1. Introduction
Gastric cancer (GC) is a major global health concern, ranking among the fifth most common malignancies and the fourth leading cause of cancer-related mortality worldwide [
1,
2]. According to 2020 estimates, GC accounted for roughly 1.1 million new cases and remains associated with significant morbidity and mortality [
3]. Risk factors for GC include chronic
Helicobacter pylori (
H. pylori) infection, dietary components high in salt and nitrites, and tobacco use [
4,
5,
6]. The World Health Organization has classified
H. pylori as a Class I carcinogen, and epidemiological studies consistently link this pathogen to non-cardia gastric cancer (NCGC), with a more variable association in cardia gastric cancer (CGC) [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16].
The COVID-19 pandemic introduced unprecedented challenges to healthcare. Many centers postponed or limited non-emergency endoscopies, which potentially delayed GC diagnoses [
17,
18,
19]. In Romania, organizational changes and patient avoidance of hospitals out of fear of infection further decreased endoscopic procedures and early detection of GC. Late presentation is highly detrimental, as advanced-stage disease significantly diminishes survival [
16].
Upper gastrointestinal (GI) bleeding is a life-threatening complication in GC, occurring in 10–58% of patients depending on tumor location and extent [
9,
20]. Antiplatelet or anticoagulant therapies, while essential for cardiovascular protection, may exacerbate bleeding in the presence of a tumor [
21,
22,
23,
24]. Balancing these therapies against bleeding risk is an ongoing clinical dilemma.
The nature of gastric cancer care is multifaceted—where staging, Helicobacter pylori status, and bleeding risk frequently intersect in clinical practice. Therefore, we aimed (1) to evaluate changes in GC staging before, during, and after the COVID-19 pandemic, (2) to analyze H. pylori prevalence in CGC versus NCGC, and (3) to assess the impact of antiplatelet/anticoagulant use on upper GI bleeding risk. By comparing three time periods (pre-pandemic, pandemic, and post-pandemic), we offer insights into how healthcare disruptions affected GC diagnosis and outcomes.
2. Materials and Methods
2.1. Study Design and Setting
The PICO statement of this study was considered as follows: Population (P): adult patients (n = 121) with confirmed gastric cancer in a tertiary Gastroenterology Unit in Western Romania. Intervention (I): comparison of gastric cancer diagnosis and care before, during, and after the COVID-19 pandemic. Comparison (C): three time periods (pre-pandemic, pandemic, and post-pandemic) reflecting variations in healthcare access. Outcome (O): tumor stage at presentation, Helicobacter pylori infection rates in cardia and non-cardia gastric cancers, risk of upper GI bleeding, and one-year mortality.
This retrospective cohort study was conducted at a tertiary Gastroenterology Unit in Western Romania, focusing on adult patients (≥18 years) diagnosed with gastric cancer over a six-year interval (March 2018–February 2024). We divided this timeframe into three distinct periods to capture any impact of the COVID-19 pandemic: pre-pandemic (March 2018–February 2020), pandemic (March 2020–February 2022), and post-pandemic (March 2022–February 2024). As a tertiary referral center, our facility receives patients from smaller regional hospitals, expanding the representativeness of the study population.
All patients underwent esophagogastroduodenoscopy (EGD) for evaluation of upper GI symptoms (including alarm features such as anemia, weight loss, and dysphagia) or acute bleeding episodes. EGDs were performed using Olympus Evis EXERA III (CV-190) and GIF-HQ190 endoscopes (Olympus corp., Tokyo, Japan). During the endoscopic procedures, the patients were sedated with benzodiazepines (Midazolam), fentanyl, and propofol, and at least four biopsies were taken from the tumor site for histopathological confirmation of GC.
H. pylori status was assessed via histology only. Tumor staging was completed using the TNM classification system (8th edition) [
1,
2], confirmed by computed tomography (CT) of the abdomen and pelvis to evaluate local extension and distant metastases.
The latest TNM classification for gastric cancer, as outlined by the American Joint Committee on Cancer (AJCC), organizes the disease based on three key factors: the extent of the primary tumor (T), the absence or presence of regional lymph node involvement (N), and the absence or presence of distant metastasis (M). The primary tumor (T) is categorized from T1, indicating tumor invasion into the lamina propria or submucosa, to T4a and T4b, where T4a involves tumor penetration through the serosa without invasion into adjacent structures and T4b indicates invasion into nearby structures. Lymph node involvement (N) is classified from N0, with no regional lymph node metastasis, to N3, which is further subdivided into N3a (7–15 metastatic nodes) and N3b (more than 15). Metastasis (M) is categorized as M0, indicating no distant metastasis, or M1, where there is evidence of distant metastasis.
