Low Carotid Mean Flow Velocity: A Noninvasive Marker for Coronary Heart Disease—A Community-Based Study
Abstract
1. Introduction
2. Methods
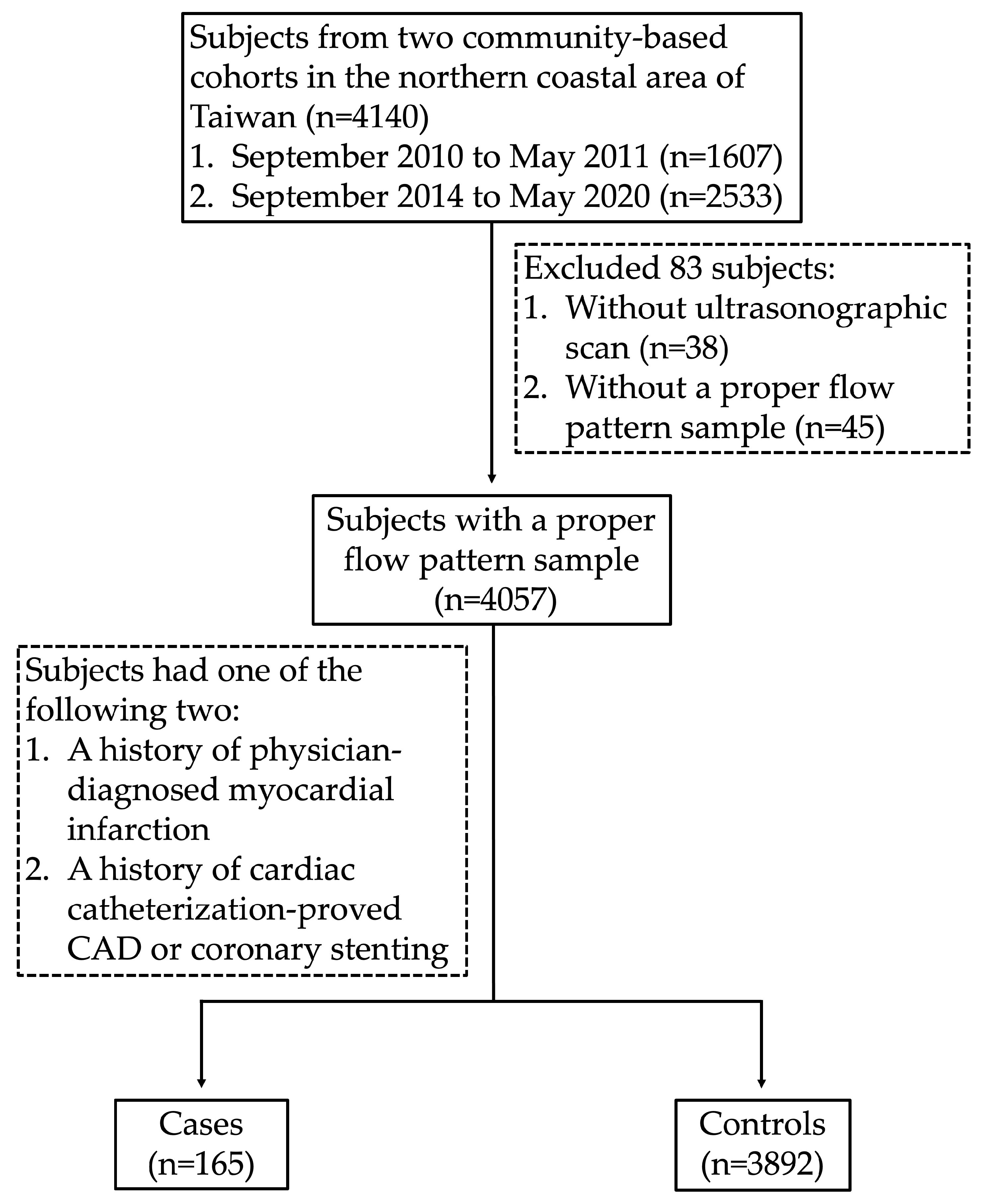
2.1. Study Subjects
2.2. Anthropometric and Biochemical Measurements
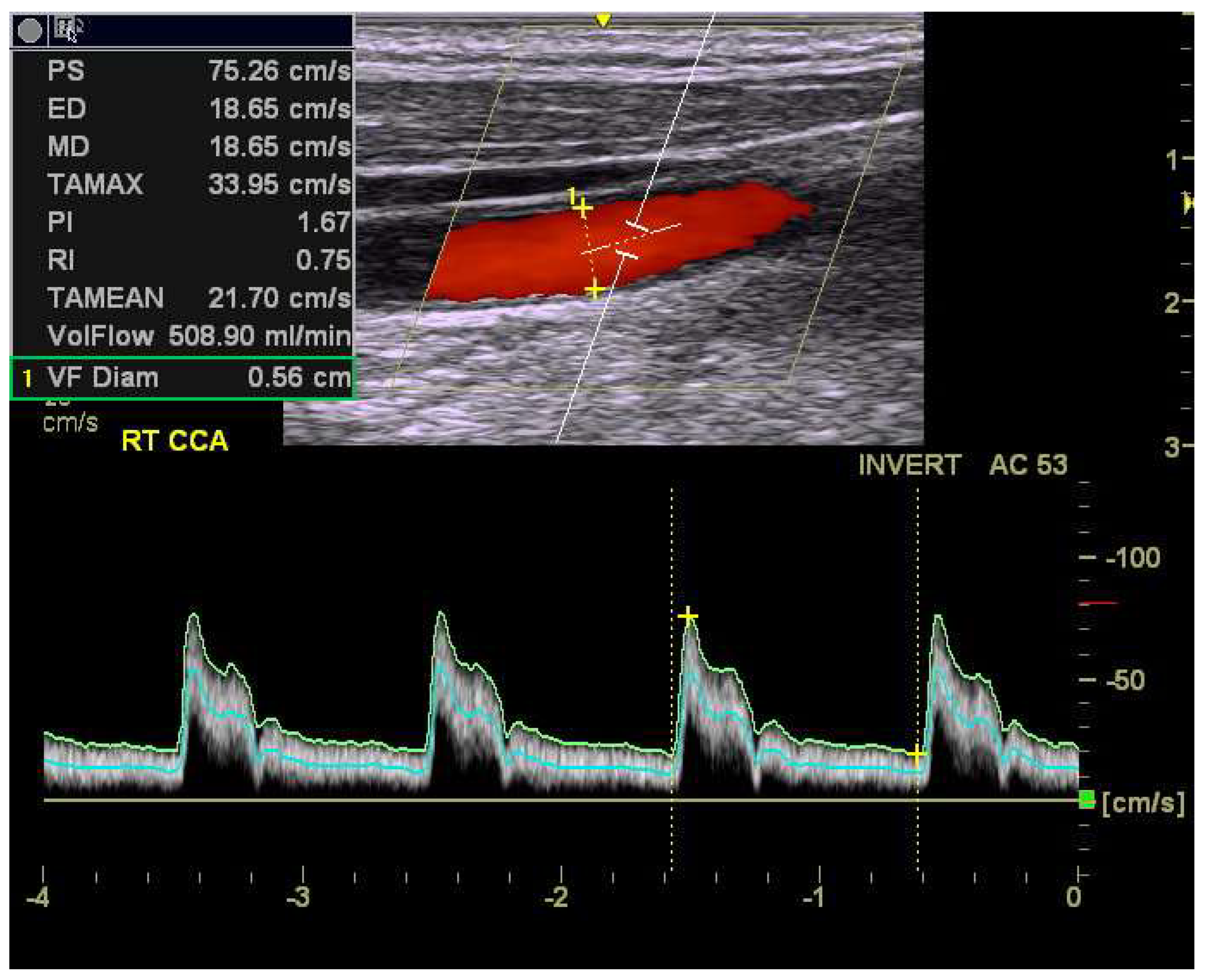
2.3. Ultrasonographic Measurements of Carotid Blood Flow
2.4. Statistical Analyses
3. Results
3.1. Baseline Clinical Characteristics and Carotid Flow Parameters Between CHD and Non-CHD Subjects
3.2. Univariate and Multivariate Analyses for the Carotid Flow Parameters in Predicting the Presence of CHD
3.3. Right CCA MFV and Hypertension Jointly Predict the Presence of CHD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCVDs | Atherosclerosis-related cardiovascular diseases |
| CHD | Coronary heart disease |
| IMT | Intima-media thickness |
| CA | Carotid artery |
| CCA | Common carotid artery |
| ICA | Internal carotid artery |
| MFV | Mean flow velocity |
| PSV | Peak flow velocity |
| EDV | End diastolic flow velocity |
| PI | Pulsatility index |
| RI | Resistance index |
| CAD | Coronary artery disease |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
References
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Satiroglu, O.; Kocaman, S.A.; Karadag, Z.; Temiz, A.; Cetin, M.; Canga, A.; Erdogan, T.; Bostan, M.; Cicek, Y.; Durakoglugil, E.; et al. Relationship of the angiographic extent of peripheral arterial disease with coronary artery involvement. J. Pak. Med. Assoc. 2012, 62, 644–649. [Google Scholar] [PubMed]
- Amarenco, P.; Lavallée, P.C.; Labreuche, J.; Ducrocq, G.; Juliard, J.-M.; Feldman, L.; Cabrejo, L.; Meseguer, E.; Guidoux, C.; Adraï, V.; et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke 2011, 42, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.; Béjot, Y.; Rothwell, P.M.; Touzé, E. Long-term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: Systematic review and meta-analysis. J. Am. Heart Assoc. 2018, 7, e007267. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Ren, T.; Wang, X.; Liu, D.; Xu, M.; Han, L.; Wu, Z.; Li, H.; Zhu, Y.; et al. Associations between carotid artery plaque ccore, carotid hemodynamics and coronary heart disease. Int. J. Environ. Res. Public Health 2015, 12, 14275–14284. [Google Scholar] [CrossRef]
- Chuang, S.-Y.; Bai, C.-H.; Cheng, H.-M.; Chen, J.-R.; Yeh, W.-T.; Hsu, P.-F.; Liu, W.-L.; Pan, W.-H. Common carotid artery end-diastolic velocity is independently associated with future cardiovascular events. Eur. J. Prev. Cardiol. 2016, 23, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.B.; Wang, X.L.; An, Z.J.; Zhu, D.L.; Xu, L.; Xu, T.; Wang, D.; Qu, Y.; Li, N.; Li, L.H. Predicting coronary artery disease by carotid color doppler ultrasonography. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11713–11721. [Google Scholar] [PubMed]
- Zócalo, Y.; Bia, D. Sex- and age-related physiological profiles for brachial, vertebral, carotid, and femoral arteries blood flow velocity parameters during growth and aging (4–76 years): Comparison with clinical cut-off levels. Front. Physiol. 2021, 12, 729309. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-L.; Wu, Y.-J.; Hung, C.-L.; Liu, C.-C.; Wang, S.-D.; Wu, T.-W.; Wang, L.-Y.; Yeh, H.-I. Segment-specific prevalence of carotid artery plaque and stenosis in middle-aged adults and elders in Taiwan: A community-based study. J. Formos. Med. Assoc. 2018, 118, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Jung, Y.H.; Kim, K.H.; Kim, J.Y.; Min, P.K.; Yoon, Y.W.; Lee, B.K.; Hong, B.K.; Rim, S.J.; Kwon, H.M.; et al. Carotid artery end-diastolic velocity and future cerebro-cardiovascular events in asymptomatic high risk patients. Korean Circ. J. 2016, 46, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Wu, C.M.; Chu, C.S.; Lee, K.T.; Sheu, S.H.; Lai, W.T. Association of carotid hemodynamics with risk of coronary heart disease in a Taiwanese population with essential hypertension. Am. J. Hypertens. 