Magnetic Resonance Imaging in the Neuroimaging of Progressive Supranuclear Palsy—Parkinsonism Predominant: Limitations and Strengths in Clinical Evaluation
Abstract
1. Introduction
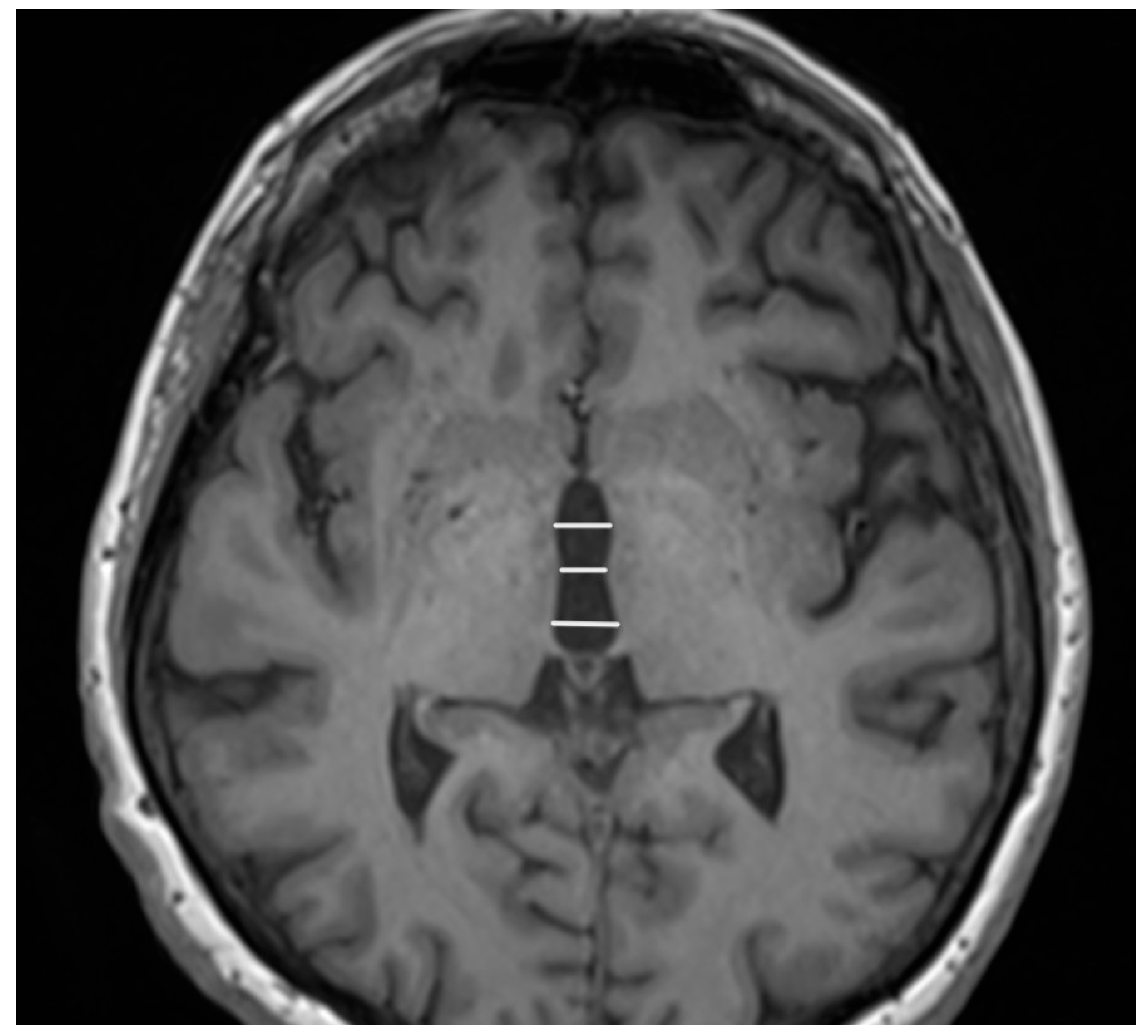
2. Magnetic Resonance Imaging (MRI)
3. Discussion
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badihian, N.; Tosakulwong, N.; Weigand, S.D.; Ali, F.; Clark, H.M.; Stierwalt, J.; Botha, H.; Savica, R.; Dickson, D.W.; Whitwell, J.L.; et al. Relationships between regional burden of tau pathology and age at death and disease duration in PSP. Parkinsonism Relat. Disord. 2024, 127, 107109. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Koziorowski, D.; Figura, M.; Milanowski, Ł.M.; Szlufik, S.; Alster, P.; Madetko, N.; Friedman, A. Mechanisms of Neurodegeneration in Various Forms of Parkinsonism-Similarities and Differences. Cells 2021, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Madetko-Alster, N.; Otto-Ślusarczyk, D.; Wiercińska-Drapało, A.; Koziorowski, D.; Szlufik, S.; Samborska-Ćwik, J.; Struga, M.; Friedman, A.; Alster, P. Clinical Phenotypes of Progressive Supranuclear Palsy-The Differences in Interleukin Patterns. Int. J. Mol. Sci. 2023, 24, 15135. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Otto-Ślusarczyk, D.; Kutyłowski, M.; Migda, B.; Wiercińska-Drapało, A.; Jabłońska, J.; Struga, M.; Madetko-Alster, N. The associations between common neuroimaging parameters of Progressive Supranuclear Palsy in magnetic resonance imaging and non-specific inflammatory factors—Pilot study. Front. Immunol. 2024, 15, 1458713. [Google Scholar] [CrossRef] [PubMed]
- Couto, B.; Di Luca, D.G.; Antwi, J.; Bhakta, P.; Fox, S.; Tartaglia, M.C.; Kovacs, G.G.; Lang, A.E. Ethnic background and distribution of clinical phenotypes in patients with probable progressive supranuclear palsy. Parkinsonism Relat. Disord. 2024, 123, 106955. [Google Scholar] [CrossRef]
- Inci, I.; Kusbeci, O.Y.; Eskut, N. The neutrophil-to-lymphocyte ratio as a marker of peripheral inflammation in progressive supranuclear palsy: A retrospective study. Neurol. Sci. 2020, 41, 1233–1237. [Google Scholar] [CrossRef]
- Greten, S.; Wegner, F.; Jensen, I.; Krey, L.; Rogozinski, S.; Fehring, M.; Heine, J.; Doll-Lee, J.; Pötter-Nerger, M.; Zeitzschel, M.; et al. The comorbidity and co-medication profile of patients with progressive supranuclear palsy. J. Neurol. 2024, 271, 782–793. [Google Scholar] [CrossRef]
- Colosimo, C.; Osaki, Y.; Vanacore, N.; Lees, A.J. Lack of association between progressive supranuclear palsy and arterial hypertension: A clinicopathological study. Mov. Disord. 2003, 18, 694–697. [Google Scholar] [CrossRef]
- Koga, S.; Roemer, S.F.; Kasanuki, K.; Dickson, D.W. Cerebrovascular pathology presenting as corticobasal syndrome: An autopsy case series of “vascular CBS”. Parkinsonism Relat. Disord. 2019, 68, 79–84. [Google Scholar] [CrossRef]
- Wang, H.; Chang, T.S.; Dombroski, B.A.; Cheng, P.L.; Patil, V.; Valiente-Banuet, L.; Farrell, K.; Mclean, C.; Molina-Porcel, L.; Rajput, A.; et al. Whole-genome sequencing analysis reveals new susceptibility loci and structural variants associated with progressive supranuclear palsy. Mol. Neurodegener. 2024, 19, 61. [Google Scholar] [CrossRef]
- Jackson, R.J.; Melloni, A.; Fykstra, D.P.; Serrano-Pozo, A.; Shinobu, L.; Hyman, B.T. Astrocyte tau deposition in progressive supranuclear palsy is associated with dysregulation of MAPT transcription. Acta Neuropathol. Commun. 2024, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.; Giese, A.; Grossman, M.; Kurz, C.; et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef]
- Planche, V.; Mansencal, B.; Manjon, J.V.; Meissner, W.G.; Tourdias, T.; Coupé, P. Staging of progressive supranuclear palsy-Richardson syndrome using MRI brain charts for the human lifespan. Brain Commun. 2024, 6, fcae055. [Google Scholar] [CrossRef] [PubMed]
- Necpál, J.; Borsek, M.; Jeleňová, B. “Parkinson’s disease” on the way to progressive supranuclear palsy: A review on PSP-parkinsonism. Neurol. Sci. 2021, 42, 4927–4936. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yang, Q.; Jiao, B.; Zhang, W.; Lin, J.; Zhu, Y.; Xu, Q.; Zhou, H.; Weng, L.; Liao, X.; et al. Clinical features of progressive supranuclear palsy. Front. Aging Neurosci. 2023, 15, 1229491. [Google Scholar] [CrossRef]
