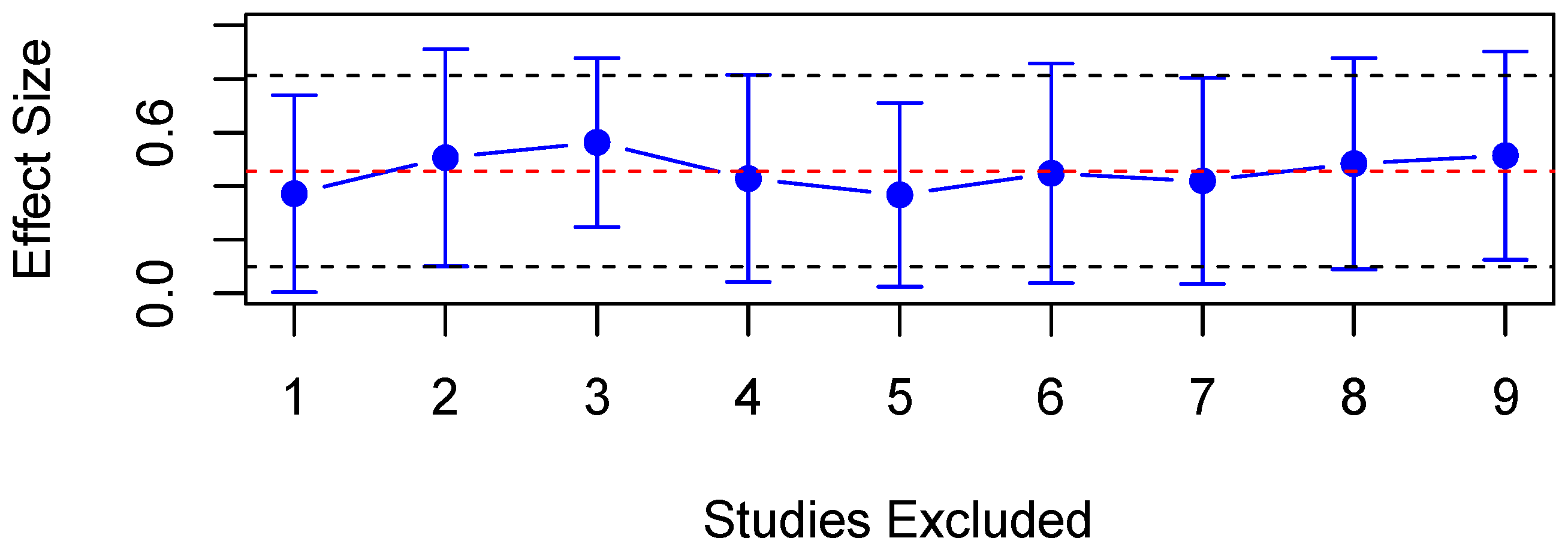
3.5. Pupillary Unrest
4. Discussion
4.1. PLR Parameters and Pupillary Unrest as PD Biomarkers
4.1.1. Findings of the Qualitative Synthesis
4.1.2. Effect Size
4.2. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronym | Meaning |
| AHRQ | Agency for Healthcare Research and Quality |
| AMax | Maximum Acceleration of Constriction |
| CAmp | Constriction Amplitude |
| CBD | Corticobasal Disease |
| CI | Confidence Interval |
| CL | Constriction Latency |
| DaT-SPECT | Dopamine Transporter Single-Photon Emission Computed Tomography |
| DLB | Dementia with Lewy Bodies |
| DPE | Dilated Pupil Equilibrium |
| DPE | Dipivefrine Hydrochloride |
| H&Y | Hoehn and Yahr |
| HC | Healthy Controls |
| ICA | Initial Constriction Amplitude |
| ipRGCs | Intrinsically Photosensitive Retinal Ganglion Cells |
| L-DOPA | Levodopa |
| MMSE | Mini-Mental State Examination |
| MRI | Magnetic Resonance Imaging |
| MSA | Multiple System Atrophy |
| NCI | Normalised Constriction Index |
| PD | Parkinson’s Disease |
| PL | Pilocarpine Hydrochloride |
| PLR | Pupillary Light Reflex |
| PSP | Progressive Supranuclear Palsy |
| REM | Rapid Eye Movements |
| RM | Response Mean |
| RMax | Maximum Response Pupil Size |
| SMD | Standardised Mean Difference |
| SMD | Standardised Mean Difference |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| VMax | Maximum Constriction Velocity |
References
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-Synuclein: Pathology, Mitochondrial Dysfunction and Neuroinflammation in Parkinson’s Disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef]
- Bonifati, V. Genetics of Parkinson’s Disease—State of the Art, 2013. Park. Relat Disord 2014, 20, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Singleton, A.B.; Farrer, M.J.; Bonifati, V. The Genetics of Parkinson’s Disease: Progress and Therapeutic Implications. Mov. Disord. 2013, 28, 14–23. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Durcan, R.; Wiblin, L.; Lawson, R.A.; Khoo, T.K.; Yarnall, A.J.; Duncan, G.W.; Brooks, D.J.; Pavese, N.; Burn, D.J.; ICICLE-PD Study Group. Prevalence and Duration of Non-Motor Symptoms in Prodromal Parkinson’s Disease. Eur. J. Neurol. 2019, 26, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Suwijn, S.R.; van Boheemen, C.J.M.; de Haan, R.J.; Tissingh, G.; Booij, J.; de Bie, R.M.A. The Diagnostic Accuracy of Dopamine Transporter SPECT Imaging to Detect Nigrostriatal Cell Loss in Patients with Parkinson’s Disease or Clinically Uncertain Parkinsonism: A Systematic Review. EJNMMI Res. 2015, 5, 12. [Google Scholar] [CrossRef]
- Prange, S.; Metereau, E.; Thobois, S. Structural Imaging in Parkinson’s Disease: New Developments. Curr. Neurol. Neurosci. Rep. 2019, 19, 50. [Google Scholar] [CrossRef]
- Tan, A.H.; Lim, S.Y.; Lang, A.E. The Microbiome-Gut-Brain Axis in Parkinson Disease—From Basic Research to the Clinic. Nat. Rev. Neurol. 2022, 18, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Kluge, A.; Bunk, J.; Schaeffer, E.; Drobny, A.; Xiang, W.; Knacke, H.; Bub, S.; Lückstädt, W.; Arnold, P.; Lucius, R.; et al. Detection of Neuron-Derived Pathological α-Synuclein in Blood. Brain 2022, 145, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Stower, H. Skin Test for Parkinson’s Disease. Nat. Med. 2020, 26, 1677. [Google Scholar] [CrossRef] [PubMed]
- Linder, J.; Libelius, R.; Nordh, E.; Holmberg, B.; Stenlund, H.; Forsgren, L. Anal Sphincter Electromyography in Patients with Newly Diagnosed Idiopathic Parkinsonism. Acta Neurol. Scand. 2012, 126, 248–255. [Google Scholar] [CrossRef]
- Paviour, D.C.; Williams, D.; Fowler, C.J.; Quinn, N.P.; Lees, A.J. Is Sphincter Electromyography a Helpful Investigation in the Diagnosis of Multiple System Atrophy? A Retrospective Study with Pathological Diagnosis. Mov. Disord. 2005, 20, 1425–1430. [Google Scholar] [CrossRef]
- Stankovic, I.; Fanciulli, A.; Kostic, V.S.; Krismer, F.; Meissner, W.G.; Palma, J.A.; Panicker, J.N.; Seppi, K.; Wenning, G.K. Laboratory-Supported Multiple System Atrophy beyond Autonomic Function Testing and Imaging: A Systematic Review by the MoDiMSA Study Group. Mov. Disord. Clin. Pract. 2021, 8, 322–340. [Google Scholar] [CrossRef]
- Pellecchia, M.T.; Stankovic, I.; Fanciulli, A.; Krismer, F.; Meissner, W.G.; Palma, J.; Panicker, J.N.; Seppi, K.; Wenning, G.K. Can Autonomic Testing and Imaging Contribute to the Early Diagnosis of Multiple System Atrophy? A Systematic Review and Recommendations by the Movement Disorder Society Multiple System Atrophy Study Group. Mov. Disord. Clin. Pract. 2020, 7, 750–762. [Google Scholar] [CrossRef]
- Leys, F.; Fanciulli, A.; Ndayisaba, J.-P.; Granata, R.; Struhal, W.; Wenning, G.K. Cardiovascular Autonomic Function Testing in Multiple System Atrophy and Parkinson’s Disease: An Expert-Based Blinded Evaluation. Clin. Auton. Res. 2020, 30, 255–263. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic Control of the Eye. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 5, pp. 439–473. [Google Scholar] [CrossRef]
- Hall, C.A.; Chilcott, R.P. Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics. Diagnostics 2018, 8, 19. [Google Scholar] [CrossRef]
- Wang, C.A.; McInnis, H.; Brien, D.C.; Pari, G.; Munoz, D.P. Disruption of Pupil Size Modulation Correlates with Voluntary Motor Preparation Deficits in Parkinson’s Disease. Neuropsychologia 2016, 80, 176–184. [Google Scholar] [CrossRef]
- Mailankody, P.; Battu, R.; Lenka, A.; Mohammed Shereef, P.M.; Thennarasu, K.; Yadav, R.; Pal, P.K. Retinal Changes in Parkinson’s Disease: A Longitudinal Follow-up Study. Neurol. India 2022, 70, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B.; Dumpala, S.; Kerr, G.K.; Zele, A.J. Melanopsin Cell Dysfunction Is Involved in Sleep Disruption in Parkinson’s Disease. J. Park. Dis. 2020, 10, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.; Bradley, M.M.; Okun, M.S.; Bowers, D. Emotion and Ocular Responses in Parkinson’s Disease. Neuropsychologia 2011, 49, 3247–3253. [Google Scholar] [CrossRef]
- Fotiou, D.F.; Stergiou, V.; Tsiptsios, D.; Lithari, C.; Nakou, M.; Karlovasitou, A. Cholinergic Deficiency in Alzheimer’s and Parkinson’s Disease: Evaluation with Pupillometry. Int. J. Psychophysiol. 2009, 73, 143–149. [Google Scholar] [CrossRef]
- Giza, E.; Fotiou, D.; Bostantjopoulou, S.; Katsarou, Z.; Karlovasitou, A. Pupil Light Reflex in Parkinson’s Disease: Evaluation with Pupillometry. Int. J. Neurosci. 2011, 121, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Giza, E.; Fotiou, D.; Bostantjopoulou, S.; Katsarou, Z.; Gerasimou, G.; Gotzamani-Psarrakou, A.; Karlovasitou, A. Pupillometry and 123I-DaTSCAN Imaging in Parkinson’s Disease: A Comparison Study. Int. J. Neurosci. 2012, 122, 26–34. [Google Scholar] [CrossRef]
- Stergiou, V.; Fotiou, D.; Tsiptsios, D.; Haidich, B.; Nakou, M.; Giantselidis, C.; Karlovasitou, A. Pupillometric Findings in Patients with Parkinson’s Disease and Cognitive Disorder. Int. J. Psychophysiol. 2009, 72, 97–101. [Google Scholar] [CrossRef]
- Granholm, E.; Morris, S.; Galasko, D.; Shults, C.; Rogers, E.; Vukov, B. Tropicamide Effects on Pupil Size and Pupillary Light Reflexes in Alzheimer’s and Parkinson’s Disease. Int. J. Psychophysiol. 2003, 47, 95–115. [Google Scholar] [CrossRef]
- Micieli, G.; Tassorelli, C.; Martignoni, E.; Pacchetti, C.; Bruggi, P.; Magri, M.; Nappi, G. Disordered Pupil Reactivity in Parkinson’s Disease. Clin. Auton. Res. 1991, 1, 55–58. [Google Scholar] [CrossRef]
- Mestanikova, A.; Ondrejka, I.; Mestanik, M.; Cesnekova, D.; Visnovcova, Z.; Bujnakova, I.; Oppa, M.; Calkovska, A.; Tonhajzerova, I. Pupillary Light Reflex Is Altered in Adolescent Depression. Physiol. Res. 2017, 66, S277–S284. [Google Scholar] [CrossRef]
- Im, J.J.; Jeong, H.; Chung, Y.-A.; Park, J.-S.; Heo, Y.; Oh, J.K.; Song, I.-U. Neuroprotective Effects of Rasagiline in Parkinson’s Disease: A Regional Cerebral Blood Flow Study. J. Neuroimaging 2019, 29, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Van Stavern, G.P.; Bei, L.; Shui, Y.B.; Huecker, J.; Gordon, M. Pupillary Light Reaction in Preclinical Alzheimer’s Disease Subjects Compared with Normal Ageing Controls. Br. J. Ophthalmol. 2019, 103, 971–975. [Google Scholar] [CrossRef]
- Bittner, D.M.; Wieseler, I.; Wilhelm, H.; Riepe, M.W.; Müller, N.G. Repetitive Pupil Light Reflex: Potential Marker in Alzheimer’s Disease? J. Alzheimer’s Dis. 2014, 42, 1469–1477. [Google Scholar] [CrossRef]
- Tales, A.; Troscianko, T.; Lush, D.; Haworth, J.; Wilcock, G.K.; Butler, S.R. The Pupillary Light Reflex in Aging and Alzheimer’s Disease. Aging Clin. Exp. Res. 2001, 13, 473–478. [Google Scholar]
- Prettyman, R.; Bitsios, P.; Szabadi, E. Altered Pupillary Size and Darkness and Light Reflexes in Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 665–668. [Google Scholar] [CrossRef]
- Fotiou, D.F.; Brozou, C.G.; Haidich, A.B.; Tsiptsios, D.; Nakou, M.; Kabitsi, A.; Giantselidis, C.; Fotiou, F. Pupil Reaction to Light in Alzheimer’s Disease: Evaluation of Pupil Size Changes and Mobility. Aging Clin. Exp. Res. 2007, 19, 364–371. [Google Scholar] [CrossRef]
- Bär, K.J.; Boettger, M.K.; Schulz, S.; Harzendorf, C.; Agelink, M.W.; Yeragani, V.K.; Chokka, P.; Voss, A. The Interaction between Pupil Function and Cardiovascular Regulation in Patients with Acute Schizophrenia. Clin. Neurophysiol. 2008, 119, 2209–2213. [Google Scholar] [CrossRef]
- Koller, D.; Saiz-Rodríguez, M.; Zubiaur, P.; Ochoa, D.; Almenara, S.; Román, M.; Romero-Palacián, D.; Miguel-Cáceres, A.; Martín, S.; Navares-Gómez, M.; et al. The Effects of Aripiprazole and Olanzapine on Pupillary Light Reflex and Its Relationship with Pharmacogenetics in a Randomized Multiple-dose Trial. Br. J. Clin. Pharmacol. 2020, 86, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Laurenzo, S.A.; Kardon, R.; Ledolter, J.; Poolman, P.; Schumacher, A.M.; Potash, J.B.; Full, J.M.; Rice, O.; Ketcham, A.; Starkey, C.; et al. Pupillary Response Abnormalities in Depressive Disorders. Psychiatry Res. 2016, 246, 492–499. [Google Scholar] [CrossRef]
- Yuhas, P.T.; Shorter, P.D.; McDaniel, C.E.; Earley, M.J.; Hartwick, A.T.E. Blue and Red Light-Evoked Pupil Responses in Photophobic Subjects with TBI. Optom. Vision. Sci. 2017, 94, 108–117. [Google Scholar] [CrossRef]
- Truong, J.Q.; Ciuffreda, K.J. Comparison of Pupillary Dynamics to Light in the Mild Traumatic Brain Injury (MTBI) and Normal Populations. Brain Inj. 2016, 30, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, K.J.; Joshi, N.R.; Truong, J.Q. Understanding the Effects of Mild Traumatic Brain Injury on the Pupillary Light Reflex. Concussion 2017, 2, CNC36. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.D.; Singh, V. Portable Infrared Pupillometry in Critical Care. Crit. Care 2016, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Graur, S.; Siegle, G. Pupillary Motility: Bringing Neuroscience to the Psychiatry Clinic of the Future. Curr. Neurol. Neurosci. Rep. 2013, 13, 365. [Google Scholar] [CrossRef]
- Siva kumar, A.V.; Maruthy, K.N.; Padmavathi, R.; Sowjanya, B.; MaheshKumar, K. Quantitative Determination of Pupil by Dynamic Pupillometry Using Infrared Videography—Role in Evaluation of Autonomic Activity. Clin. Epidemiol. Glob. Health 2020, 8, 728–732. [Google Scholar] [CrossRef]
- Troiani, V. The Future of Quantitative Pupillometry in Health and Disease. Clin. Auton. Res. 2020, 30, 11–12. [Google Scholar] [CrossRef]
