An Incidental Finding of Appendiceal Gastrointestinal Stromal Tumor with Abundant Skeinoid Fibers: A Rare Case Report with Insights from a Comprehensive Literature Review
Abstract
1. Introduction
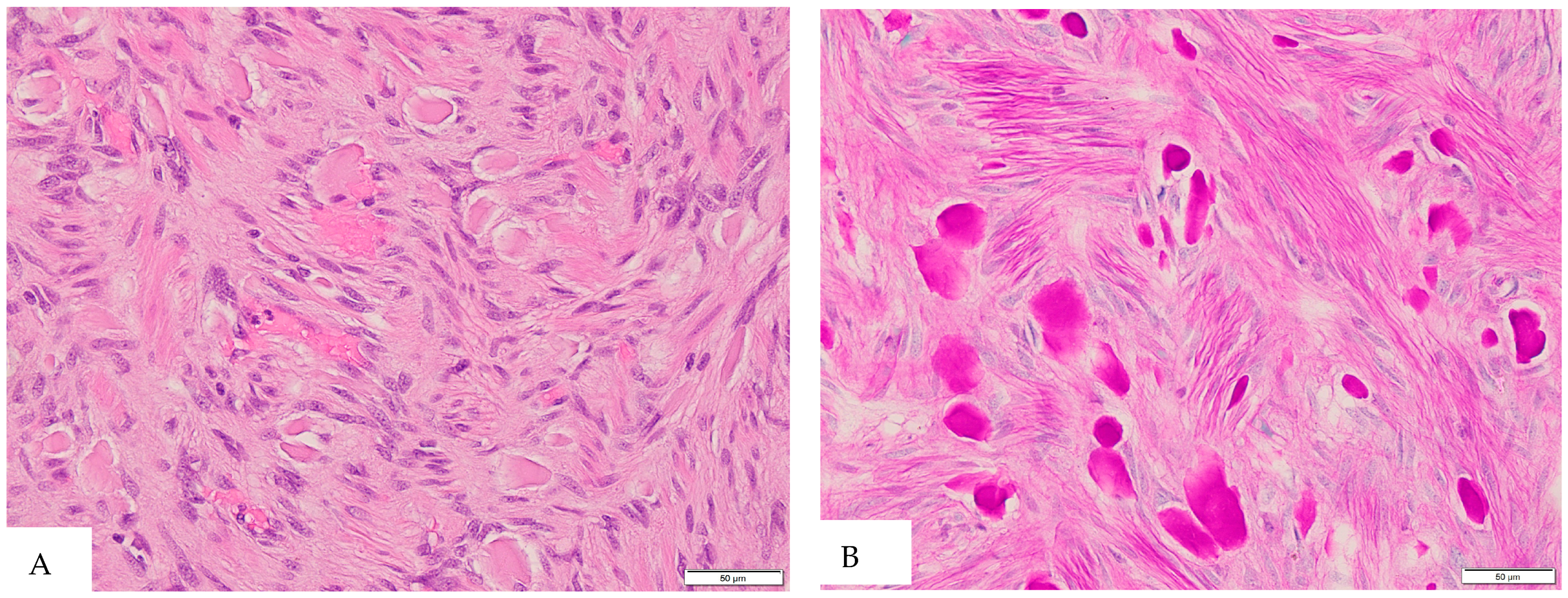
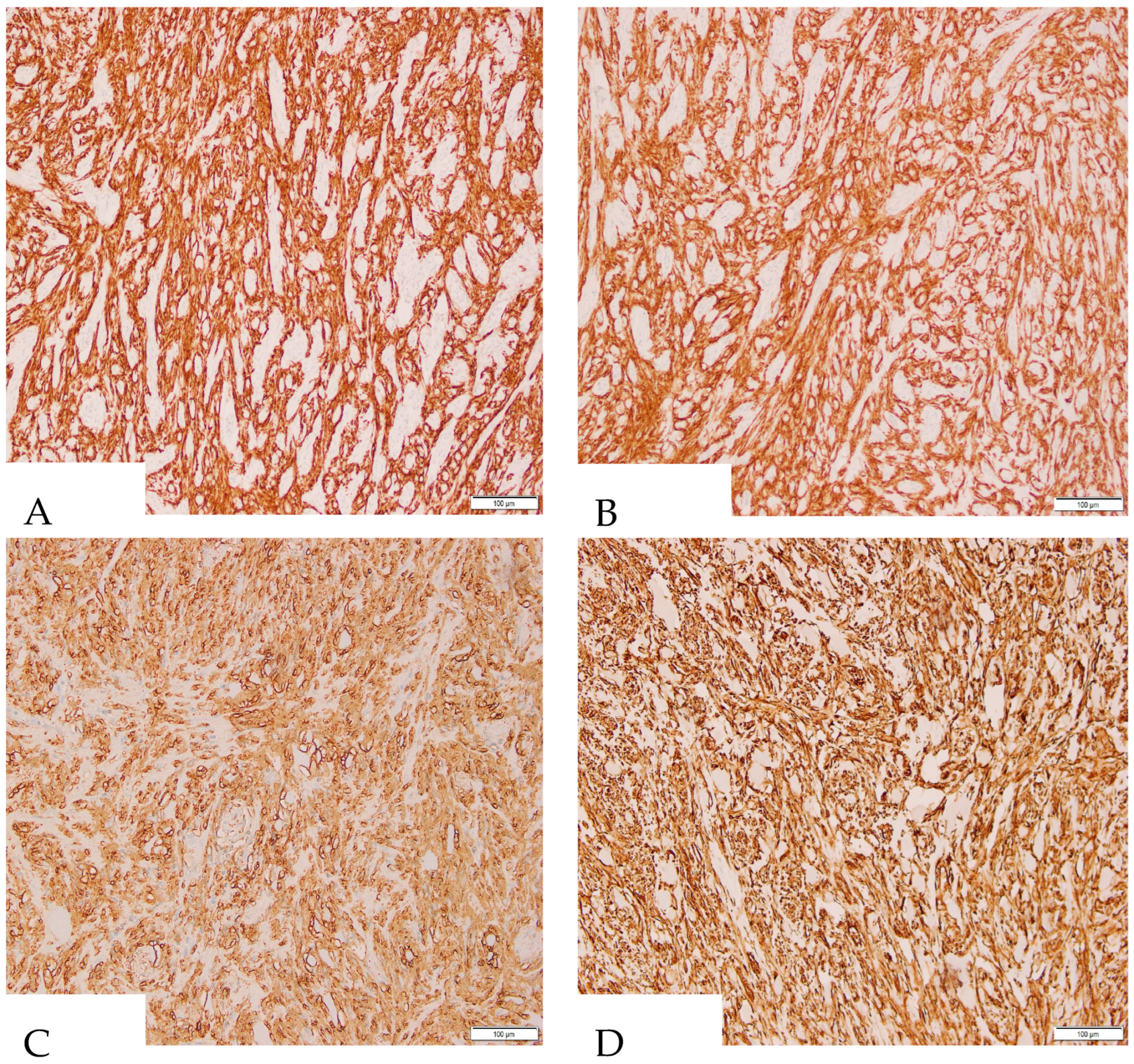
2. Case Presentation
3. Discussion and Literature Review
| Case No. | Reference | Age | Gender | Symptoms | Location | Size (cm) | Histology | Risk Classification | Coexisting Malignancy | Genotype | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Markku Miettinen et al., 2001 [6] | 64 | M | Incidental (autopsy) | Tip | 1.4 | Spindle cell: SF+ | Low risk | Metastatic pulmonary adenocarcinoma | N/A | Died (unrelated) |
| 2 | Markku Miettinen et al., 2001 [6] | 56 | M | Appendicitis-like | Proximal | 1.2 | Spindle cell; SF+ | Low risk | None | N/A | Alive at 8 Y |
| 3 | Markku Miettinen et al., 2001 [6] | 59 | M | Incidental | N/A | 0.9 | Spindle cell; SF+ | Low risk | Gastric epithelioid GIST | N/A | Died at 15 Y (liver metastasis from gastric GIST) |
| 4 | Markku Miettinen et al., 2001 [6] | 72 | M | Appendicitis | Proximal | 1.3 | Spindle cell | N/A | None | N/A | Died at 4 Y (COPD) |
| 5 | W. M. Yap et al., 2005 [8] | 66 | F | Appendicitis-like | Mid portion | 0.25 | Spindle cell | Low risk | NF2 | N/A | N/A |
| 6 | Kyu-Jong Kim et al., 2007 [20] | 56 | M | GI bleeding | Mid portion | <1 | Spindle cell | Low risk | None | N/A | No recurrence at 3 M |
| 7 | A. Agaimy et al., 2008 [4] | 72 | M | Incidental | Tip | 2.5 | Spindle cell; focal epithelioid | Low risk | Urinary bladder carcinoma | KIT inframe deletion I571_R588 (Exon 11) | No recurrence at 4 M |
| 8 | A. Agaimy et al., 2008 [4] | 78 | F | Appendicitis-like | Proximal | 0.5 | Spindle cell; SF+ | Very low risk | Endometrial adenocarcinoma | KIT K558R (Exon11) | N/A |
| 9 | A. Agaimy et al., 2008 [5] | 86 | F | N/A | N/A | <1 | Spindle cell | Very low risk | None | Wild type | N/A |
| 10 | Kurosh Rahimi et al., 2009 [7] | 65 | F | Incidental | N/A | 1.1 | Epithelioid and spindle cell; nerve differentiation | Very low risk | Mantle cell lymphoma with appendiceal involvement | Wild type | No recurrence at 24 M |