- Campagnolo, M.; Weis, L.; Fogliano, C.; Cianci, V.; Garon, M.; Fiorenzato, E.; Carecchio, M.; Ferreri, F.; Bisiacchi, P.; Antonini, A.; et al. Clinical, cognitive, and morphometric profiles of progressive supranuclear palsy phenotypes. J. Neural Transm. 2023, 130, 97–109. [Google Scholar] [CrossRef]
- Ebentheuer, J.; Canelo, M.; Trautmann, E.; Trenkwalder, C. Substantia nigra echogenicity in progressive supranuclear palsy. Mov. Disord. 2010, 25, 773–777. [Google Scholar] [CrossRef]
- Spiegel, C.; Marotta, C.; Bertram, K.; Vivash, L.; Harding, I.H. Brainstem and cerebellar radiological findings in progressive supranuclear palsy. Brain Commun. 2025, 7, fcaf051. [Google Scholar] [CrossRef]
- Watanabe, H.; Yoshida, M.; Naganawa, S.; Sobue, G. The diagnosis of neurodegenerative disorders based on clinical and pathological findings using an MRI approach. Rinsho Shinkeigaku 2011, 51, 863–864. [Google Scholar] [CrossRef][Green Version]
- Longoni, G.; Agosta, F.; Kostić, V.S.; Stojković, T.; Pagani, E.; Stošić-Opinćal, T.; Filippi, M. MRI measurements of brainstem structures in patients with Richardson’s syndrome, progressive supranuclear palsy-parkinsonism, and Parkinson’s disease. Mov. Disord. 2011, 26, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Yang, H.; Kim, Y.; Youn, J.; Park, J.; Huh, Y.E.; Kim, H.T.; Cho, J.W. Differential Progression of Midbrain Atrophy in Parkinsonism: Longitudinal MRI Study. Neurodegener. Dis. 2017, 17, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.A.; Yadav, R.; Ganeshan, M.; Saini, J.; Gupta, A.; Sandhya, M.; Pal, P.K. Correlation between qualitative balance indices, dynamic posturography and structural brain imaging in patients with progressive supranuclear palsy and its subtypes. Neurol. India 2016, 64, 633–639. [Google Scholar] [CrossRef]
- Agosta, F.; Pievani, M.; Svetel, M.; Ječmenica Lukić, M.; Copetti, M.; Tomić, A.; Scarale, A.; Longoni, G.; Comi, G.; Kostić, V.S. Diffusion tensor MRI contributes to differentiate Richardson’s syndrome from PSP-parkinsonism. Neurobiol. Aging 2012, 33, 2817–2826. [Google Scholar] [CrossRef]
- Liscic, R.M.; Srulijes, K.; Gröger, A.; Maetzler, W.; Berg, D. Differentiation of progressive supranuclear palsy: Clinical, imaging and laboratory tools. Acta Neurol. Scand. 2013, 127, 362–370. [Google Scholar] [CrossRef]
- Quattrone, A.; Morelli, M.; Williams, D.R.; Vescio, B.; Arabia, G.; Nigro, S.; Nicoletti, G.; Salsone, M.; Novellino, F.; Nisticò, R.; et al. MR parkinsonism index predicts vertical supranuclear gaze palsy in patients with PSP-parkinsonism. Neurology 2016, 87, 1266–1273. [Google Scholar] [CrossRef]
- Yamawaki, T. Diagnosis of MSA-P and PSP-P in Early Stage. Brain Nerve 2020, 72, 331–343. [Google Scholar] [CrossRef]
- Picillo, M.; Tepedino, M.F.; Abate, F.; Erro, R.; Ponticorvo, S.; Tartaglione, S.; Volpe, G.; Frosini, D.; Cecchi, P.; Cosottini, M.; et al. Midbrain MRI assessments in progressive supranuclear palsy subtypes. J. Neurol. Neurosurg. Psychiatry 2020, 91, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Quattrone, A.; Sarica, A.; Buonocore, J.; Morelli, M.; Bianco, M.G.; Calomino, C.; Aracri, F.; De Maria, M.; Vescio, B.; Vaccaro, M.G.; et al. Differentiating between common PSP phenotypes using structural MRI: A machine learning study. J. Neurol. 2023, 270, 5502–5515. [Google Scholar] [CrossRef]
- Quattrone, A.; Caligiuri, M.E.; Morelli, M.; Nigro, S.; Vescio, B.; Arabia, G.; Nicoletti, G.; Nisticò, R.; Salsone, M.; Novellino, F.; et al. Imaging counterpart of postural instability and vertical ocular dysfunction in patients with PSP: A multimodal MRI study. Parkinsonism Relat. Disord. 2019, 63, 124–130. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Tosakulwong, N.; Clark, H.M.; Ali, F.; Botha, H.; Weigand, S.D.; Sintini, I.; Machulda, M.M.; Schwarz, C.G.; Reid, R.I.; et al. Diffusion tensor imaging analysis in three progressive supranuclear palsy variants. J. Neurol. 2021, 268, 3409–3420. [Google Scholar] [CrossRef]
- Quattrone, A.; Morelli, M.; Nigro, S.; Quattrone, A.; Vescio, B.; Arabia, G.; Nicoletti, G.; Nisticò, R.; Salsone, M.; Novellino, F.; et al. A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 54, 3–8. [Google Scholar] [CrossRef]
