Maternal and Perinatal Outcomes in Pregnant Women with Cancer: A Single-Center Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Studied Variables
2.3. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
3.2. Cancer Types and Staging
3.3. Clinical and Obstetric History
3.4. Cancer Diagnosis and Treatment During Pregnancy
3.5. Maternal and Perinatal Outcomes
3.6. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Câncer José Alencar Gomes da Silva (BR). Estimativa 2020: Incidência de Câncer no Brasil [Internet]. Rio de Janeiro: 2019. 120p. Available online: https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/estimativa-2020-incidencia-de-cancer-no-brasil.pdf (accessed on 22 May 2021).
- Amant, F.; Berveiller, P.; Boere, I.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.; Hasenburg, A.; Johansson, A.; Lambertini, M.; et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Dimitrakakis, C.; Marinopoulos, S.; Tsigginou, A.; Dimopoulos, M.-A. Cancer in pregnancy: Disentangling treatment modalities. ESMO Open 2016, 1, e000016. [Google Scholar] [CrossRef] [PubMed]
- Pentheroudakis, G.; Pavlidis, N. Cancer and pregnancy: Poena magna, not anymore. Eur. J. Cancer 2006, 42, 126–140. [Google Scholar] [CrossRef]
- Stensheim, H.; Møller, B.; van Dijk, T.; Fosså, S.D. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: A registry-based cohort study. J. Clin. Oncol. 2009, 27, 45–51. [Google Scholar] [CrossRef]
- Eibye, S.; Kjær, S.K.; Mellemkjær, L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet. Gynecol. 2013, 122, 608–617. [Google Scholar] [CrossRef]
- Mitrou, S.; Zarkavelis, G.; Fotopoulos, G.; Petrakis, D.; Pavlidis, N. A mini review on pregnant mothers with cancer: A paradoxical coexistence. J. Adv. Res. 2016, 7, 559–563. [Google Scholar] [CrossRef]
- Cottreau, C.M.; Dashevsky, I.; Andrade, S.E.; Li, D.-K.; Nekhlyudov, L.; Raebel, M.A.; Ritzwoller, D.P.; Partridge, A.H.; Pawloski, P.A.; Toh, S. Pregnancy-Associated Cancer: A U.S. Population-Based Study. J. Women’s Health 2019, 28, 250–257. [Google Scholar] [CrossRef]
- Ferraz, B.E.P.L.; Filho, R.C.S.; Carvalho, L.R.B.; Almeida, M.S.; Bonetti, T.C.d.S.; Júnior, E.A.; Braga, A.; Sun, S.Y. Neoadjuvant chemotherapy with carboplatin and paclitaxel in pregnant women with advanced stage cervical cancer: Maternal and perinatal outcomes. J. Gynecol. Obstet. Hum. Reprod. 2024, 54, 102890. [Google Scholar] [CrossRef]
- IBGE: Instituto Brasileiro de Geografia e Estatística (BR). Autoidentificação, identidade étnico-racial e heteroclassificação. In: Características Étnico-Raciais da População: Classificações e Identidades [Internet]. Rio de Janeiro: IBGE; 2013. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv63405.pdf (accessed on 26 May 2021).
- Ministério da Saúde (BR). Cadernos de atenção básica: Atenção ao pré-natal de baixo risco [Internet]. Brasília: Editora do Ministério da Saúde; 2012. 318p. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/cadernos_atencao_basica_32_prenatal.pdf (accessed on 22 May 2021).