2008, 21, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.W.; Wu, Y.J.; Chou, C.L.; Cheng, C.F.; Lu, S.X.; Wang, L.Y. Hemodynamic parameters and diabetes mellitus in community-dwelling middle-aged adults and elders: A community-based study. Sci. Rep. 2024, 14, 12032. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Kleczyński, P.; Niewiara, Ł.; Podolec, J.; Badacz, R.; Gackowski, A.; Pieniążek, P.; Legutko, J.; Żmudka, K.; Przewłocki, T.; et al. Importance of increased arterial resistance in risk prediction in patients with cardiovascular risk factors and degenerative aortic stenosis. J. Clin. Med. 2021, 10, 2109. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Chen, P.-J.; Lu, S.-N.; Liang, F.-W.; Chuang, H.-Y. Comparing carotid artery velocities with current ASCVD risk stratification: A novel approach to simpler risk assessment. J. Epidemiol. Glob. Health 2024, 14, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Okura, T.; Watanabe, S.; Higaki, J. Association between carotid haemodynamics and inflammation in patients with essential hypertension. J. Hum. Hypertens. 2005, 19, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Ueng, K.C.; Chiang, C.E.; Chao, T.H.; Wu, Y.W.; Lee, W.L.; Li, Y.H.; Ting, K.H.; Su, C.H.; Lin, H.J.; Su, T.C.; et al. 2023 Guidelines of the Taiwan Society of Cardiology on the Diagnosis and Management of Chronic Coronary Syndrome. Acta Cardiol. Sin. 2023, 39, 4–96. [Google Scholar] [PubMed]
- Cheong, I.; Otero Castro, V.; Sosa, F.A.; Tort Oribe, B.; Merlo, P.M.; Tamagnone, F.M. Carotid flow as a surrogate of the left ventricular stroke volume. J. Clin. Monit. Comput. 2023, 37, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-Y.; Bai, C.-H.; Chen, J.-R.; Yeh, W.-T.; Chen, H.-J.; Chiu, H.-C.; Shiu, R.-S.; Pan, W.-H. Common carotid end-diastolic velocity and intima-media thickness jointly predict ischemic stroke in Taiwan. Stroke 2011, 42, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