| 11 | Ram Elazary et al. [19] | 57 | M | Abdominal pain and peri-appendiceal abscess | Tip | 20 | Spindle cells | Malignant (invading adjacent bowel) | None | N/A | N/A |
| 12 | Wenhua Li et al., 2012 [3] | 6 | M | Abdominal pain and hard mass | Mesoappendix | 6.7 | Spindle and epithelioid cell | Malignant | None | N/A | No recurrence at 9 M |
| 13 | M. Bouassida et al., 2013 [21] | 75 | M | Appendicitis, perforation, and acute peritonitis | Mid portion | 2.0 | Spindle cell | Low risk | None | N/A | No recurrence at 48 M |
| 14 | Nikolaos Vassos et al., 2013 [13] | 48 | M | Appendicitis-like | N/A | 3.0 | Spindle cell | High risk (tumor rupture) | None | KIT 558-559del and 559-560ins (Exon11) | No recurrence at 27 M |
| 15 | J. M. Chun and K. H. Lim, 2016 [22] | 68 | M | Appendicitis | Proximal | 3.0 | Spindle cell | Low risk | None | N/A | No recurrence at 35 M |
| 16 | M. Kaneko et al., 2017 [18] | 67 | M | Large abdominal mass | Tip | 22.0 | Spindle cell | Malignant (peritoneal metastasis) | None | N/A | No recurrence at 6 M |
| 17 | Bao Zhang et al., 2018 [23] | 59 | F | Incidental | N/A | 10 | N/A | High risk | Cervical cancer | N/A | N/A |
| 18 | N. Mourra et al., 2020 [10] | 61 | M | Appendicitis-like symptoms | Proximal | 1.0 | Spindle cell; SF+ | Very low risk | None | N/A | N/A |
| 19 | Ahmad Elnassasra et al., 2022 [24] | 75 | M | Appendicitis | Tip | <1 | Spindle cell | Low risk | None | N/A | N/A |
| 20 | Jacob D. Williams et al., 2024 [25] | 74 | M | Abdominal pain; appendiceal mass | Mid portion | 1.5 | Spindle cell | Low risk | None | N/A | No recurrence at 2 M |
| Our case | 74 | M | Incidental | Proximal | 1.2 | Spindle cell; SF+ | Very low risk | Colonic adenocarcinoma | N/A | No recurrence at 4 M |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [PubMed]
- Kalfusova, A.; Linke, Z.; Kalinova, M.; Krskova, L.; Hilska, I.; Szabova, J.; Vicha, A.; Kodet, R. Gastrointestinal stromal tumors—Summary of mutational status of the primary/secondary KIT/PDGFRA mutations, BRAF mutations and SDH defects. Pathol. Res. Pract. 2019, 215, 152708. [Google Scholar] [PubMed]