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef]
- de Haan, J.; Verheecke, M.; Van Calsteren, K.; Van Calster, B.; Shmakov, R.G.; Gziri, M.M.; Halaska, M.J.; Fruscio, R.; Lok, C.A.R.; A Boere, I.; et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: A 20-year international cohort study of 1170 patients. Lancet Oncol. 2018, 19, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Puzzi-Fernandes, C.; Surita, F.G.; Schettini, C.S.; Parpinelli, M.A.; Guida, J.P.; Costa, M.L. Awareness towards an increasing concern during pregnancy: Maternal and perinatal outcomes of women with cancer. Am. J. Obstet. Gynecol. MFM 2020, 2, 100168. [Google Scholar] [CrossRef] [PubMed]
- Van Calsteren, K.; Heyns, L.; De Smet, F.; Van Eycken, L.; Gziri, M.M.; Van Gemert, W.; Halaska, M.; Vergote, I.; Ottevanger, N.; Amant, F. Cancer during pregnancy: An analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J. Clin. Oncol. 2010, 28, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Vizcaino, A.P.; Moreno, V.; Bosch, F.X.; Muñoz, N.; Barros-Dios, X.M.; Borras, J.; Parkin, D.M. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int. J. Cancer 2000, 86, 429–435. [Google Scholar] [CrossRef]
- Gomez-Hidalgo, N.R.; Mendizabal, E.; Joigneau, L.; Pintado, P.; De Leon-Luis, J. Breast cancer during pregnancy: Results of maternal and perinatal outcomes in a single institution and systematic review of the literature. J. Obstet. Gynaecol. 2018, 39, 27–35. [Google Scholar] [CrossRef]
- García-Manero, M.; Royo, M.P.; Espinos, J.; Pina, L.; Alcazar, J.L.; López, G. Pregnancy associated breast cancer. Eur. J. Surg. Oncol. 2009, 35, 215–218. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Huang, H.F.; Lian, L.J.; Lang, J.H. Ovarian cancer in pregnancy: A clinicopathologic analysis of 22 cases and review of the literature. Int. J. Gynecol. Cancer 2006, 16, 8–15. [Google Scholar] [CrossRef]
- Prat, J.; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- Hosseini, M.M.; Zeyghami, S.; Geramizadeh, B.; Manaheji, F.; Ahmad, E.; Zand, F.; Amoee, S. Management of Bladder Tumor in Pregnancy: Report a Case and Review of the Literature. Middle East J. Cancer 2012, 3, 27–30. [Google Scholar]