- Lussier, B.L.; Olson, D.W.M.; Aiyagari, V. Automated Pupillometry in Neurocritical Care: Research and Practice. Curr. Neurol. Neurosci. Rep. 2019, 19, 71. [Google Scholar] [CrossRef]
- Mukherjee, S.; Vernino, S. Dysfunction of the Pupillary Light Reflex in Experimental Autoimmune Autonomic Ganglionopathy. Auton. Neurosci. 2007, 137, 19–26. [Google Scholar] [CrossRef]
- Park, J.C.; McAnany, J.J. Effect of Stimulus Size and Luminance on the Rod-, Cone-, and Melanopsin-Mediated Pupillary Light Reflex. J. Vis. 2015, 15, 13. [Google Scholar] [CrossRef][Green Version]
- Napieralski, P.; Rynkiewicz, F. Modeling Human Pupil Dilation to Decouple the Pupillary Light Reflex. Open Phys. 2019, 17, 458–467. [Google Scholar] [CrossRef]
- Ranchet, M.; Orlosky, J.; Morgan, J.; Qadir, S.; Akinwuntan, A.E.; Devos, H. Pupillary Response to Cognitive Workload during Saccadic Tasks in Parkinson’s Disease. Behav. Brain Res. 2017, 327, 162–166. [Google Scholar] [CrossRef]
- Manohar, S.G.; Husain, M. Reduced Pupillary Reward Sensitivity in Parkinson’s Disease. npj Park. Dis. 2015, 1, 15026. [Google Scholar] [CrossRef]
- Muhammed, K.; Manohar, S.; Ben Yehuda, M.; Chong, T.T.-J.; Tofaris, G.; Lennox, G.; Bogdanovic, M.; Hu, M.; Husain, M. Reward Sensitivity Deficits Modulated by Dopamine Are Associated with Apathy in Parkinson’s Disease. Brain 2016, 139, 2706–2721. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.S.; Muhammed, K.; Baig, F.; Kelly, M.; Saleh, Y.; Sarangmat, N.; Okai, D.; Hu, M.; Manohar, S.; Husain, M. Dopamine and Reward Hypersensitivity in Parkinson’s Disease with Impulse Control Disorder. Brain 2020, 143, 2502–2518. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.R.; Muhammed, K.; Drew, D.; Bradley, K.M.; McGowan, D.R.; Klein, J.C.; Manohar, S.G.; Hu, M.T.M.; Husain, M. Reward Insensitivity Is Associated with Dopaminergic Deficit in Rapid Eye Movement Sleep Behaviour Disorder. Brain 2023, 146, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Romagnoli, M.; Pizza, F.; Biscarini, F.; Filardi, M.; Donadio, V.; Carbonelli, M.; Amore, G.; Park, J.C.; Tinazzi, M.; et al. Chromatic Pupillometry in Isolated Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2022, 37, 205–210. [Google Scholar] [CrossRef]
- Gaynes, B.I.; Zaffer, A.; Yousefzai, R.; Chazaro-Cortes, M.; Colletta, K.; Kletzel, S.L.; Jost, M.B.; Park, Y.; Chawla, J.; Albert, M.V.; et al. Variable Abnormality of the Melanopsin-Derived Portion of the Pupillary Light Reflex (PLR) in Patients with Parkinson’s Disease (PD) and Parkinsonism Features. Neurol. Sci. 2022, 43, 349–356. [Google Scholar] [CrossRef]
- Park, J.C.; Moura, A.L.; Raza, A.S.; Rhee, D.W.; Kardon, R.H.; Hood, D.C. Toward a Clinical Protocol for Assessing Rod, Cone, and Melanopsin Contributions to the Human Pupil Response. Invest. Ophthalmol. Vis. Sci. 2011, 52, 6624–6635. [Google Scholar] [CrossRef]
- Guo, L.; Normando, E.M.; Shah, P.A.; De Groef, L.; Cordeiro, M.F. Oculo-Visual Abnormalities in Parkinson’s Disease: Possible Value as Biomarkers. Mov. Disord. 2018, 33, 1390–1406. [Google Scholar] [CrossRef]
- Wang, Y.; Zekveld, A.A.; Naylor, G.; Ohlenforst, B.; Jansma, E.P.; Lorens, A.; Lunner, T.; Kramer, S.E. Parasympathetic Nervous System Dysfunction, as Identified by Pupil Light Reflex, and Its Possible Connection to Hearing Impairment. PLoS ONE 2016, 11, e0153566. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Controlling the Risk of Spurious Findings from Meta-Regression. Stat. Med. 2004, 23, 1663–1682. [Google Scholar] [CrossRef]
- Tobias, A. Assessing the Influence of a Single Study in the Meta-Anyalysis Estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. Available online: http://www.rstudio.com/ (accessed on 15 March 2023).