- Quattrone, A.; Bianco, M.G.; Antonini, A.; Vaillancourt, D.E.; Seppi, K.; Ceravolo, R.; Strafella, A.P.; Tedeschi, G.; Tessitore, A.; Cilia, R.; et al. Development and Validation of Automated Magnetic Resonance Parkinsonism Index 2.0 to Distinguish Progressive Supranuclear Palsy-Parkinsonism from Parkinson’s Disease. Mov. Disord. 2022, 37, 1272–1281. [Google Scholar] [CrossRef]
- Madetko, N.; Alster, P.; Kutyłowski, M.; Migda, B.; Nieciecki, M.; Koziorowski, D.; Królicki, L. Is MRPI 2.0 More Useful than MRPI and M/P Ratio in Differential Diagnosis of PSP-P with Other Atypical Parkinsonisms? J. Clin. Med. 2022, 11, 2701. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Nieciecki, M.; Migda, B.; Kutyłowski, M.; Madetko, N.; Duszyńska-Wąs, K.; Charzyńska, I.; Koziorowski, D.; Królicki, L.; Friedman, A. The Strengths and Obstacles in the Differential Diagnosis of Progressive Supranuclear Palsy-Parkinsonism Predominant (PSP-P) and Multiple System Atrophy (MSA) Using Magnetic Resonance Imaging (MRI) and Perfusion Single Photon Emission Computed Tomography (SPECT). Diagnostics 2022, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L.; Tosakulwong, N.; Botha, H.; Ali, F.; Clark, H.M.; Duffy, J.R.; Utianski, R.L.; Stevens, C.A.; Weigand, S.D.; Schwarz, C.G.; et al. Brain volume and flortaucipir analysis of progressive supranuclear palsy clinical variants. Neuroimage Clin. 2020, 25, 102152. [Google Scholar] [CrossRef] [PubMed]
- Amrami, A.; Singh, N.A.; Ali, F.; Pham, N.T.T.; Stephens, Y.C.; Josephs, K.A.; Whitwell, J.L. Clinical Utility of Tectal Plate Measurements on Magnetic Resonance Imaging in Progressive Supranuclear Palsy. Mov. Disord. 2024, 39, 1402–1407. [Google Scholar] [CrossRef]
- Picillo, M.; Tepedino, M.F.; Abate, F.; Ponticorvo, S.; Erro, R.; Cuoco, S.; Oksuz, N.; Di Salle, G.; Di Salle, F.; Esposito, F.; et al. Uncovering clinical and radiological asymmetry in progressive supranuclear palsy-Richardson’s syndrome. Neurol. Sci. 2022, 43, 3677–3682. [Google Scholar] [CrossRef]