- de Moura, A.C.; Delamain, M.T.; Duarte, G.B.O.; Lorand-Metze, I.; de Souza, C.A.; Pagnano, K.B.B. Management of chronic myeloid leukemia during pregnancy: A retrospective analysis at a single center. Hematol. Transfus. Cell Ther. 2019, 41, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kal, H.B.; Struikmans, H. Radiotherapy during pregnancy: Fact and fiction. Lancet Oncol. 2005, 6, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Deckers, S.; Van Calsteren, K.; Loibl, S.; Halaska, M.; Brepoels, L.; Beijnen, J.; Cardoso, F.; Gentilini, O.; Lagae, L.; et al. Breast cancer in pregnancy: Recommendations of an international consensus meeting. Eur. J. Cancer 2010, 46, 3158–3168. [Google Scholar] [CrossRef] [PubMed]
- Cubillo, A.; Morales, S.; Goñi, E.; Matute, F.; Muñoz, J.L.; Pérez-Díaz, D.; de Santiago, J.; Rodríguez-Lescure, Á. Multidisciplinary consensus on cancer management during pregnancy. Clin. Transl. Oncol. 2020, 23, 1054–1066. [Google Scholar] [CrossRef]
- Cardonick, E.; Iacobucci, A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004, 5, 283–291. [Google Scholar] [CrossRef]
- Amant, F.; Vandenbroucke, T.; Verheecke, M.; Fumagalli, M.; Halaska, M.J.; Boere, I.; Han, S.; Gziri, M.M.; Peccatori, F.; Rob, L.; et al. Pediatric Outcome after Maternal Cancer Diagnosed during Pregnancy. N. Engl. J. Med. 2015, 373, 1824–1834. [Google Scholar] [CrossRef]
- Halaska, M.J.; Uzan, C.; Han, S.N.; Fruscio, R.; Steffensen, K.D.; Van Calster, B.; Stankusova, H.; De Marchette, M.L.; Mephon, A.; Rouzier, R.; et al. Characteristics of patients with cervical cancer during pregnancy: A multicenter matched cohort study. An initiative from the International Network on Cancer, Infertility and Pregnancy. Int. J. Gynecol. Cancer 2019, 29, 676–682. [Google Scholar] [CrossRef]
- Rabaiotti, E.; Girardelli, S.; Valsecchi, L.; Bergamini, A.; Petrone, M.; Mangili, G.; Candiani, M. Carboplatin Use in Pregnancy for Stage IB3 Cervical Cancer: Case Report and Review of the Literature. J. Adolesc. Young-Adult Oncol. 2020, 9, 445–448. [Google Scholar] [CrossRef]
- Azim, H.A., Jr.; Santoro, L.; Russell-Edu, W.; Pentheroudakis, G.; Pavlidis, N.; Peccatori, F.A. Prognosis of pregnancy-associated breast cancer: A meta-analysis of 30 studies. Cancer Treat. Rev. 2012, 38, 834–842. [Google Scholar] [CrossRef]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.-S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]

| Sociodemographic Characteristics | Group 1 (N = 28) | Group 2 (N = 11) | Total Cases (N = 39) | ||||
|---|---|---|---|---|---|---|---|
| No of Cases (N) | % | No of Cases (N) | % | No of Cases (N) | % | p * | |
| Ethnicity | 0.333 | ||||||
| White | 13 | 46.4% | 8 | 72.7% | 21 | 53.9% | |
| Mixed | 13 | 46.4% | 3 | 27.3% | 16 | 41% | |
| Black | 2 | 7.2% | 0 | 0 | 2 | 5.1% | |
| Marital status | 1.000 | ||||||