- Beaumont, S.; Harris, J.; Leendertz, J.; Phillipson, O. The Pupillary Light Reflex in Mild Parkinsons-Disease. Clin. Vision. Sci. 1987, 2, 123–129. [Google Scholar]
- Ørskov, L.; Jakobsen, J.; Dupont, E.; de Fine Olivarius, B.; Christensen, N.J. Autonomic Function in Parkinsonian Patients Relates to Duration of Disease. Neurology 1987, 37, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Utsumi, T. Pupillary Dynamics in Parkinson’s Disease. Neuro-Ophthalmol. 1990, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Herting, B.; Prieur, S.; Junghanns, S.; Schweitzer, K.; Globas, C.; Schöls, L.; Antoni, S.; Ferger, D.; Reichmann, H.; et al. Pupil Diameter in Darkness Differentiates Progressive Supranuclear Palsy (PSP) from Other Extrapyramidal Syndromes. Mov. Disord. 2007, 22, 2123–2126. [Google Scholar] [CrossRef]
- Hori, N.; Takamori, M.; Hirayama, M.; Watanabe, H.; Nakamura, T.; Yamashita, F.; Ito, H.; Mabuchi, N.; Sobue, G. Pupillary Supersensitivity and Visual Disturbance in Parkinson’s Disease. Clin. Auton. Res. 2008, 18, 20–27. [Google Scholar] [CrossRef]
- Yamashita, F.; Hirayama, M.; Nakamura, T.; Takamori, M.; Hori, N.; Uchida, K.; Hama, T.; Sobue, G. Pupillary Autonomic Dysfunction in Multiple System Atrophy and Parkinson’s Disease: An Assessment by Eye-Drop Tests. Clin. Auton. Res. 2010, 20, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Siegle, G.J.; Gu, C.; Moore, C.G.; Ivanco, L.S.; Studenski, S.; Greenamyre, J.T.; Steinhauer, S.R. Pupillary Unrest Correlates with Arousal Symptoms and Motor Signs in Parkinson Disease. Mov. Disord. 2011, 26, 1344–1347. [Google Scholar] [CrossRef]
- Jain, S.; Siegle, G.J.; Gu, C.; Moore, C.G.; Ivanco, L.S.; Jennings, J.R.; Steinhauer, S.R.; Studenski, S.; Greenamyre, J.T. Autonomic Insufficiency in Pupillary and Cardiovascular Systems in Parkinson’s Disease. Park. Relat. Disord. 2011, 17, 119–122. [Google Scholar] [CrossRef][Green Version]
- Kang, P.; Kloke, J.; Jain, S. Olfactory Dysfunction and Parasympathetic Dysautonomia in Parkinson’s Disease. Clin. Auton. Res. 2012, 22, 161–166. [Google Scholar] [CrossRef]
- Bartošová, O.; Bonnet, C.; Ulmanová, O.; Šíma, M.; Perlík, F.; Růžička, E.; Slanař, O. Pupillometry as an Indicator of L-DOPA Dosages in Parkinson’s Disease Patients. J. Neural Transm. 2018, 125, 699–703. [Google Scholar] [CrossRef]
- Kahya, M.; Moon, S.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.E.; Devos, H. Pupillary Response to Cognitive Demand in Parkinson’s Disease: A Pilot Study. Front. Aging Neurosci. 2018, 10, 90. [Google Scholar] [CrossRef]
- Schwartz, R.; Rothermich, K.; Kotz, S.A.; Pell, M.D. Unaltered Emotional Experience in Parkinson’s Disease: Pupillometry and Behavioral Evidence. J. Clin. Exp. Neuropsychol. 2018, 40, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Goyal, V.; Sood, S.K.; Sharma, R. Reduced Saccadic Velocity and Pupillary Width in Young Onset Parkinson’s Disease. Neurol. Psychiatry Brain Res. 2018, 27, 17–20. [Google Scholar] [CrossRef]
- Araga, T.; Shiraishi, M.; Hasegawa, Y. Video Pupillometric Evaluation of the Pupillary Reflex as a Test for the Clinical Manifestations of Parkinson’s Disease. J. St. Marian. Univ. 2019, 10, 19–26. [Google Scholar] [CrossRef]
- Kahya, M.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.E.; He, J.; Devos, H. Reliability and Validity of Pupillary Response During Dual-Task Balance in Parkinson Disease. Arch. Phys. Med. Rehabil. 2020, 102, 448–455. [Google Scholar] [CrossRef]
- Moon, S.; Kahya, M.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.E.; Devos, H. Cognitive Workload during Verbal Abstract Reasoning in Parkinson’s Disease: A Pilot Study. Int. J. Neurosci. 2020, 131, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.I.; Whitsett, T.L. Cardiovascular Effects of Levodopa. Clin. Pharmacol. Ther. 1971, 12, 376–382. [Google Scholar] [CrossRef]
- Wang, C.-A.; Munoz, D.P. Coordination of Pupil and Saccade Responses by the Superior Colliculus. J. Cogn. Neurosci. 2021, 33, 919–932. [Google Scholar] [CrossRef]