- Lenka, A.; Pasha, S.A.; Mangalore, S.; George, L.; Jhunjhunwala, K.R.; Bagepally, B.S.; Naduthota, R.M.; Saini, J.; Yadav, R.; Pal, P.K. Role of Corpus Callosum Volumetry in Differentiating the Subtypes of Progressive Supranuclear Palsy and Early Parkinson’s Disease. Mov. Disord. Clin. Pract. 2017, 4, 552–558. [Google Scholar] [CrossRef]
- Grijalva, R.M.; Pham, N.T.T.; Huang, Q.; Martin, P.R.; Ali, F.; Clark, H.M.; Duffy, J.R.; Utianski, R.L.; Botha, H.; Machulda, M.M.; et al. Brainstem Biomarkers of Clinical Variant and Pathology in Progressive Supranuclear Palsy. Mov. Disord. 2022, 37, 702–712. [Google Scholar] [CrossRef]
- Agosta, F.; Kostić, V.S.; Galantucci, S.; Mesaros, S.; Svetel, M.; Pagani, E.; Stefanova, E.; Filippi, M. The in vivo distribution of brain tissue loss in Richardson’s syndrome and PSP-parkinsonism: A VBM-DARTEL study. Eur. J. Neurosci. 2010, 32, 640–647. [Google Scholar] [CrossRef]
- Seki, M.; Seppi, K.; Mueller, C.; Potrusil, T.; Goebel, G.; Reiter, E.; Nocker, M.; Steiger, R.; Wildauer, M.; Gizewski, E.R.; et al. Diagnostic potential of dentatorubrothalamic tract analysis in progressive supranuclear palsy. Parkinsonism Relat. Disord. 2018, 49, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Agosta, F.; Ječmenica-Lukić, M.; Petrović, I.; Meani, A.; Kostic, V.S.; Filippi, M. Progression of white matter damage in progressive supranuclear palsy with predominant parkinsonism. Parkinsonism Relat. Disord. 2018, 49, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Nigro, S.; Barbagallo, G.; Bianco, M.G.; Morelli, M.; Arabia, G.; Quattrone, A.; Gasparini, S.; Cascini, G.L.; Quattrone, A. Track density imaging: A reliable method to assess white matter changes in Progressive Supranuclear Palsy with predominant parkinsonism. Parkinsonism Relat. Disord. 2019, 69, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Potrusil, T.; Krismer, F.; Beliveau, V.; Seppi, K.; Müller, C.; Troger, F.; Göbel, G.; Steiger, R.; Gizewski, E.R.; Poewe, W.; et al. Diagnostic potential of automated tractography in progressive supranuclear palsy variants. Parkinsonism Relat. Disord. 2020, 72, 65–71. [Google Scholar] [CrossRef]