| Single | 7 | 25% | 2 | 18.2% | 9 | 23.1% | |
| Married | 21 | 75% | 9 | 81.8% | 30 | 76.9% | |
| State of residence | 0.545 | ||||||
| São Paulo | 25 | 89.3% | 11 | 100% | 36 | 92.3% | |
| Other | 3 | 10.7% | 0 | 0 | 3 | 7.7% | |
| Education level | 0.013 | ||||||
| Incomplete primary school | 8 | 28.6% | 0 | 0 | 8 | 20.5% | |
| Complete primary school | 2 | 7.1% | 5 | 45.5% | 7 | 17.9% | |
| Incomplete high School | 15 | 53.6% | 6 | 54.5% | 21 | 53.9% | |
| Complete high school | 3 | 10.7% | 0 | 0 | 3 | 7.7% | |
| Type of Cancer | Group 1 (N = 28) | Group 2 (N = 11) | Total (N = 39) | ||||
|---|---|---|---|---|---|---|---|
| No of Case (N) | % | No of Case (N) | % | No of Case (N) | % | p * | |
| Cases (N) | 0.353 | ||||||
| Breast Cancer | 11 | 39.3% | 3 | 27.3% | 14 | 35.9% | |
| Cervical Cancer | 10 | 35.8% | 3 | 27.3% | 13 | 33.4% | |
| Ovarian Cancer | 2 | 7.1% | 1 | 9.1% | 3 | 7.7% | |
| Hematologic Cancer | 2 | 7.1% | 4 | 36.3% | 6 | 15.4% | |
| Gastrointestinal Cancer | 2 | 7.1% | 0 | 0% | 2 | 5.1% | |
| Bladder Cancer | 1 | 3.6% | 0 | 0% | 1 | 2.5% | |
| Staging vs. Type of Neoplasm | Group 1 (N = 28) | Group 2 (N = 11) | Total (N = 39) | ||||
|---|---|---|---|---|---|---|---|
| No of Cases (N) | % | No of Cases (N) | % | No of Cases (N) | % | p * | |
| Breast cancer | N = 11 | N = 3 | N = 14 | 0.835 | |||
| Stage I | 1 | 9.1% | 1 | 33.3% | 2 | 14.3% | |
| Stage II | 3 | 27.3% | 1 | 33.3% | 4 | 28.6% | |
| Stage III | 5 | 45.4% | 1 | 33.4% | 6 | 42.8% | |
| Stage IV | 2 | 18.2% | 0 | 0% | 2 | 14.3% | |
| Cervical cancer | N = 10 | N = 3 | N = 13 | 0.255 | |||
| Stage I | 3 | 30% | 3 | 100% | 6 | 46.2% | |
| Stage II | 4 | 40% | 0 | 0% | 4 | 30.7% | |
| Stage III | 3 | 30% | 0 | 0% | 3 | 23.1% | |
| Ovarian cancer | N = 2 | N = 1 | N = 3 | 1.000 | |||
| IA Stage | 1 | 50% | 1 | 100% | 2 | 66.7% | |
| IC3 Stage | 1 | 50% | 0 | 0% | 1 | 33.3% | |
| Hematologic cancer | N = 2 | N = 4 | N = 6 | - | |||
| BVI | 0 | 0% | 1 | 25% | 1 | 16.7% | |
| Not applicable | 1 | 50% | 3 | 75% | 4 | 66.6% | |
| Not available | 1 | 50% | 0 | 0% | 1 | 16.7% | |
| Gastrointestinal cancer | N = 2 | N = 0 | N = 2 | - | |||
| Stage III | 2 | 100% | 0 | 0% | 2 | 100% | |
| Bladder cancer | N = 1 | N = 0 | N = 1 | - | |||
| Ta Stage | 1 | 100% | 0 | 0% | 1 | 100% | |
| Patient ID | Cancer Type | Stage | GA at Diagnosis (w) | Treatment | GA at Delivery (w) | Birth Weight | Neonatal Outcome | Maternal Outcome |
|---|---|---|---|---|---|---|---|---|
| G1-1 | Breast | T1cN0M0 | 10 | Surgery + Chemo | 37 | SGA | Discharged | Alive |
| G1-2 | Breast | T2N0M0 | 1 | Neoadjuvant Chemo | 38 | AGA | Discharged | Alive |
| G1-3 | Breast | T3N0M0 | 17 | Surgery | 41 | AGA | Discharged | Alive |
| G1-4 | Breast | T3N0M0 | 28 | Surgery | 39 | AGA | Discharged | Death |
| G1-5 | Breast | T3N0M0 | 2 | Surgery + Chemo | 35 | AGA | Discharged | Alive |
| G1-6 | Breast | T3N1aM0 | 17 | Surgery + Chemo | 37 | AGA | Discharged | Death |
| G1-7 | Breast | T3N2M0 | 8 | Neoadjuvant Chemo + Surgery | 39 | SGA | Discharged | Death |
| G1-8 | Breast | T3N3cM0 | 19 | Neoadjuvant Chemo | 36 | AGA | Discharged | Death |
| G1-9 | Breast | T4bN1M0 | 23 | Neoadjuvant Chemo | 35 | SGA | NICU | Death |
| G1-10 | Breast | T4bN3aM1 | 7 | Surgery + Chemo | 35 | SGA | Discharged | Alive |
| G1-11 | Breast | TxNxM1 | 27 | Palliative Chemo | 30 | AGA | NICU | Death |
| G1-12 | Cervical | IB1 | 7 | Surgery | 39 | AGA | Discharged | Alive |
| G1-13 | Cervical | IB3 | 21 | None | 24 | AGA | Death | Death |
| G1-14 | Cervical | IB3 | 26 | Neoadjuvant Chemo | 37 | AGA | Discharged | Alive |
| G1-15 | Cervical | IIA1 | 25 | Neoadjuvant Chemo | 36 | AGA | Discharged | Alive |
| G1-16 | Cervical | IIA2 | 20 | Neoadjuvant Chemo | 38 | AGA | Discharged | Alive |
| G1-17 | Cervical | IIB | 13 | Neoadjuvant Chemo | 34 | AGA | Discharged | Alive |
| G1-18 | Cervical | IIB | 1 | Neoadjuvant Chemo | 33 | AGA | NICU | Alive |
| G1-19 | Cervical | IIIC1 | 25 | Neoadjuvant Chemo | 37 | AGA | Discharged | Death |
| G1-20 | Cervical | IIIC1 | 22 | Neoadjuvant Chemo | 29 | Fetal death | - | Alive |
| G1-21 | Cervical | IIIC1 | Postpartum | - | 37 | AGA | Discharged | Death |
| G1-22 | Ovarian | IC3 | 13 | Surgery + Chemo | 37 | AGA | Discharged | Alive |
| G1-23 | Ovarian | IA | 18 | Surgery | 41 | AGA | Discharged | Alive |
| G1-24 | Leukemia | - | 9 | Chemo | Abortion | - | - | Alive |
| G1-25 | Lymphoma | - | 7 | None | 37 | AGA | NICU | Alive |
| G1-26 | Gastrointestinal | IIIB | 27 | None | 36 | AGA | Discharged | Alive |
| G1-27 | Gastrointestinal | T4N0M0 | 27 | None | 30 | AGA | NICU | Death |
| G1-28 | Bladder | TaG1 | 5 | Surgery | 41 | AGA | Discharged | Alive |
| G2-1 | Breast | T1bN0M0 | Before pregnancy | None during pregnancy | 40 | AGA | Discharged | Alive |
| G2-2 | Breast | T1N3M0 | Before pregnancy | Incidental Radiotherapy | 29 | SGA | NICU | Death |
| G2-3 | Breast | T2N1M0 | Before pregnancy | Surgery + Chemo | 37 | AGA | NICU | Death |
| G2-4 | Cervical | IB1 | Before pregnancy | None during pregnancy | 38 | AGA | Discharged | Alive |