- Zangemeister, W.H.; Gronow, T.; Grzyska, U. Pupillary Responses to Single and Sinusoidal Light Stimuli in Diabetic Patients. Neurol. Int. 2009, 1, 19. [Google Scholar] [CrossRef]
- Yan, Y.-J.; Chen, C.-N.; Ou-Yang, M. Using System Identification to Construct an Inherent Model of Pupillary Light Reflex to Explore Diabetic Neuropathy. Brain Sci. 2021, 11, 852. [Google Scholar] [CrossRef]
- Stark, L. Stability, Oscillations, and Noise in the Human Pupil Servomechanism. Proc. IRE 1959, 47, 1925–1939. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Figorilli, M.; Meloni, M.; Lanza, G.; Casaglia, E.; Lecca, R.; Saibene, F.L.; Congiu, P.; Puligheddu, M. Considering REM Sleep Behavior Disorder in the Management of Parkinson’s Disease. Nat. Sci. Sleep. 2023, 15, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D. Advances in Markers of Prodromal Parkinson Disease. Nat. Rev. Neurol. 2016, 12, 622–634. [Google Scholar] [CrossRef]
- McCarter, S.J.; Gehrking, T.L.; St. Louis, E.K.; Suarez, M.D.; Boeve, B.F.; Silber, M.H.; Low, P.A.; Singer, W. Autonomic Dysfunction and Phenoconversion in Idiopathic REM Sleep Behavior Disorder. Clin. Auton. Res. 2020, 30, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Otano, J.; Llansó, L.; Alejaldre, A.; Diez, L.; Santamaría, J.; Iranzo, A. Autonomic Nervous System Dysfunction in Idiopathic REM Sleep Behavior Disorder as a Short-Term Risk for a Synucleinopathy. J. Neurol. 2025, 272, 1. [Google Scholar] [CrossRef]
- Riboldi, G.M.; Russo, M.J.; Pan, L.; Watkins, K.; Kang, U.J. Dysautonomia and REM Sleep Behavior Disorder Contributions to Progression of Parkinson’s Disease Phenotypes. npj Park. Dis. 2022, 8, 110. [Google Scholar] [CrossRef]

| Reference | Population Characteristics | Study Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Participants (PD/HC) | Mean Age (Years) | % Male | Mean Duration of Disease (Years) | Mean UPDRS III Score | Mean H&Y Score | Levodopa Equivalent Dose (mg/Day) | Pupillometric Device | Stimulus Used | Main Findings | Limitations | |
| Beaumont et al., 1987 [72] | 12/11 | 61.3 | - | - | - | - | - | IR-sensitive TV camera | IR light | Longer CL and decreased CAmp in PD patients. | - |
| Orskov et al., 1987 [73] | 23/10 | 62.4 | - | 6.5 | - | 2.7 | - | IR-sensitive camera | Photostimulator | No difference in CL, VMax, and CAmp between PD patients and controls. | - |
| Sugiyama and Utsumi, 1989 [74] | 24/30 | 64.0 | - | 4.70 | - | 2.4 | - | IR video pupillography | IR light | Lower AMax, CAmp, and VMax in PD patients. | - |
| Micieli et al., 1991 [30] | 23/26 | 56.2 | 71.4 | 2.3 | - | 1.8 | - | IR binocular TV pupilometer | Photostimulator | Longer CL and greater CAmp, CT and greater light adaptation in PD patients. | - |
| Granholm et al., 2003 [29] | 15/15 | 69.8 | 53.3 | - | - | - | - | Binocular IR pupillograph | Photostimulator | Greater RMin and decreased CAmp in PD patients. | Small sample size; potential effects of medication. |
| Schmidt et al., 2007 [75] | 19/27 | 62.0 | 60.9 | 3.50 | - | 2.0 | 488 | Compact integrated pupillograph | Photostimulator | Lower CAmp, ID and CT in PD patients. | - |
| Hori et al., 2008 [76] | 40/17 | 62.5 | 63.0 | 8.30 | - | 2.9 | - | IR-sensitive camera | IR iriscorder | Pupillary sensitivity to PL and DPE greater in PD patients. | Did not assess the effect of corneal permeability. |
| Stergiou et al., 2009 [28] | 22/11 | 72.7 | 45.5 | 5.10 | - | - | - | Charged coupled device | IR light | Lower AMax and VMax in PD patients. Lower AMax, VMax, and CAmp in PD-CI compared to PD-NCI. | - |
| Yamashita et al., 2010 [77] | 40/20 | 63.7 | 53.3 | 2.70 | - | - | - | Binocular Iriscorder | - | Pupillary sensitivity to DPE and PL greater in PD patients. | - |
| Dietz et al., 2011 [24] | 14/12 | 71.7 | 65.4 | - | 25.1 | 2.3 | 737.1 | Eye tracker | IR light | Attenuated light reflex in PD patients. | Potential effects of medication. |
| Giza et al., 2011 [26] | 66/44 | 64.2 | 58.2 | 4.7 | 21.2 | 2.0 | - | Charge-coupled high-speed digital camera | Photostimulator | Longer CL and decreased CAmp, VMax, and AMax in PD patients. | - |
