Early Alterations in De Novo Parkinson’s Disease Revealed by Diffusion Tensor Imaging: Preliminary Study
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. MRI Images Acquisition
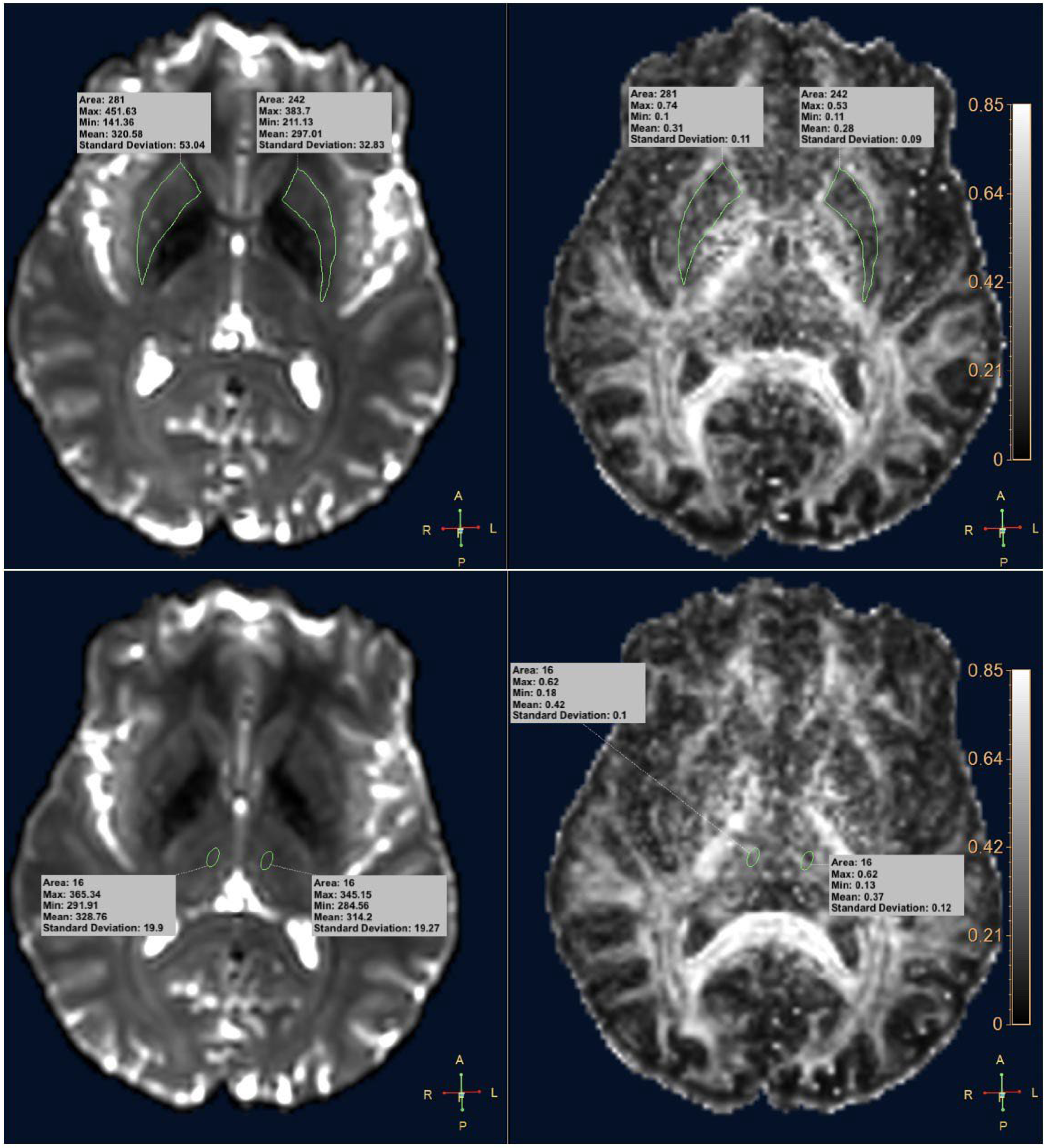
2.3. DTI Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Fereshtehnejad, S.; Berg, D.; Bohnen, N.I.; Dujardin, K.; Erro, R.; Espay, A.J.; Halliday, G.; Van Hilten, J.J.; Hu, M.T.; et al. Transitioning from Subtyping to Precision Medicine in Parkinson’s Disease: A Purpose-Driven Approach. Mov. Disord. 2024, 39, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Maftei, D.; Passali, F.M.; Grillo, P.; Zenuni, H.; Mascioli, D.; Maurizi, R.; Loccisano, L.; Vincenzi, M.; Rinaldi, A.M.; et al. Olfactory Neuron Prokineticin-2 as a Potential Target in Parkinson’s Disease. Ann. Neurol. 2023, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Tagliaferro, P.; Burke, R.E. Retrograde Axonal Degeneration in Parkinson Disease. J. Parkinsons Dis. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Nigro, S.; Riccelli, R.; Passamonti, L.; Arabia, G.; Morelli, M.; Nisticò, R.; Novellino, F.; Salsone, M.; Barbagallo, G.; Quattrone, A. Characterizing structural neural networks in de novo Parkinson disease patients using diffusion tensor imaging. Hum. Brain Mapp. 2016, 37, 4500–4510. [Google Scholar] [CrossRef]
- Rektor, I.; Svátková, A.; Vojtíšek, L.; Zikmundová, I.; Vaníček, J.; Király, A.; Szabó, N. White matter alterations in Parkinson’s disease with normal cognition precede grey matter atrophy. PLoS ONE 2018, 13, e0187939. [Google Scholar] [CrossRef]
- Park, C.-H.; Shin, N.-Y.; Yoo, S.-W.; Seo, H.; Yoon, U.; Yoo, J.-Y.; Ahn, K.; Kim, J.-S. Simulating the progression of brain structural alterations in Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 86. [Google Scholar] [CrossRef]
- Marino, S.; Ciurleo, R.; Di Lorenzo, G.; Barresi, M.; De Salvo, S.; Giacoppo, S.; Bramanti, A.; Lanzafame, P.; Bramanti, P. Magnetic resonance imaging markers for early diagnosis of Parkinson’s disease. Neural Regen. Res. 2012, 7, 611–619. [Google Scholar] [CrossRef]
- Modrego, P.J.; Fayed, N.; Artal, J.; Olmos, S. Correlation of Findings in Advanced MRI Techniques with Global Severity Scales in Patients with Parkinson Disease. Acad. Radiol. 2011, 18, 235–241. [Google Scholar] [CrossRef]
- Basser, P.J.; Mattiello, J.; Lebihan, D. Estimation of the Effective Self-Diffusion Tensor from the NMR Spin Echo. J. Magn. Reson. B 1994, 103, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Burock, M.A. Diffusion Tensor Imaging in Parkinson’s Disease and Parkinsonian Syndrome: A Systematic Review. Front. Neurol. 2020, 11, 531993. [Google Scholar] [CrossRef]
- Grillo, P.; Sancesario, G.M.; Mascioli, D.; Geusa, L.; Zenuni, H.; Giannella, E.; