The Role of Near-Infrared Spectroscopy (NIRS) in Neurological and Neurodegenerative Diseases as Support to Clinical Practice: An Overview of the Literature
Abstract
1. Introduction
2. Methods
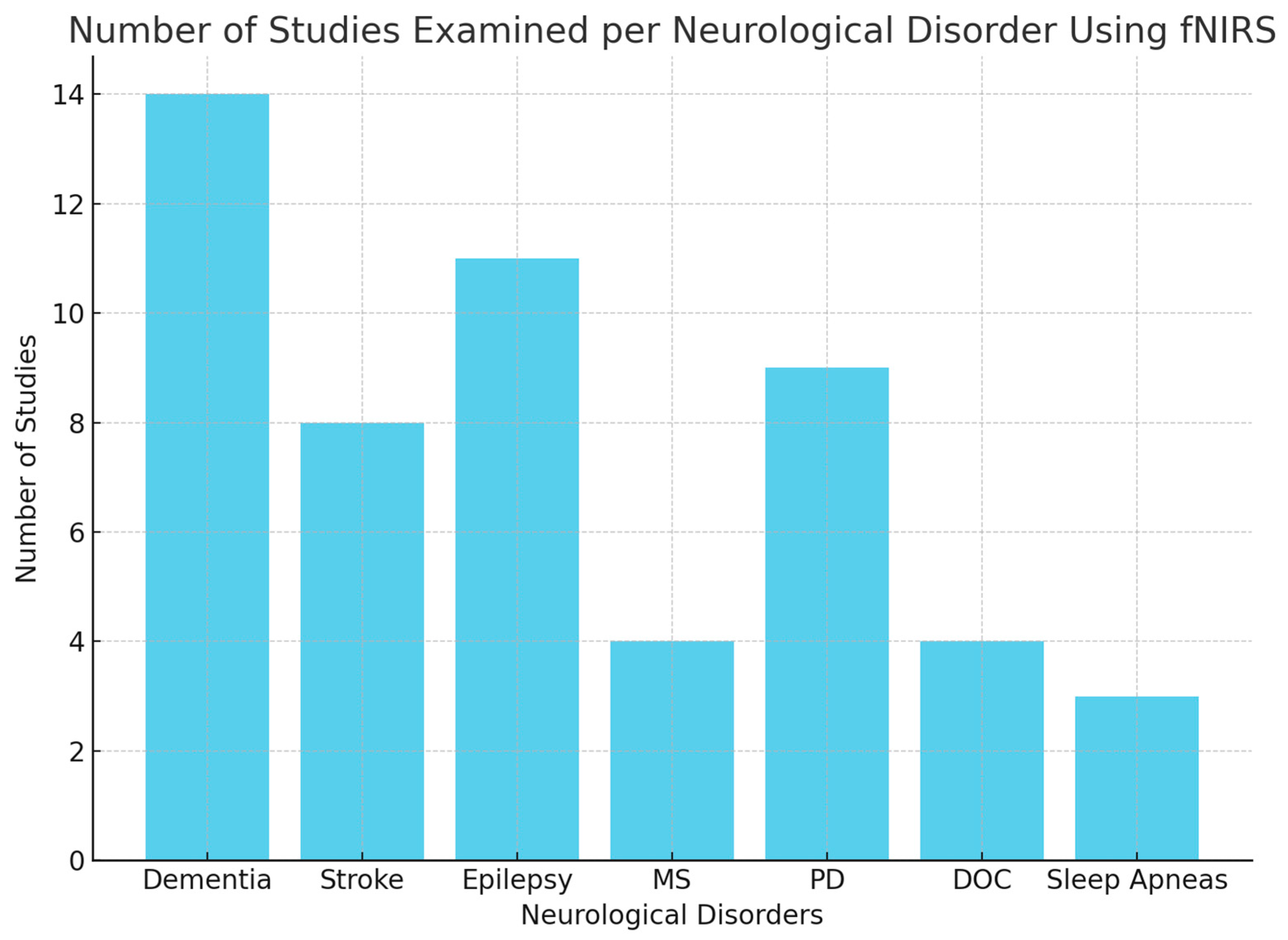
3. Results
3.1. Dementia
3.2. Stroke
3.3. Epilepsy
3.4. Multiple Sclerosis
3.5. Parkinson’s Disease
3.6. Disorders of Consciousness
3.7. Sleep Apneas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NIRS | Near-Infrared Spectroscopy |
| fNIRS | Functional Near-Infrared Spectroscopy |
| Oxy-HB | Oxygenated hemoglobin |
| Deoxy-Hb | Deoxygenated hemoglobin |
| mBLL | Modified Beer–Lambert law |
| HD-EEG | High-Density Electroencephalogram |
| EEG | Electroencephalography |
| MRI | Magnetic Resonance Imaging |
| TMS | Transcranial Magnetic Stimulation |
| AD | Alzheimer’s Disease |
| MCI | Mild Cognitive Impairment |
| VFT | Verbal Fluency Task |
| DTW | Dual-Task Walking |
| PD | Parkinson’s Disease |
| PDD | Parkinson’s Disease Dementia |
| MS | Multiple Sclerosis |
| StO2 | Tissue oxygen saturation |
| FES | Functional electrical stimulation |
| GPi | Globus pallidus internus |
| VIM | Ventralis Intermedium Nucleus |
| DOC | Disorder of Consciousness |
| VS | Vegetative state |
| MCS | Minimally conscious state |
| SCS | Spinal cord stimulation |
| OSA | Obstructive sleep apnea |
| SpO2 | Saturation of peripheral oxygen |
| tDCS | Transcranial Direct Current Stimulation |
References
- Pinti, P.; Aichelburg, C.; Gilbert, S.; Hamilton, A.; Hirsch, J.; Burgess, P.; Tachtsidis, I. A Review on the Use of Wearable Functional Near-Infrared Spectroscopy in Naturalistic Environments. Jpn. Psychol. Res. 2018, 60, 347–373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huo, C.; Xu, G.; Xie, H.; Chen, T.; Shao, G.; Wang, J.; Li, W.; Wang, D.; Li, Z. Functional near-infrared spectroscopy in non-invasive neuromodulation. Neural Regen. Res. 2024, 19, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K.; Chan, A.S. Functional near-infrared spectroscopy reveals decreased resting oxygenation levels and task-related oxygenation changes in mild cognitive impairment and dementia: A systematic review. J. Psychiatr. Res. 2020, 124, 58–76. [Google Scholar]
- Sutin, A.R.; Stephan, Y.; Terracciano, A. Verbal Fluency and Risk of Dementia. Int. J. Geriatr. Psychiatry 2019, 34, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Fallgatter, A.J.; Roesler, M.; Sitzmann, L.; Heidrich, A.; Mueller, T.J.; Strik, W.K. Loss of functional hemispheric asymmetry in Alzheimer’s dementia assessed with near-infrared spectroscopy. Cogn. Brain Res. 1997, 6, 67–72. [Google Scholar]
- Arai, H.; Takano, M.; Miyakawa, K.; Ota, T.; Takahashi, T.; Asaka, H.; Kawaguchi, T. A quantitative near-infrared spectroscopy study: A decrease in cerebral hemoglobin oxygenation in Alzheimer’s disease and mild cognitive impairment. Brain Cogn. 2006, 61, 189–194. [Google Scholar]