- Li, W.; Cui, Y.; Ren, G.; Wang, J.; Wu, X. Extragastrointestinal stromal tumor of the mesoappendix: CT findings and a review of the literature. World J. Surg. Oncol. 2012, 10, 211. [Google Scholar] [PubMed]
- Agaimy, A.; Pelz, A.F.; Wieacker, P.; Roessner, A.; Wunsch, P.H.; Schneider-Stock, R. Gastrointestinal stromal tumors of the vermiform appendix: Clinicopathologic, immunohistochemical, and molecular study of 2 cases with literature review. Hum. Pathol. 2008, 39, 1252–1257. [Google Scholar]
- Agaimy, A.; Wunsch, P.H.; Dirnhofer, S.; Bihl, M.P.; Terracciano, L.M.; Tornillo, L. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: A clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am. J. Surg. Pathol. 2008, 32, 867–873. [Google Scholar] [CrossRef]
- Miettinen, M.; Sobin, L.H. Gastrointestinal stromal tumors in the appendix: A clinicopathologic and immunohistochemical study of four cases. Am. J. Surg. Pathol. 2001, 25, 1433–1437. [Google Scholar]
- Rahimi, K.; Gologan, A.; Haliotis, T.; Lamoureux, E.; Chetty, R. Gastrointestinal stromal tumor with autonomic nerve differentiation and coexistent mantle cell lymphoma involving the appendix. Int. J. Clin. Exp. Pathol. 2009, 2, 608–613. [Google Scholar]
- Yap, W.M.; Tan, H.W.; Goh, S.G.; Chuah, K.L. Appendiceal gastrointestinal stromal tumor. Am. J. Surg. Pathol. 2005, 29, 1545–1547. [Google Scholar]
- Miettinen, M.; Fetsch, J.F.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: A clinicopathologic and molecular genetic study of 45 cases. Am. J. Surg. Pathol. 2006, 30, 90–96. [Google Scholar]
- Mourra, N.; Matalka, I.; Arrive, L. Incidental Finding of Gastrointestinal Stromal Tumor in the Appendix. Appl. Immunohistochem. Mol. Morphol. 2020, 28, e31–e32. [Google Scholar]
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008, 39, 1411–1419. [Google Scholar] [PubMed]