| Variables | Non-CHD Controls (n = 3892) | CHD Patients (n = 165) | p-Value | ||
|---|---|---|---|---|---|
| Continuous variables | Mean | SD | Mean | SD | |
| Age (years) | 56.0 | 8.9 | 61.3 | 8.5 | <0.0001 |
| Body mass index (kg/m2) | 24.6 | 3.6 | 25.2 | 3.7 | 0.041 |
| Waist circumference (cm) | 85.6 | 10.1 | 88.6 | 9.8 | 0.0002 |
| HIP (cm) | 96.5 | 7.2 | 96.9 | 6.7 | 0.44 |
| Waist-to-hip ratio (%) | 88.6 | 7.1 | 91.3 | 6.4 | <0.0001 |
| SBP (mmHg) | 126.5 | 18.7 | 127.6 | 17.1 | 0.46 |
| DBP (mmHg) | 76.3 | 12.6 | 75.5 | 11.4 | 0.38 |
| Total cholesterol (mg/dL) | 204.8 | 38.6 | 184.1 | 39.3 | <0.0001 |
| LDL (mg/dL) | 121.4 | 32.5 | 104.9 | 31.5 | <0.0001 |
| HDL (mg/dL) | 55.7 | 15.0 | 51.4 | 15.5 | 0.0004 |
| Log (Trigelyceride) | 4.6 | 0.6 | 4.7 | 0.6 | 0.11 |
| Categorical variables | n | % | n | % | |
| Female | 2523 | 64.8 | 86 | 52.1 | 0.0008 |
| Hypertension | 922 | 23.7 | 93 | 56.4 | <0.0001 |
| Hyperlipidemia | 1124 | 28.9 | 82 | 49.7 | <0.0001 |
| Diabetes mellitus | 417 | 10.7 | 39 | 23.6 | <0.0001 |
| Cigarette smoking | 827 | 21.3 | 52 | 31.5 | 0.0019 |
| Alcohol drinking | 498 | 12.8 | 28 | 17.0 | 0.12 |
| Variables | Non-CHD Controls (n = 3892) | CHD Patients (n = 165) | p-Value | ||
|---|---|---|---|---|---|
| Right CCA | Mean | SD | Mean | SD | |
| PSV (cm/s) | 86.2 | 20.2 | 79.6 | 19.4 | <0.0001 |
| EDV (cm/s) | 23.4 | 6.4 | 20.1 | 6.4 | <0.0001 |
| MFV (cm/s) | 40.7 | 9.2 | 35.8 | 9.2 | <0.0001 |
| RI | 0.73 | 0.06 | 0.75 | 0.06 | <0.0001 |
| PI | 1.57 | 0.36 | 1.70 | 0.39 | <0.0001 |
| Left CCA | |||||
| PSV (cm/s) | 85.6 | 19.0 | 80.9 | 19.0 | 0.002 |
| EDV (cm/s) | 24.5 | 6.5 | 21.6 | 7.0 | <0.0001 |
| MFV (cm/s) | 41.6 | 9.2 | 37.8 | 9.7 | <0.0001 |
| RI | 0.71 | 0.06 | 0.73 | 0.06 | <0.0001 |