- Beliveau, V.; Müller, C.; Steiger, R.; Gizewski, E.R.; Poewe, W.; Seppi, K.; Scherfler, C. Characterization and diagnostic potential of R2* in early-stage progressive supranuclear palsy variants. Parkinsonism Relat. Disord. 2022, 101, 43–48. [Google Scholar] [CrossRef]
- Mangalore, S.; Kumar, M.; Pal, P.; Saini, J.; Pasha, S.; Yadav, R. Role of Multivoxel MR Spectroscopy Progressive Supranuclear Palsy—A Preliminary Study. Neurol. India 2022, 70, 2388–2391. [Google Scholar] [CrossRef]
- Satoh, R.; Weigand, S.D.; Pham, N.T.T.; Ali, F.; Arani, A.; Senjem, M.L.; Jack, C.R., Jr.; Whitwell, J.L.; Josephs, K.A. Magnetic Susceptibility in Progressive Supranuclear Palsy Variants, Parkinson’s Disease, and Corticobasal Syndrome. Mov. Disord. 2023, 38, 2282–2290. [Google Scholar] [CrossRef]
- Kawazoe, M.; Koga, S.; Sekiya, H.; Josephs, K.A.; Graff-Radford, N.R.; Dickson, D.W. Disproportionately Enlarged Subarachnoid-Space Hydrocephalus on MRI in Pathologically Confirmed Progressive Supranuclear Palsy. Neurol. Clin. Pract. 2025, 15, e200431. [Google Scholar] [CrossRef]
- Georgiopoulos, C.; Papadimitriou, S.; Nyholm, D.; Kilander, L.; Löwenmark, M.; Fällmar, D.; Virhammar, J. Quantitative brain stem assessment in discriminating neurodegenerative disorders from normal pressure hydrocephalus. J. Neuroimaging 2024, 34, 612–618. [Google Scholar] [CrossRef]
- Alster, P.; Otto-Ślusarczyk, D.; Szlufik, S.; Duszyńska-Wąs, K.; Drzewińska, A.; Wiercińska-Drapało, A.; Struga, M.; Kutyłowski, M.; Friedman, A.; Madetko-Alster, N. The significance of glial cell line-derived neurotrophic factor analysis in Progressive Supranuclear Palsy. Sci. Rep. 2024, 14, 2805. [Google Scholar] [CrossRef]
- Bianco, M.G.; Cristiani, C.M.; Scaramuzzino, L.; Sarica, A.; Augimeri, A.; Chimento, I.; Buonocore, J.; Parrotta, E.I.; Quattrone, A.; Cuda, G.; et al. Combined blood Neurofilament light chain and third ventricle width to differentiate Progressive Supranuclear Palsy from Parkinson’s Disease: A machine learning study. Parkinsonism Relat. Disord. 2024, 123, 106978. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, K.; Barthel, H.; Brendel, M.; Scherlach, C.; Hoffmann, K.T.; Rauchmann, B.S.; Rullmann, M.; Marek, K.; Villemagne, V.L.; Rumpf, J.J.; et al. 18F-PI-2620 Tau PET Improves the Imaging Diagnosis of Progressive Supranuclear Palsy. J. Nucl. Med. 2022, 63, 1754–1760. [Google Scholar] [CrossRef]