2.2. Patient Selection, Inclusion/Exclusion Criteria, and Definitions
The inclusion criteria were (1) histologically confirmed gastric adenocarcinoma or other malignant GC subtypes (e.g., lymphoma, undifferentiated carcinoma); (2) availability of complete medical records detailing demographic variables, comorbidities, presenting symptoms, H. pylori status, staging, and treatments; and (3) admission dates falling within the designated study intervals. Patients were categorized as having cardia gastric cancer (CGC) if the tumor primarily involved the gastroesophageal junction/cardia region, whereas non-cardia gastric cancer (NCGC) encompassed lesions arising from the body, antrum, or pylorus.
The exclusion criteria included (1) histologically negative or inconclusive biopsy results; (2) incomplete clinical data (e.g., missing endoscopic or imaging reports); (3) concurrent malignant conditions of other GI sites (e.g., esophageal cancer) that could confound staging and outcomes; and (4) patients lost to follow-up within 14 days of diagnosis, preventing reliable staging or outcome assessments.
Upper GI bleeding was defined as the presence of hematemesis, melena, or endoscopic evidence of active bleeding. “Advanced stage” referred to TNM Stage III or IV, while “early stage” comprised Stages I or II. Anemia was defined by a hemoglobin level < 12 g/dL in women or <13 g/dL in men. Antiplatelet/anticoagulant use included aspirin, clopidogrel, vitamin K antagonists (VKA), and direct oral anticoagulants (DOACs) initiated prior to or during admission.
2.3. Data Collection Procedures and Variables
Data were extracted from both paper-based and electronic health records by two independent reviewers trained in epidemiologic methods. Demographic variables included age, gender, and body mass index (BMI). Clinical variables encompassed presenting symptoms (weight loss, dysphagia, vomiting, early satiety, weakness), vital signs, and relevant laboratory parameters (hemoglobin, platelets, international normalized ratio [INR]). We also captured comorbidities such as hypertension, diabetes, and heart failure, as well as habits like smoking and alcohol consumption.
Histopathological categories were grouped as adenocarcinoma (including tubular, papillary, mucinous, mixed subtypes), signet-ring (poorly cohesive) cell carcinoma, lymphoma (diffuse large B-cell or follicular), and undifferentiated carcinoma. Tumor localization was designated as either CGC or NCGC based on endoscopic and radiologic findings. H. pylori status was determined via histological staining of biopsy specimens. Upper GI bleeding was recorded if documented in the endoscopy or clinical notes. Antiplatelet and anticoagulant therapies were noted at admission, with additional details about dosage and indication where available.
2.4. Statistical Analysis
A priori power analysis was conducted using G*Power software (version 3.1, Heinrich Heine University, Düsseldorf, Germany) to estimate the required sample size for detecting a clinically meaningful shift in the proportion of advanced-stage (Stage III–IV) gastric cancer cases across the three study periods. Based on prior regional data suggesting a 20% increase in advanced-stage disease post-pandemic, a power of 80%, and a two-sided alpha of 0.05, the calculation yielded a minimum total sample size of approximately 110 participants. Ultimately, 121 patients met the inclusion criteria, exceeding this threshold and thus providing adequate power to detect statistically significant differences in tumor stage and associated variables.
All statistical analyses were performed using IBM SPSS Statistics (version 27) and Stata/SE (version 18). Descriptive statistics were presented as means (±standard deviations) or medians (IQR) for continuous variables and counts (percentages) for categorical variables. When comparing three groups (pre-pandemic, pandemic, post-pandemic), we used one-way ANOVA for normally distributed continuous variables or the Kruskal–Wallis test if distributions were skewed. For categorical comparisons, we used chi-square or Fisher’s exact tests, as appropriate, with a significance threshold of p < 0.05. For each row in the multi-row tables, a separate p-value was calculated to examine differences among the three time periods.
We conducted logistic regression to evaluate factors associated with upper GI bleeding, incorporating significant variables from univariate analyses (p < 0.10) into a multivariable model. Cox proportional hazards models assessed one-year mortality, reporting hazard ratios (HRs) with 95% confidence intervals (CIs). The variables tested included age, advanced stage, H. pylori infection, tumor location, and antithrombotic therapy. The proportional hazards assumption was verified graphically and via goodness-of-fit tests. Statistical significance was determined at p < 0.05 for all analyses, and all tests were two-sided.