- Herrmann, M.J.; Langer, J.B.; Jacob, C.; Ehlis, A.C.; Fallgatter, A.J. Reduced prefrontal oxygenation in Alzheimer disease during verbal fluency tasks. Am. J. Geriatr. Psychiatry 2008, 16, 125–135. [Google Scholar]
- Richter, M.M.; Herrmann, M.J.; Ehlis, A.C.; Plichta, M.M.; Fallgatter, A.J. Brain activation in elderly people with and without dementia: Influences of gender and medication. World J. Biol. Psychiatry 2007, 8, 23–29. [Google Scholar]
- Yeung, M.K.; Sze, S.L.; Woo, J.; Kwok, T.; Shum, D.H.; Yu, R.; Chan, A.S. Altered Frontal Lateralization Underlies the Category Fluency Deficits in Older Adults with Mild Cognitive Impairment: A Near-Infrared Spectroscopy Study. Front. Aging Neurosci. 2016, 8, 59. [Google Scholar]
- Hock, C.; Villringer, K.; Müller-Spahn, F.; Hofmann, M.; Schuh-Hofer, S.; Heekeren, H.; Wenzel, R.; Dirnagl, U.; Villringer, A. Near infrared spectroscopy in the diagnosis of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1996, 777, 22–29. [Google Scholar]
- Niu, H.J.; Li, X.; Chen, Y.J.; Ma, C.; Zhang, J.Y.; Zhang, Z.J. Reduced frontal activation during a working memory task in mild cognitive impairment: A non-invasive near-infrared spectroscopy study. CNS Neurosci. Ther. 2013, 19, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Shimada, H.; Doi, T.; Makizako, H.; Tsutsumimoto, K.; Park, H.; Suzuki, T. Reduced prefrontal oxygenation in mild cognitive impairment during memory retrieval. Int. J. Geriatr. Psychiatry 2016, 31, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Makizako, H.; Shimada, H.; Park, H.; Tsutsumimoto, K.; Uemura, K.; Suzuki, T. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: A fNIRS study. Aging Clin. Exp. Res. 2013, 25, 539–544. [Google Scholar] [CrossRef]
- Zeller, J.B.; Herrmann, M.J.; Ehlis, A.C.; Polak, T.; Fallgatter, A.J. Altered parietal brain oxygenation in Alzheimer’s disease as assessed with near-infrared spectroscopy. Am. J. Geriatr. Psychiatry 2010, 18, 433–441. [Google Scholar] [CrossRef]
- Kito, H.; Ryokawa, A.; Kinoshita, Y.; Sasayama, D.; Sugiyama, N.; Ogihara, T.; Yasaki, T.; Hagiwara, T.; Inuzuka, S.; Takahashi, T.; et al. Comparison of alterations in cerebral hemoglobin oxygenation in late life depression and Alzheimer’s disease as assessed by near-infrared spectroscopy. Behav. Brain Funct. 2014, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Zou, C.J.; Hu, J.; Liu, X.L.; Zheng, C.Y.; Zhou, D.S. Functional near-infrared spectroscopy in elderly patients with four types of dementia. World J. Psychiatry 2023, 13, 203–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.; Lee, H.; Lee, J.; Rhee, S.Y.; Shin, J.I.; Lee, S.W.; Cho, W.; Min, C.; Kwon, R.; Kim, J.G.; et al. Quantification of identifying cognitive impairment using olfactory-stimulated functional near-infrared spectroscopy with machine learning: A post hoc analysis of a diagnostic trial and validation of an external additional trial. Alzheimers Res. Ther. 2023, 15, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Y.; Ma, L.; Cai, W.; Shao, X. Interaction between tau and water during the induced aggregation revealed by near-infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118046. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, G.; Zanoletti, M.; Re, R.; Germinario, B.; Contini, D.; Spinelli, L.; Torricelli, A.; Roveri, L. Time-domain near-infrared spectroscopy in acute ischemic stroke patients. Neurophotonics 2019, 6, 015003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obrig, H.; Steinbrink, J. Non-invasive optical imaging of stroke. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4470–4494. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Z.; Yuan, T.; Feng, W.; Wang, P. A Systemic Review of Functional Near-Infrared Spectroscopy for Stroke: Current Application and Future Directions. Front. Neurol. 2019, 10, 58. [Google Scholar]
- Formica, C.; De Salvo, S.; Muscarà, N.; Bonanno, L.; Arcadi, F.A.; Buono, V.L.; Acri, G.; Quartarone, A.; Marino, S. Applications of Near Infrared Spectroscopy and Mirror Therapy for Upper Limb Rehabilitation in Post-Stroke Patients: A Brain Plasticity Pilot Study. J. Clin. Med. 2024, 13, 6612. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Izumiyama, M.; Koizumi, H.; Takahashi, A.; Itoyama, Y. Near-infrared spectroscopic topography as a tool to monitor motor reorganization after hemiparetic stroke: A comparison with functional MRI. Stroke 2002, 33, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I. Review of Functional Near-Infrared Spectroscopy in Neurorehabilitation. Neurophotonics 2016, 3, 031414. [Google Scholar] [PubMed]
- Liu, Q.; Wang, B.; Liu, Y.; Lv, Z.; Li, W.; Li, Z.; Fan, Y. Frequency-specific Effective Connectivity in Subjects with Cerebral Infarction as Revealed by NIRS Method. Neuroscience 2018, 373, 169–181. [Google Scholar] [CrossRef]
- Su, H.; Huo, C.; Wang, B.; Li, W.; Xu, G.; Liu, Q.; Li, Z. Alterations in the coupling functions between cerebral oxyhaemoglobin and arterial blood pressure signals in post-stroke subjects. PLoS ONE 2018, 13, e0195936. [Google Scholar] [CrossRef]
- Tung, H.; Lin, W.H.; Lan, T.H.; Hsieh, P.F.; Chiang, M.C.; Lin, Y.Y.; Peng, S.J. Network reorganization during verbal fluency task in fronto-temporal epilepsy: A functional near-infrared spectroscopy study. J. Psychiatr. Res. 2021, 138, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Adelson, P.D.; Nemoto, E.; Scheuer, M.; Painter, M.; Morgan, J.; Yonas, H. Noninvasive continuous monitoring of cerebral oxygenation periictally using near-infrared spectroscopy: A preliminary report. Epilepsia 1999, 40, 1484–1489. [Google Scholar]
- Watanabe, E.; Maki, A.; Kawaguchi, F.; Yamashita, Y.; Koizumi, H.; Mayanagi, Y. Noninvasive cerebral blood volume measurement during seizures using multichannel near infrared spectroscopic topography. J. Biomed. Opt. 2000, 5, 287–290. [Google Scholar]