| Jain et al., 2011 a [78] | 14/17 | 61.7 | 58.1 | - | 10.1 | 1.7 | - | IR video pupillography | IR light | Pupillary unrest greater in PD patients. | Small sample size; limited range of PD severity; limited range of arousal symptoms; cross-sectional design. |
| Jain et al., 2011 b [79] | 17/18 | 62.5 | 62.9 | - | 11.1 | 1.7 | 516 | IR video pupillography | IR light | Pupillary unrest greater in PD patients. No difference in VMax. | Small sample size; limited range of PD severity; residual effects of medication (withheld for 12 h before testing). |
| Giza et al., 2012 [27] | 35/44 | 63.7 | 58.2 | - | - | - | - | Charge-coupled high-speed digital camera | Photostimulator | Longer CL and decreased CAmp, VMax, and AMax in PD patients. | - |
| Kang et al., 2012 [80] | 15/18 | 62.8 | 69.7 | - | 10.7 | 1.7 | 516 | - | Photostimulator | No difference in VMax between PD patients and controls. | Small sample size; cross-sectional design; limited range in PD severity; possibility of residual effects of longer-acting anti-Parkinsonian medications affecting results after withholding medications for 12 h. |
| Manohar and Husain 2015 [53] | 16/22 | 63.8 | 46.3 | - | 23.1 | 1.8 | - | Eye tracker | Photostimulator | Greater ID in PD patients. No difference in CAmp. | - |
| Muhammed et al., 2016 [54] | 40/20 | 52.9 | 42.9 | 5.0 | 19.4 | 1.3 | - | Eye tracker | Auditory cue | No difference in ID and reward sensitivity between PD patients and controls. | - |
| Wang et al., 2016 [21] | 22/19 | 68 | 56.1 | 6.10 | 29.5 | 2.4 | 685 | Eye tracker | Visual stimulus | TMiosis longer and ID greater and dilation size smaller in PD patients. | - |
| Ranchet et al., 2017 [52] | 21/16 | 67 | 64 | 5.2 | 27.6 | 2.0 | 300 (CI), 463 (NCI) | Eye tracker | Prosaccade & antisaccade tasks | Greater cognitive workload for PD-CI in pro-saccade task and for PD-NCI in anti-saccade task. | Potential effects of medication; large heterogeneity in CI group; task design. |
| Bartosova et al., 2018 [81] | 16/14 | 58.2 | 70.0 | 10.0 | - | - | 532 | Monocular IR pupillograph | Photostimulator | Higher RMin and RMax in PD patients, no difference in VMax and CL. | - |
| Kahya et al., 2018 [82] | 16/10 | - | 46.2 | 6.0 | 30.0 | 2.3 | 750 | Eye tracker | - | No difference in cognitive load between PD patients and controls. | Small sample size; groups not matched for sex. |
| Schwartz et al., 2018 [83] | 17/22 | 63.6 | 51.3 | 9.90 | - | - | - | Eye tracker | Emotional video stimuli | No differenced in pupil dilation and diameter between PD patients and controls. | Unable to fully control the brightness and contrast between video clips. |
| Srivastava et al., 2018 [84] | 12/10 | 36.1 | 100.0 | 5.2 | - | - | - | Eye tracker | Visual stimulus | Reduced pupil width in PD patients. | Small sample size; influence of medication not taken into account. |
| Araga et al., 2019 [85] | 45/20 | 64.3 | 50.8 | - | 28.9 | 2.9 | 644.3 | Eye tracker | IR light | Reduced RMin in PD patients, no difference in ID. | Did not evaluate retinal function; did not compare any other diseases with Parkinsonism syndrome. |
| Drew et al., 2020 [55] | 26/31 | - | - | 4.9 | 22.9 | 1.3 | 497.15 | Eye tracker | Visual targets | ID greater in PD patients ON compared to OFF medication. | Patient groups might not be representative of typical cases; sample sizes relatively small; potentially heterogeneous; baseline factors that might also have been potential confounding factors. |
| Kahya et al., 2020 [86] | 33/35 | 69.0 | 48.5 | - | 44.0 | 2.3 | 302.8 | IR camera and eye tracker | - | Greater ICA in PD patients with which correlates with pupillary response in PD patients. | Fluctuations in cognitive and motor performance in PD; relies on self-report. |
| Moon et al., 2020 [87] | 19/10 | 66.2 | 58.6 | 5.70 | 30.9 | 2.1 | 749.3 | Eye tracker | Cognitive tasks | Greater cognitive load in PD patients. | Small sample size; potential effects of medication; unmatched sex distribution; unequal participant numbers; cross-sectional design. |