| PI | 1.49 | 0.34 | 1.61 | 0.37 | <0.0001 |
| Average of right and left CCA | |||||
| PSV (cm/s) | 85.9 | 18.3 | 80.2 | 18.2 | 0.0001 |
| EDV (cm/s) | 23.9 | 5.9 | 20.8 | 6.3 | <0.0001 |
| MFV (cm/s) | 41.1 | 8.5 | 36.8 | 8.9 | <0.0001 |
| RI | 0.71 | 0.06 | 0.73 | 0.06 | <0.0001 |
| PI | 1.53 | 0.32 | 1.66 | 0.35 | <0.0001 |
| Univariable | Multivariable Adjusted 1 | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p | OR | (95% CI) | p | |
| Right CCA | ||||||
| PSV (per 5 cm/s) | 0.92 | (0.88–0.95) | <0.0001 | 0.96 | (0.92–1.01) | 0.094 |
| EDV (per 5 cm/s) | 0.64 | (0.56–0.73) | <0.0001 | 0.82 | (0.70–0.95) | 0.011 |
| MFV (per 5 cm/s) | 0.73 | (0.67–0.80) | <0.0001 | 0.85 | (0.77–0.95) | 0.0038 |
| RI (per 0.1) | 1.94 | (1.46–2.57) | <0.0001 | 1.32 | (0.97–1.78) | 0.073 |
| PI (per 1.0) | 2.22 | (1.55–3.19) | <0.0001 | 1.50 | (0.98–2.29) | 0.065 |
| Left CCA | ||||||
| PSV (per 5 cm/s) | 0.93 | (0.90–0.98) | 0.0019 | 0.98 | (0.93–1.02) | 0.35 |
| EDV (per 5 cm/s) | 0.69 | (0.60–0.78) | <0.0001 | 0.86 | (0.74–0.99) | 0.037 |
| MFV (per 5 cm/s) | 0.79 | (0.72–0.86) | <0.0001 | 0.91 | (0.82–1.01) | 0.069 |
| RI (per 0.1) | 2.01 | (1.53–2.65) | <0.0001 | 1.41 | (1.04–1.90) | 0.027 |
| PI (per 1.0) | 2.14 | (1.49–3.06) | <0.0001 | 1.60 | (1.03–2.50) | 0.037 |
| Average of right and left CCA | ||||||
| PSV (per 5 cm/s) | 0.91 | (0.87–0.96) | <0.0001 | 0.97 | (0.92–1.01) | 0.15 |
| EDV (per 5 cm/s) | 0.62 | (0.54–0.71) | <0.0001 | 0.81 | (0.68–0.95) | 0.011 |
| MFV (per 5 cm/s) | 0.73 | (0.66–0.80) | <0.0001 | 0.86 | (0.77–0.97) | 0.0098 |
| RI (per 0.1) | 2.23 | (1.65–3.01) | <0.0001 | 1.46 | (1.04–2.04) | 0.027 |
| PI (per 1.0) | 2.64 | (1.73–3.97) | <0.0001 | 1.72 | (1.05–2.83) | 0.032 |
| CHD Cases | Model 1 1 | Model 2 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | No. | % | OR | (95% CI) | p | OR | (95% CI) | p | ||
| Hypertension | ||||||||||
| No | 3042 | 72 | 2.4 | 1.00 | - | |||||