- Buchheim, K.; Obrig, H.; Pannwitz, W.V.; Müller, A.; Heekeren, H.; Villringer, A.; Meierkord, H. Decrease in haemoglobin oxygenation during absence seizures in adult humans. Neurosci. Lett. 2004, 354, 119–122. [Google Scholar] [CrossRef]
- Villringer, A.; Planck, J.; Stodieck, S.; Bötzel, K.; Schleinkofer, L.; Dirnagl, U. Noninvasive assessment of cerebral hemodynamics and tissue oxygenation during activation of brain cell function in human adults using near infrared spectroscopy. Adv. Exp. Med. Biol. 1994, 345, 559–565. [Google Scholar]
- Sokol, D.K.; Markand, O.N.; Daly, E.C.; Luerssen, T.G.; Malkoff, M.D. Near infrared spectroscopy (NIRS) distinguishes seizure types. Seizure 2000, 9, 323–327. [Google Scholar] [PubMed]
- Haginoya, K.; Munakata, M.; Kato, R.; Yokoyama, H.; Ishizuka, M.; Iinuma, K. Ictal cerebral haemodynamics of childhood epilepsy measured with near-infrared spectrophotometry. Brain 2002, 125 Pt 9, 1960–1971. [Google Scholar] [PubMed]
- Roche-Labarbe, N.; Zaaimi, B.; Berquin, P.; Nehlig, A.; Grebe, R.; Wallois, F. NIRS-measured oxy- and deoxyhemoglobin changes associated with EEG spike-and-wave discharges in children. Epilepsia 2008, 49, 1871–1880. [Google Scholar]
- Seyal, M. Frontal hemodynamic changes precede EEG onset of temporal lobe seizures. Clin. Neurophysiol. 2014, 125, 442–448. [Google Scholar] [PubMed]
- Obrig, H. NIRS in Clinical Neurology—A ‘Promising’ Tool? Neuroimage 2014, 85 Pt 1, 535–546. [Google Scholar]
- Watanabe, E.; Maki, A.; Kawaguchi, F.; Takashiro, K.; Yamashita, Y.; Koizumi, H.; Mayanagi, Y. Non-invasive assessment of language dominance with near-infrared spectroscopic mapping. Neurosci. Lett. 1998, 256, 49–52. [Google Scholar]
- Gallagher, A.; Béland, R.; Lassonde, M. The contribution of functional near-infrared spectroscopy (fNIRS) to the presurgical assessment of language function in children. Brain Lang. 2012, 121, 124–129. [Google Scholar]
- Yang, R.; Dunn, J.F. Reduced cortical microvascular oxygenation in multiple sclerosis: A blinded, case-controlled study using a novel quantitative near-infrared spectroscopy method. Sci. Rep. 2015, 5, 16477. [Google Scholar]
- Malagoni, A.M.; Felisatti, M.; Lamberti, N.; Basaglia, N.; Manfredini, R.; Salvi, F.; Zamboni, P.; Manfredini, F. Muscle oxygen consumption by NIRS and mobility in multiple sclerosis patients. BMC Neurol. 2013, 13, 52. [Google Scholar]
- Harp, M.A.; McCully, K.K.; Moldavskiy, M.; Backus, D. Skeletal muscle mitochondrial capacity in people with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2016, 2, 2055217316678020. [Google Scholar]
- Reynolds, M.A.; McCully, K.; Burdett, B.; Manella, C.; Hawkins, L.; Backus, D. Pilot study: Evaluation of the effect of functional electrical stimulation cycling on muscle metabolism in nonambulatory people with multiple sclerosis. Arch. Phys. Med. Rehabil. 2015, 96, 627–632. [Google Scholar] [PubMed]