- Gerhard, A.; Trender-Gerhard, I.; Turkheimer, F.; Quinn, N.P.; Bhatia, K.P.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in progressive supranuclear palsy. Mov. Disord. 2006, 21, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, H.; Shin, J.H.; Lee, J.Y.; Kim, H.J.; Kim, J.M.; Jeon, B. Eye movements and association with regional brain atrophy in clinical subtypes of progressive supranuclear palsy. J. Neurol. 2021, 268, 967–977. [Google Scholar] [CrossRef]
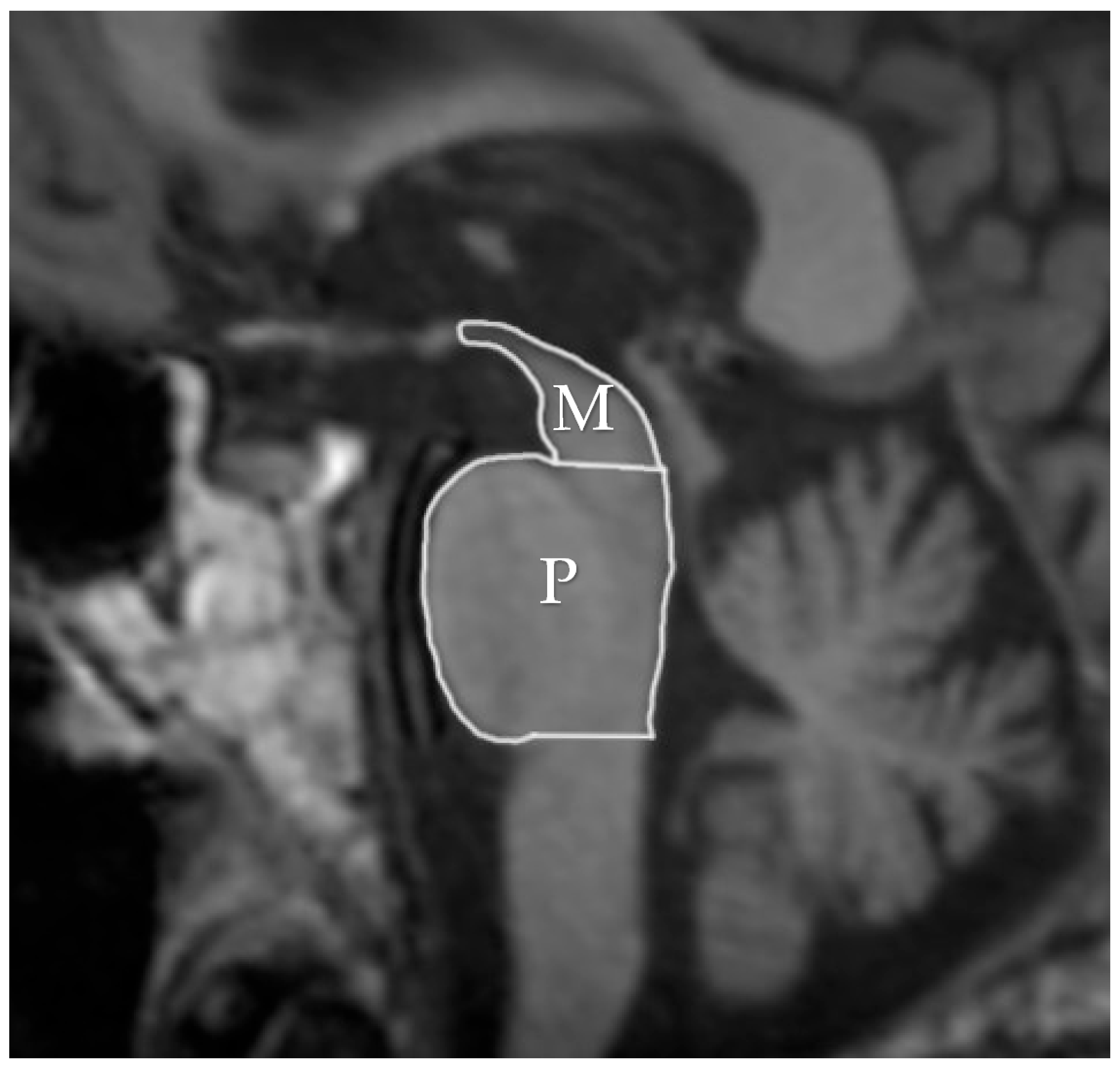
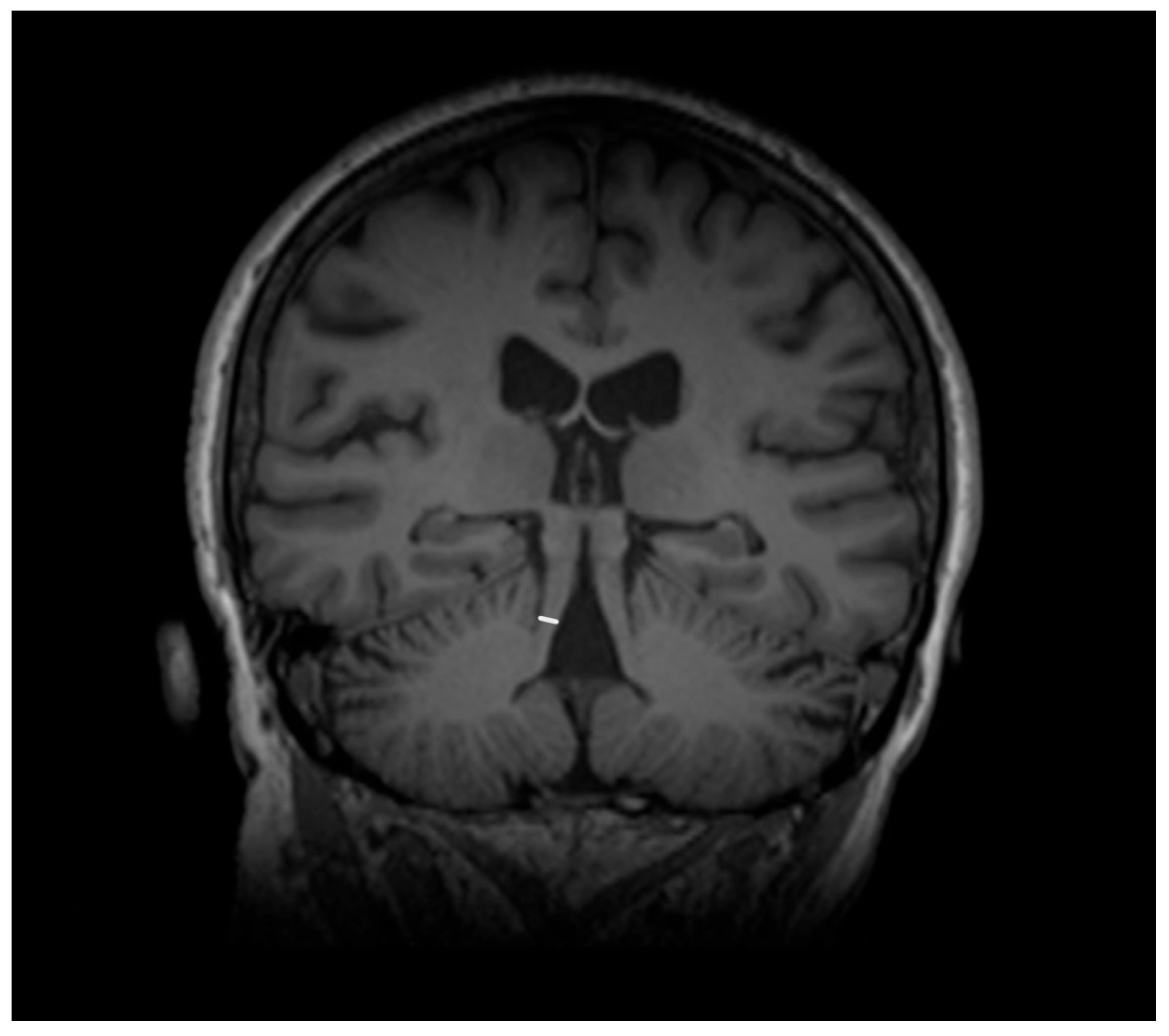
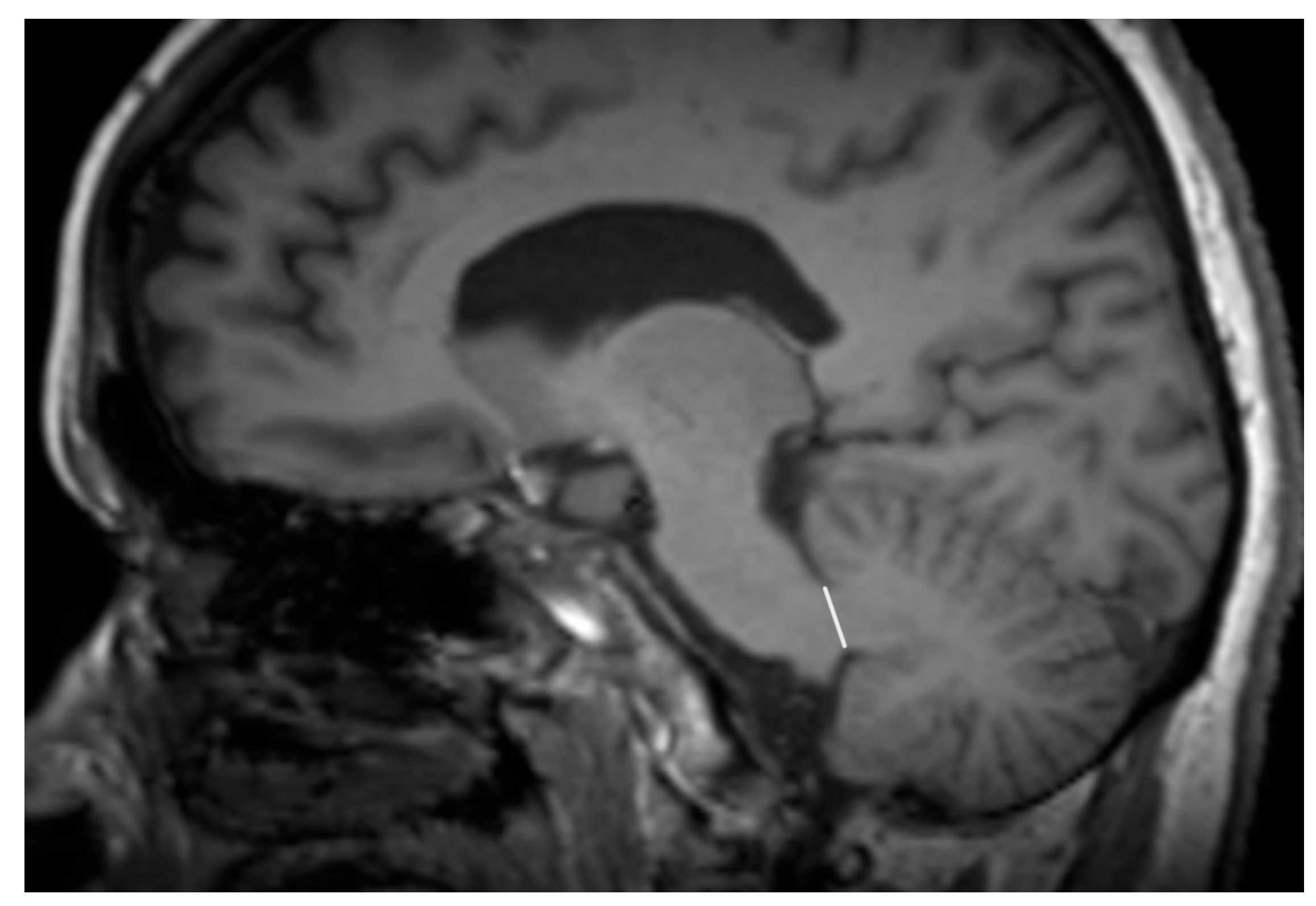
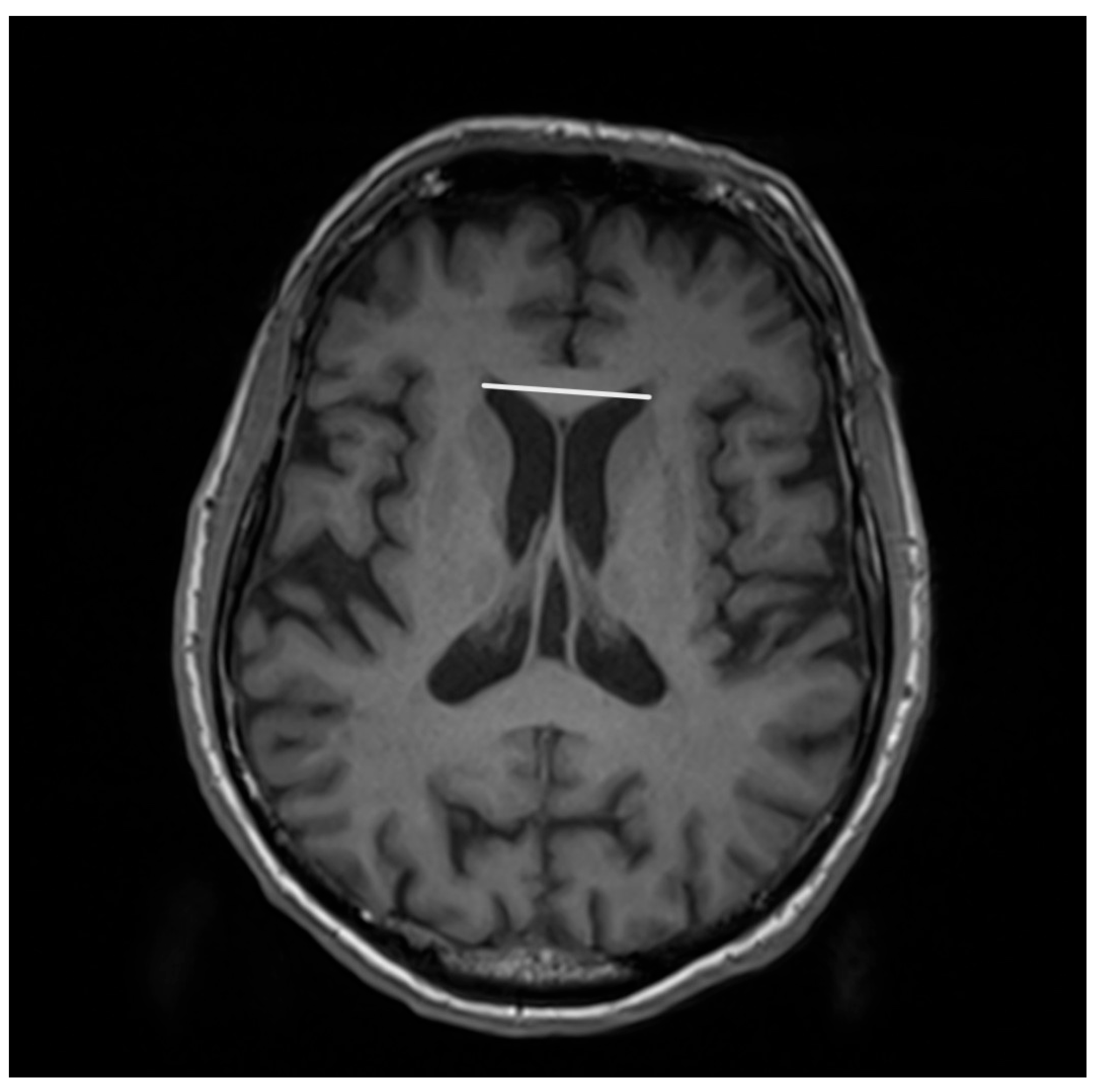
| Entity Differentiated with PSP-P | Significance in MRI |
|---|---|
| PD |
|
| PSP-RS |
|
| MSA-P | Midbrain to pons ratio MRPI |
| Other | Spectroscopy with PSP-C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alster, P.; Kutyłowski, M.; Madetko-Alster, N. Magnetic Resonance Imaging in the Neuroimaging of Progressive Supranuclear Palsy—Parkinsonism Predominant: Limitations and Strengths in Clinical Evaluation. Diagnostics 2025, 15, 945. https://doi.org/10.3390/diagnostics15080945
Alster P, Kutyłowski M, Madetko-Alster N. Magnetic Resonance Imaging in the Neuroimaging of Progressive Supranuclear Palsy—Parkinsonism Predominant: Limitations and Strengths in Clinical Evaluation. Diagnostics. 2025; 15(8):945. https://doi.org/10.3390/diagnostics15080945
Chicago/Turabian StyleAlster, Piotr, Michał Kutyłowski, and Natalia Madetko-Alster. 2025. "Magnetic Resonance Imaging in the Neuroimaging of Progressive Supranuclear Palsy—Parkinsonism Predominant: Limitations and Strengths in Clinical Evaluation" Diagnostics 15, no. 8: 945. https://doi.org/10.3390/diagnostics15080945
APA StyleAlster, P., Kutyłowski, M., & Madetko-Alster, N. (2025). Magnetic Resonance Imaging in the Neuroimaging of Progressive Supranuclear Palsy—Parkinsonism Predominant: Limitations and Strengths in Clinical Evaluation. Diagnostics, 15(8), 945. https://doi.org/10.3390/diagnostics15080945