| Yes | 1015 | 93 | 9.2 | 2.84 | (1.95–4.13) | <0.0001 | - | |||
| Right CCA MFV (cm/s) | ||||||||||
| ≥44.2 | 1350 | 30 | 2.2 | 1.00 | - | |||||
| 36.0~44.1 | 1365 | 40 | 2.9 | 1.06 | (0.65–1.74) | 0.81 | - | |||
| <36.0 | 1342 | 95 | 7.1 | 1.79 | (1.13–2.84) | 0.013 | - | |||
| Hypertension | MFV (cm/s) | |||||||||
| No | ≥44.2 | 1152 | 20 | 1.7 | - | 1.00 | ||||
| No | 36.0~44.1 | 1061 | 23 | 2.2 | - | 1.13 | (0.61–2.08) | 0.71 | ||
| No | <36.0 | 829 | 29 | 3.5 | - | 1.63 | (0.89–2.98) | 0.11 | ||
| Yes | ≥44.2 | 198 | 10 | 5.1 | - | 2.47 | (1.10–5.56) | 0.029 | ||
| Yes | 36.0~44.1 | 304 | 17 | 5.6 | - | 2.46 | (1.20–5.03) | 0.014 | ||
| Yes | <36.0 | 513 | 66 | 12.9 | - | 4.79 | (2.63–8.72) | <0.0001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.-C.; Chou, C.-L.; Wu, S.-H.; Wu, T.-W.; Lan, W.-R.; Cheng, C.-F.; Lu, S.-X.; Wu, Y.-J.; Wang, L.-Y. Low Carotid Mean Flow Velocity: A Noninvasive Marker for Coronary Heart Disease—A Community-Based Study. Diagnostics 2025, 15, 1005. https://doi.org/10.3390/diagnostics15081005
Wu L-C, Chou C-L, Wu S-H, Wu T-W, Lan W-R, Cheng C-F, Lu S-X, Wu Y-J, Wang L-Y. Low Carotid Mean Flow Velocity: A Noninvasive Marker for Coronary Heart Disease—A Community-Based Study. Diagnostics. 2025; 15(8):1005. https://doi.org/10.3390/diagnostics15081005
Chicago/Turabian StyleWu, Li-Chih, Chao-Liang Chou, Shu-Hao Wu, Tzu-Wei Wu, Wei-Ren Lan, Chun-Fang Cheng, Shu-Xin Lu, Yih-Jer Wu, and Li-Yu Wang. 2025. "Low Carotid Mean Flow Velocity: A Noninvasive Marker for Coronary Heart Disease—A Community-Based Study" Diagnostics 15, no. 8: 1005. https://doi.org/10.3390/diagnostics15081005
APA StyleWu, L.-C., Chou, C.-L., Wu, S.-H., Wu, T.-W., Lan, W.-R., Cheng, C.-F., Lu, S.-X., Wu, Y.-J., & Wang, L.-Y. (2025). Low Carotid Mean Flow Velocity: A Noninvasive Marker for Coronary Heart Disease—A Community-Based Study. Diagnostics, 15(8), 1005. https://doi.org/10.3390/diagnostics15081005