3. Results
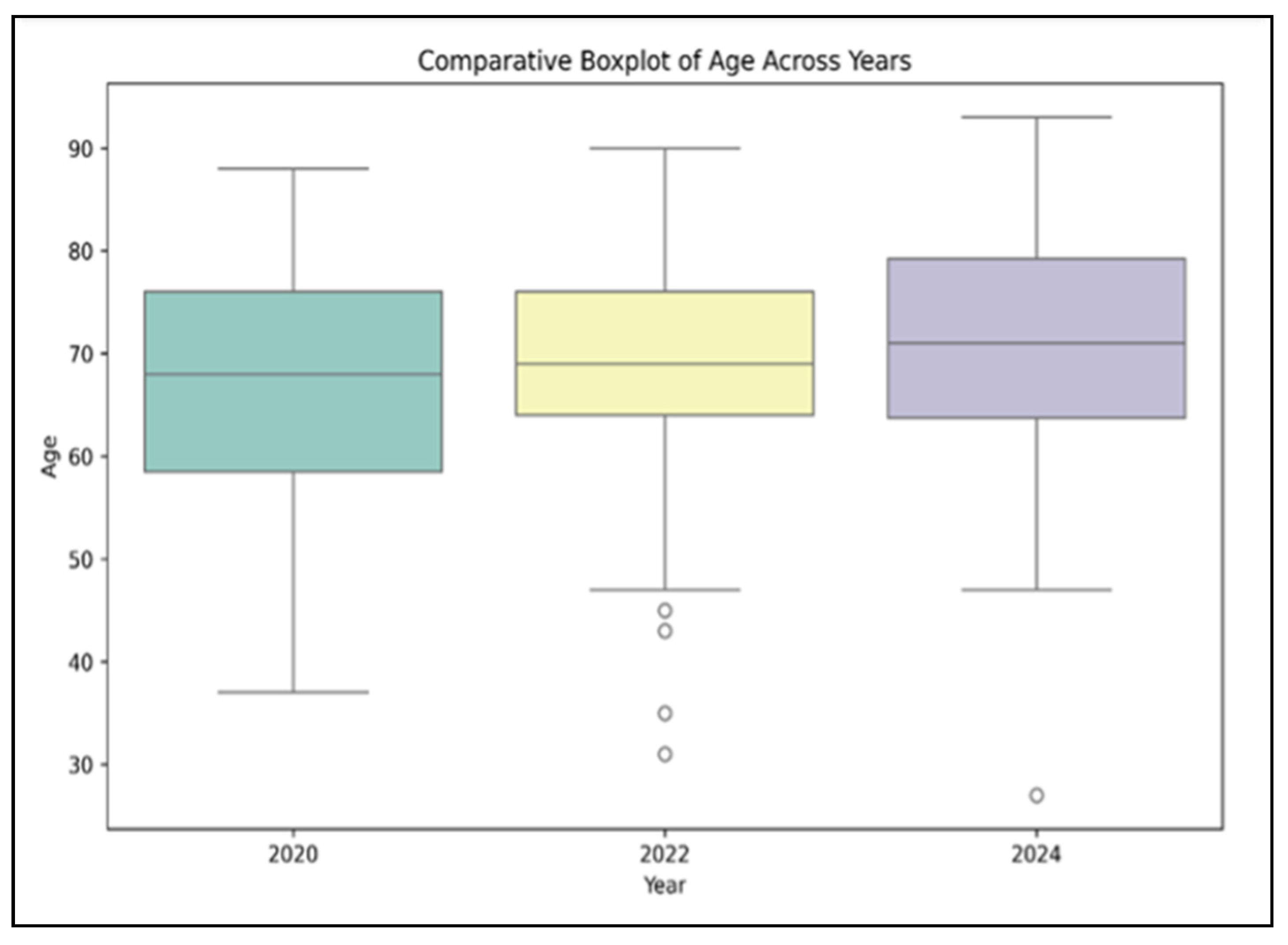
Regarding patient demographics, the mean age of patients increased slightly across the three cohorts, from 66.2 years (±9.7) in the pre-pandemic group (
n = 45) to 68.5 years (±10.3) during the pandemic (
n = 40) and 70.4 years (±11.2) in the post-pandemic period (
n = 36), although this incremental rise did not reach statistical significance (
p = 0.149), as presented in
Figure 1. Male sex predominated in all three groups (64.4% pre-pandemic; 70.0% pandemic; 75.0% post-pandemic), but again, no statistically significant difference was observed (
p = 0.417).