- Stuart, S.; Vitorio, R.; Morris, R.; Martini, D.N.; Fino, P.C.; Mancini, M. Cortical activity during walking and balance tasks in older adults and in people with Parkinson’s disease: A structured review. Maturitas 2018, 113, 53–72. [Google Scholar] [PubMed]
- Mahoney, J.R.; Holtzer, R.; Izzetoglu, M.; Zemon, V.; Verghese, J.; Allali, G. The role of prefrontal cortex during postural control in Parkinsonian syndromes a functional near-infrared spectroscopy study. Brain Res. 2016, 1633, 126–138. [Google Scholar] [PubMed]
- Maidan, I.; Bernad-Elazari, H.; Gazit, E.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: An fNIRS study of transient motor-cognitive failures. J. Neurol. 2015, 262, 899–908. [Google Scholar]
- Maidan, I.; Nieuwhof, F.; Bernad-Elazari, H.; Reelick, M.F.; Bloem, B.R.; Giladi, N.; Deutsch, J.E.; Hausdorff, J.M.; Claassen, J.A.; Mirelman, A. The Role of the Frontal Lobe in Complex Walking Among Patients with Parkinson’s Disease and Healthy Older Adults: An fNIRS Study. Neurorehabilit. Neural Repair. 2016, 30, 963–971. [Google Scholar]
- Stuart, S.; Mancini, M. Prefrontal Cortical Activation with Open and Closed-Loop Tactile Cueing When Walking and Turning in Parkinson Disease: A Pilot Study. J. Neurol. Phys. Ther. 2020, 44, 121–131. [Google Scholar]
- Maidan, I.; Nieuwhof, F.; Bernad-Elazari, H.; Bloem, B.R.; Giladi, N.; Hausdorff, J.M.; Claassen, J.A.H.R.; Mirelman, A. Evidence for Differential Effects of 2 Forms of Exercise on Prefrontal Plasticity During Walking in Parkinson’s Disease. Neurorehabilit. Neural Repair. 2018, 32, 200–208. [Google Scholar]
- Thumm, P.C.; Maidan, I.; Brozgol, M.; Shustak, S.; Gazit, E.; Shema Shiratzki, S.; Bernad-Elazari, H.; Beck, Y.; Giladi, N.; Hausdorff, J.M.; et al. Treadmill walking reduces pre-frontal activation in patients with Parkinson’s disease. Gait Posture 2018, 62, 384–387. [Google Scholar]
- Sakatani, K.; Katayama, Y.; Yamamoto, T.; Suzuki, S. Changes in cerebral blood oxygenation of the frontal lobe induced by direct electrical stimulation of thalamus and globus pallidus: A near infrared spectroscopy study. J. Neurol. Neurosurg. Psychiatry 1999, 67, 769–773. [Google Scholar]
- Murata, Y.; Katayama, Y.; Oshima, H.; Kawamata, T.; Yamamoto, T.; Sakatani, K.; Suzuki, S. Changes in cerebral blood oxygenation induced by deep brain stimulation: Study by near-infrared spectroscopy (NIRS). Keio J. Med. 2000, 49 (Suppl. S1), A61–A63. [Google Scholar] [PubMed]
- De Salvo, S.; Caminiti, F.; Bonanno, L.; De Cola, M.C.; Corallo, F.; Caizzone, A.; Rifici, C.; Bramanti, P.; Marino, S. Neurophysiological assessment for evaluating residual cognition in vegetative and minimally conscious state patients: A pilot study. Funct. Neurol. 2015, 30, 237–244. [Google Scholar] [PubMed]
- Molteni, E.; Arrigoni, F.; Bardoni, A.; Galbiati, S.; Villa, F.; Colombo, K.; Strazzer, S. Bedside assessment of residual functional activation in minimally conscious state using NIRS and general linear models. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 3551–3554. [Google Scholar]