- Miettinen, M.; Makhlouf, H.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the jejunum and ileum: A clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am. J. Surg. Pathol. 2006, 30, 477–489. [Google Scholar] [PubMed]
- Vassos, N.; Agaimy, A.; Gunther, K.; Hohenberger, W.; Schneider-Stock, R.; Croner, R.S. A novel complex KIT mutation in a gastrointestinal stromal tumor of the vermiform appendix. Hum. Pathol. 2013, 44, 651–655. [Google Scholar]
- Comandone, A.; Boglione, A. The importance of mutational status in prognosis and therapy of GIST. Recenti Prog. Med. 2015, 106, 17–22. [Google Scholar]
- Astolfi, A.; Indio, V.; Nannini, M.; Saponara, M.; Schipani, A.; De Leo, A.; Altimari, A.; Vincenzi, B.; Comandini, D.; Grignani, G.; et al. Targeted Deep Sequencing Uncovers Cryptic KITMutations in KIT/PDGFRA/SDH/RAS-PWild-Type GIST. Front. Oncol. 2020, 10, 504. [Google Scholar]
- Benesch, M.; Wardelmann, E.; Ferrari, A.; Brennan, B.; Verschuur, A. Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatr. Blood Cancer 2009, 53, 1171–1179. [Google Scholar]
- Min, V.; Corradini, N.; Macagno, N.; Orbach, D.; Reguerre, Y.; Petit, P.; Blay, J.-Y.; Verschuur, A. Gastrointestinal stromal tumours (GIST) in children: An update of this orphan disease. Bull. Cancer 2025, 112, 348–357. [Google Scholar]
- Kaneko, M.; Kawai, K.; Murono, K.; Nishikawa, T.; Sasaki, K.; Otani, K.; Yasuda, K.; Tanaka, T.; Kiyomatsu, T.; Hata, K.; et al. Giant gastrointestinal stromal tumor of the vermiform appendix: A case report. Mol. Clin. Oncol. 2017, 7, 399–403. [Google Scholar]
- Elazary, R.; Schlager, A.; Khalaileh, A.; Appelbaum, L.; Bala, M.; Abu-Gazala, M.; Khatib, A.; Neuman, T.; Rivkind, A.I.; Almogy, G. Malignant appendiceal GIST: Case report and review of the literature. J. Gastrointest. Cancer 2010, 41, 9–12. [Google Scholar]
- Kim, K.J.; Moon, W.; Park, M.I.; Park, S.J.; Lee, S.H.; Chun, B.K. Gastrointestinal stromal tumor of appendix incidentally diagnosed by appendiceal hemorrhage. World J. Gastroenterol. 2007, 13, 3265–3267. [Google Scholar]
- Bouassida, M.; Chtourou, M.F.; Chalbi, E.; Chebbi, F.; Hamzaoui, L.; Sassi, S.; Charifi, L.; Mighri, M.M.; Touinsi, H.; Sassi, A. Appendiceal GIST: Report of an exceptional case and review of the literature. Pan Afr. Med. J. 2013, 15, 85. [Google Scholar] [PubMed]