In terms of comorbid conditions, hypertension was present in roughly half to two-thirds of participants (62.2% pre-pandemic; 55.0% pandemic; 69.4% post-pandemic), and heart failure was documented in 40–44% of individuals across the three groups. Diabetes rates remained relatively similar (20.0% pre-pandemic; 22.5% pandemic; 19.4% post-pandemic). Anemia affected more than three-quarters of patients in each cohort, with percentages ranging from 80.6% to 85.0%. Obesity (BMI > 30) and smoking also showed consistent prevalence with no significant differences: obesity hovered around 30–33%, and smoking varied between 38 and 45%. Overall, the distribution of comorbidities and lifestyle factors did not vary significantly across the three timeframes, as indicated by
p-values exceeding 0.05 (
Table 1).
In the pre-pandemic cohort (
n = 45), the distribution by stage was as follows: Stage I in 5 patients (11.1%), Stage IIA in 8 (17.8%), Stage IIB in 6 (13.3%), Stage III in 9 (20.0%), Stage IVA in 13 (28.9%), and Stage IVB in 4 (8.9%), with a
p-value of 0.679 for Stage I comparisons. During the pandemic period (
n = 40), the numbers were Stage I in 3 patients (7.5%), Stage IIA in 7 (17.5%), Stage IIB in 5 (12.5%), Stage III in 10 (25.0%), Stage IVA in 5 (12.5%), and Stage IVB in 10 (25.0%), with corresponding
p-values of 0.837 and 0.726 for Stages IIA and IIB. For the post-pandemic period (
n = 36), the distribution was Stage I in 2 patients (5.6%), Stage IIA in 5 (13.9%), Stage IIB in 3 (8.3%), Stage III in 16 (44.4%), Stage IVA in 1 (2.8%), and Stage IVB in 9 (25.0%). The
p-values for Stages III, IVA, and IVB were 0.031, 0.002, and 0.043, respectively, indicating differences in the advanced stages across periods (
Table 2), with stage IVB being significantly higher in the pandemic period, increasing by 16.1%.
Adenocarcinoma (tubular, papillary, mucinous, or mixed subtypes) was the most frequent histopathological finding, reported in 34 of 45 patients (75.6%) pre-pandemic, 28 of 40 (70.0%) during the pandemic, and 27 of 36 (75.0%) in the post-pandemic cohort (
p = 0.659). Signet-ring cell (poorly cohesive) carcinoma comprised 6 of 45 cases (13.3%) in the pre-pandemic period, 7 of 40 (17.5%) during the pandemic, and 5 of 36 (13.9%) post-pandemic (
p = 0.779). Lymphoma (diffuse large B-cell or follicular) was reported in 3 patients each (6.7% and 7.5%, respectively) in the pre-pandemic and pandemic groups, with a slight decrease to 2 out of 36 (5.6%) post-pandemic (
p = 0.911). Undifferentiated carcinoma remained relatively rare across all three periods, detected in two patients (4.4%) pre-pandemic, two patients (5.0%) during the pandemic, and two patients (5.6%) in the post-pandemic cohort (
p = 0.955). Overall, these findings suggest stable histopathological distributions despite the disruptions caused by the pandemic (
Table 3).
For non-cardia gastric cancer (NCGC), the pre-pandemic group showed
H. pylori positivity in 24 out of 33 patients (72.7%), during the pandemic, this was 20 out of 29 patients (69.0%), and post-pandemic, this was 19 out of 27 patients (70.4%), with an overall
p-value of 0.915. In contrast, for cardia gastric cancer (CGC),
H. pylori was positive in 5 out of 12 patients (41.7%) pre-pandemic, 4 out of 11 (36.4%) during the pandemic, and 5 out of 9 (55.6%) post-pandemic, with a
p-value of 0.357. The overall comparison between NCGC and CGC across the time periods showed
H. pylori prevalence rates of 72.7% vs. 41.7% in the pre-pandemic phase, 69.0% vs. 36.4% in the pandemic phase, and 70.4% vs. 55.6% in the post-pandemic phase (
Table 4).
Overall, the proportion of patients presenting with upper GI bleeding differed significantly among the three periods (
p = 0.046), peaking at 55.0% during the pandemic (versus 37.8% pre-pandemic and 44.4% post-pandemic). Among the bleeding cohort, antiplatelet or anticoagulant use was also highest in the pandemic group (59.1%,
p = 0.029). Additionally, patients with bleeding during the pandemic had a significantly higher mean age (71.1 ± 9.5 years) compared to those pre- and post-pandemic (
p = 0.045), as seen in
Table 5.