- Kempny, A.M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.; Playford, E.D.; Leff, A.P. Functional near infrared spectroscopy as a probe of brain function in people with prolonged disorders of consciousness. Neuroimage Clin. 2016, 12, 312–319. [Google Scholar] [PubMed]
- Zhang, Y.; Yang, Y.; Si, J.; Xia, X.; He, J.; Jiang, T. Influence of inter-stimulus interval of spinal cord stimulation in patients with disorders of consciousness: A preliminary functional near-infrared spectroscopy study. Neuroimage Clin. 2017, 17, 1–9. [Google Scholar]
- Zhang, Z.; Qi, M.; Hügli, G.; Khatami, R. Predictors of changes in cerebral perfusion and oxygenation during obstructive sleep apnea. Sci. Rep. 2021, 11, 23510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pizza, F.; Biallas, M.; Wolf, M.; Werth, E.; Bassetti, C.L. Nocturnal cerebral hemodynamics in snorers and in patients with obstructive sleep apnea: A near-infrared spectroscopy study. Sleep 2010, 33, 205–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, A.; Yokoyama, N.; Yonetani, M.; Uetani, Y.; Nakamura, H.; Nakao, H. Evaluation of change of cerebral circulation by SpO2 in preterm infants with apneic episodes using near infrared spectroscopy. Pediatr. Int. 2003, 45, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Michael, F. Functional Near-Infrared Spectroscopy: Potential and Limitations in Neuroimaging Studies. Int. Rev. Neurobiol. 2005, 66, 237–266. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gjonaj, E.; Formica, C.; Cartella, E.; Muscarà, N.; Marino, S.; Quartarone, A.; De Salvo, S. The Role of Near-Infrared Spectroscopy (NIRS) in Neurological and Neurodegenerative Diseases as Support to Clinical Practice: An Overview of the Literature. Diagnostics 2025, 15, 869. https://doi.org/10.3390/diagnostics15070869
Gjonaj E, Formica C, Cartella E, Muscarà N, Marino S, Quartarone A, De Salvo S. The Role of Near-Infrared Spectroscopy (NIRS) in Neurological and Neurodegenerative Diseases as Support to Clinical Practice: An Overview of the Literature. Diagnostics. 2025; 15(7):869. https://doi.org/10.3390/diagnostics15070869
Chicago/Turabian StyleGjonaj, Elvira, Caterina Formica, Emanuele Cartella, Nunzio Muscarà, Silvia Marino, Angelo Quartarone, and Simona De Salvo. 2025. "The Role of Near-Infrared Spectroscopy (NIRS) in Neurological and Neurodegenerative Diseases as Support to Clinical Practice: An Overview of the Literature" Diagnostics 15, no. 7: 869. https://doi.org/10.3390/diagnostics15070869
APA StyleGjonaj, E., Formica, C., Cartella, E., Muscarà, N., Marino, S., Quartarone, A., & De Salvo, S. (2025). The Role of Near-Infrared Spectroscopy (NIRS) in Neurological and Neurodegenerative Diseases as Support to Clinical Practice: An Overview of the Literature. Diagnostics, 15(7), 869. https://doi.org/10.3390/diagnostics15070869