- Chun, J.M.; Lim, K.H. Gastrointestinal stromal tumor of the vermiform appendix mimicking Meckel’s diverticulum: Case report with literature review. Int. J. Surg. Case Rep. 2016, 21, 20–22. [Google Scholar] [PubMed]
- Zhang, B.; Zheng, G.L.; Zhu, H.T.; Zhao, Y.; Zheng, Z.C. Clinicopathological characteristics and prognosis of primary appendiceal stromal tumors. World J. Surg. Oncol. 2018, 16, 225. [Google Scholar]
- Elnassasra, A.; Hershkovitz, Y.; Zager, Y.; Lavy, R. Gastrointestinal Stromal Tumor of the Appendix. Isr. Med. Assoc. J. 2022, 24, 677–678. [Google Scholar]
- Williams, J.D.; Kong, C.Y.; Schmigylski, R.; Nale, K.; Mirza, M. Gastrointestinal Stromal Tumour of the Appendix: A Very Rare Entity. Cureus 2024, 16, e59780. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Y.; Feroze, A.; Fu, Z. An Incidental Finding of Appendiceal Gastrointestinal Stromal Tumor with Abundant Skeinoid Fibers: A Rare Case Report with Insights from a Comprehensive Literature Review. Diagnostics 2025, 15, 924. https://doi.org/10.3390/diagnostics15070924
Liu Y, Chen Y, Feroze A, Fu Z. An Incidental Finding of Appendiceal Gastrointestinal Stromal Tumor with Abundant Skeinoid Fibers: A Rare Case Report with Insights from a Comprehensive Literature Review. Diagnostics. 2025; 15(7):924. https://doi.org/10.3390/diagnostics15070924
Chicago/Turabian StyleLiu, Yu, Yaomin Chen, Asra Feroze, and Zhiyan Fu. 2025. "An Incidental Finding of Appendiceal Gastrointestinal Stromal Tumor with Abundant Skeinoid Fibers: A Rare Case Report with Insights from a Comprehensive Literature Review" Diagnostics 15, no. 7: 924. https://doi.org/10.3390/diagnostics15070924
APA StyleLiu, Y., Chen, Y., Feroze, A., & Fu, Z. (2025). An Incidental Finding of Appendiceal Gastrointestinal Stromal Tumor with Abundant Skeinoid Fibers: A Rare Case Report with Insights from a Comprehensive Literature Review. Diagnostics, 15(7), 924. https://doi.org/10.3390/diagnostics15070924