| Reference | Selection | Comparability | Exposure | Quality Score (AHRQ Standard) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adequacy of Case Definition | Representativeness of Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls | Ascertainment of Exposure | Method of Ascertainment | Non-Response Rate | ||
| Beaumont et al., 1987 [72] | * | * | - | * | - | * - | * | - | Poor |
| Orskov et al., 1987 [73] | * | * | - | * | ** (age, sex) | * - | * | - | Good |
| Sugiyama and Utsumi, 1989 [74] | * | * | - | * | ** (age, sex) | * - | * | - | Good |
| Micieli et al., 1991 [30] | - | * | - | * | - | - - | * | - | Poor |
| Granholm et al., 2003 [29] | * | * | - | * | - | * - | * | - | Poor |
| Schmidt et al., 2007 [75] | * | * | - | * | ** (age, sex) | * - | * | - | Good |
| Hori et al., 2008 [76] | * | * | - | * | ** (age, sex) | * - | * | - | Good |
| Stergiou et al., 2009 [28] | * | * | * | * | ** (age, sex) | * - | * | - | Good |
| Yamashita et al., 2010 [77] | * | * | - | * | - | * - | * | - | Poor |
| Dietz et al., 2011 [24] | * | * | * | * | - | * * | * | - | Poor |
| Giza et al., 2011 [26] | * | * | - | * | * (age) | * - | * | - | Good |
| Jain et al., 2011 a [78] | * | * | - | * | ** (age, sex) | * * | * | - | Good |
| Jain et al., 2011 b [79] | * | * | - | * | ** (age, sex) | * * | * | - | Good |
| Giza et al., 2012 [27] | * | * | - | * | * (age) | * - | * | - | Good |
| Kang et al., 2012 [80] | * | * | - | * | ** (age, sex) | * - | * | - | Good |
| Manohar and Hussain 2015 [53] | * | * | - | * | * (age) | * - | * | - | Good |
| Muhammed et al., 2016 [54] | * | * | * | * | * (age) | * - | * | - | Good |
| Wang et al., 2016 [21] | * | * | * | * | * (age) | * - | * | - | Good |
| Ranchet et al., 2017 [52] | * | * | * | * | -*(sex) | * - | * | - | Poor |
| Bartosova et al., 2018 [81] | * | * | - | * | - | * - | * | - | Poor |
| Kahya et al., 2018 [82] | * | * | * | * | ** (age, education) | * - | * | - | Good |
| Schwartz et al., 2018 [83] | * | * | - | * | ** (age, education) | * * | * | - | Good |
| Srivastava et al., 2018 [84] | * | * | * | * | * (age) | * * | * | - | Good |
| Araga et al., 2019 [85] | * | * | - | * | - | * - | * | - | Poor |
| Drew et al., 2020 [55] | * | * | - | * | - | * - | * | - | Poor |
| Kahya et al., 2020 [86] | * | * | * | * | ** (age, sex) | * - | * | - | Good |
| Moon et al., 2020 [87] | * | * | * | * | * (age) | * - | * | - | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawidziuk, A.; Butters, E.; Lindegger, D.J.; Foubister, C.; Chrost, H.; Wlodarski, M.; Grogan, J.; Rowicka, P.A.; Bremner, F.; Manohar, S.G. Can the Pupillary Light Reflex and Pupillary Unrest Be Used as Biomarkers of Parkinson’s Disease? A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 1167. https://doi.org/10.3390/diagnostics15091167
Dawidziuk A, Butters E, Lindegger DJ, Foubister C, Chrost H, Wlodarski M, Grogan J, Rowicka PA, Bremner F, Manohar SG. Can the Pupillary Light Reflex and Pupillary Unrest Be Used as Biomarkers of Parkinson’s Disease? A Systematic Review and Meta-Analysis. Diagnostics. 2025; 15(9):1167. https://doi.org/10.3390/diagnostics15091167
Chicago/Turabian StyleDawidziuk, Aleksander, Emilia Butters, Daniel Josef Lindegger, Campbell Foubister, Hugo Chrost, Michal Wlodarski, John Grogan, Paulina A Rowicka, Fion Bremner, and Sanjay G Manohar. 2025. "Can the Pupillary Light Reflex and Pupillary Unrest Be Used as Biomarkers of Parkinson’s Disease? A Systematic Review and Meta-Analysis" Diagnostics 15, no. 9: 1167. https://doi.org/10.3390/diagnostics15091167
APA StyleDawidziuk, A., Butters, E., Lindegger, D. J., Foubister, C., Chrost, H., Wlodarski, M., Grogan, J., Rowicka, P. A., Bremner, F., & Manohar, S. G. (2025). Can the Pupillary Light Reflex and Pupillary Unrest Be Used as Biomarkers of Parkinson’s Disease? A Systematic Review and Meta-Analysis. Diagnostics, 15(9), 1167. https://doi.org/10.3390/diagnostics15091167