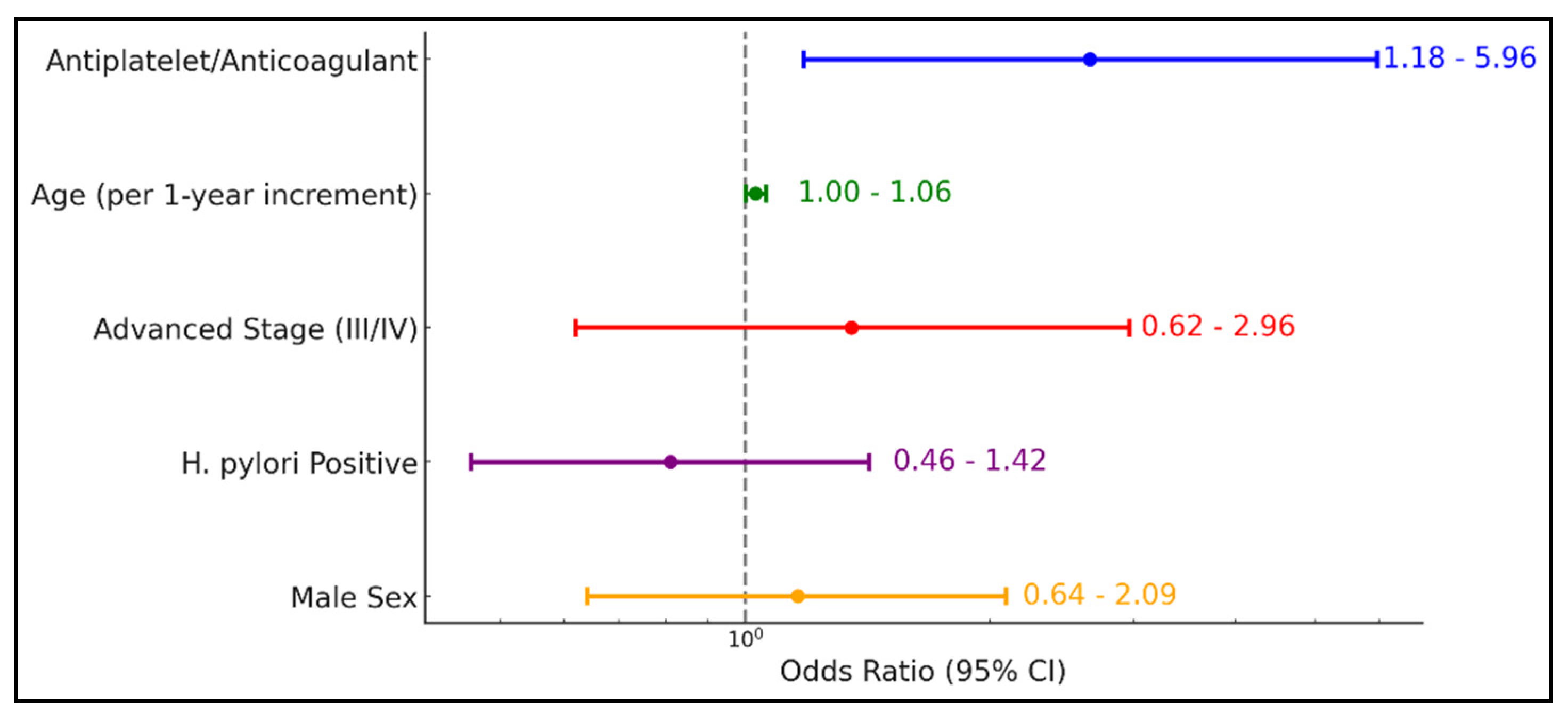
Table 6 and
Figure 2 demonstrate that antiplatelet or anticoagulant therapy significantly increases the odds of upper GI bleeding by more than twofold (OR = 2.65, 95% CI 1.18–5.96;
p = 0.018). While age exhibited a borderline effect (OR = 1.03, 95% CI 1.00–1.06;
p = 0.065), advanced disease stage (III/IV), H. pylori positivity, and male sex did not show statistically significant associations with bleeding risk (
Table 6).
Table 7 indicates that advanced tumor stage (III/IV) is the strongest predictor of one-year mortality (HR = 2.74, 95% CI 1.42–5.12;
p = 0.002), followed by a diagnosis made during the COVID-19 pandemic (HR = 1.66, 95% CI 1.19–3.61;
p = 0.010) and older age (≥70 years) (HR = 1.88, 95% CI 1.02–3.46;
p = 0.043). Neither H. pylori positivity (HR = 0.82, 95% CI 0.47–1.42;
p = 0.474) nor antiplatelet/anticoagulant use (HR = 1.26, 95% CI 0.73–2.17;
p = 0.415) significantly affected mortality risk, and tumor location (cardia vs. non-cardia) also showed no significant impact (HR = 1.15, 95% CI 0.63–2.09;
p = 0.642).