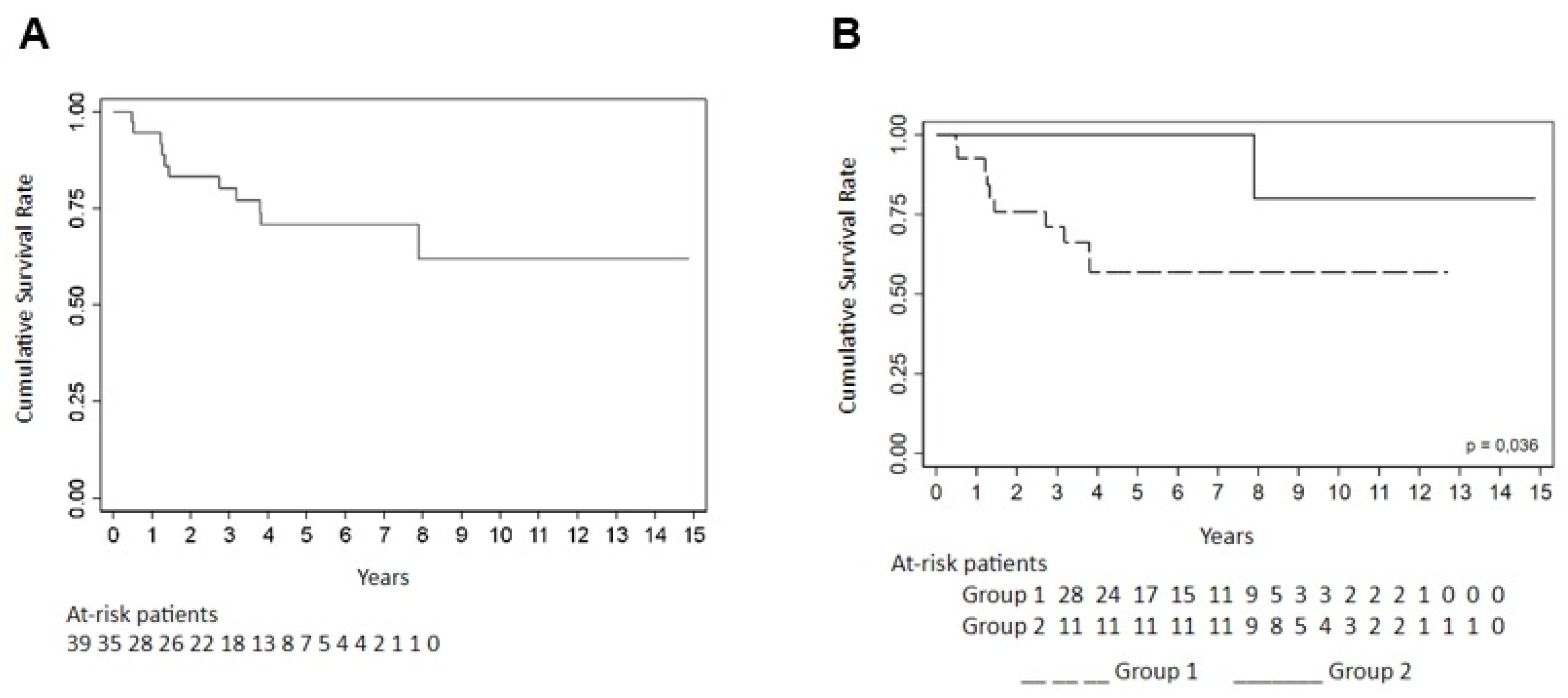
| Cumulative % Survival | p * | ||||||
|---|---|---|---|---|---|---|---|
| 6 Months | 1 Year | 2 Years | 3 Years | 4 Years | 5 Years | ||
| Total | 66.67 ± 7.55 | 61.33 ± 7.83 | 55.87 ± 8.03 | 55.87 ± 8.03 | 55.87 ± 8.03 | 55.87 ± 8.03 | |
| Group | 0.052 | ||||||
| 1 | 60.71 ± 9.23 | 53.13 ± 9.51 | 45.22 ± 9.60 | 45.22 ± 9.60 | 45.22 ± 9.60 | 45.22 ± 9.60 | |
| 2 | 81.82 ± 11.63 | 81.82 ± 11.63 | 81.82 ± 11.63 | 81.82 ± 11.63 | 81.82 ± 11.63 | 81.82 ±11.63 | |
| Type of tumor | 0.006 | ||||||
| Gastrointestinal tract | (1) | (1) | (1) | (1) | (1) | (1) | |
| Bladder | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | |
| Cervix | 76.92 ± 11.69 | 76.92 ± 11.69 | 58.61 ± 14.45 | 58.61 ± 14.45 | 58.61 ± 14.45 | 58.61 ± 14.45 | |
| Breast | 42.86 ± 13.23 | 28.57 ± 12.07 | 28.57 ± 12.07 | 28.57 ± 12.07 | 28.57 ± 12.07 | 28.57 ± 12.07 | |
| Ovary | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | |
| Hematological | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | 100.00 (-) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes Ferraz, B.E.P.; Signorini Filho, R.C.; Carvalho, L.R.B.; Almeida, M.S.; Bonetti, T.C.d.S.; Araujo Júnior, E.; Braga, A.; Sun, S.Y.; Granese, R. Maternal and Perinatal Outcomes in Pregnant Women with Cancer: A Single-Center Retrospective Cohort Study. Diagnostics 2025, 15, 1012. https://doi.org/10.3390/diagnostics15081012
Lopes Ferraz BEP, Signorini Filho RC, Carvalho LRB, Almeida MS, Bonetti TCdS, Araujo Júnior E, Braga A, Sun SY, Granese R. Maternal and Perinatal Outcomes in Pregnant Women with Cancer: A Single-Center Retrospective Cohort Study. Diagnostics. 2025; 15(8):1012. https://doi.org/10.3390/diagnostics15081012
Chicago/Turabian StyleLopes Ferraz, Bruna Elias Parreira, Roney César Signorini Filho, Lucas Ribeiro Borges Carvalho, Michelle Samora Almeida, Tatiana Carvalho de Souza Bonetti, Edward Araujo Júnior, Antonio Braga, Sue Yazaki Sun, and Roberta Granese. 2025. "Maternal and Perinatal Outcomes in Pregnant Women with Cancer: A Single-Center Retrospective Cohort Study" Diagnostics 15, no. 8: 1012. https://doi.org/10.3390/diagnostics15081012
APA StyleLopes Ferraz, B. E. P., Signorini Filho, R. C., Carvalho, L. R. B., Almeida, M. S., Bonetti, T. C. d. S., Araujo Júnior, E., Braga, A., Sun, S. Y., & Granese, R. (2025). Maternal and Perinatal Outcomes in Pregnant Women with Cancer: A Single-Center Retrospective Cohort Study. Diagnostics, 15(8), 1012. https://doi.org/10.3390/diagnostics15081012









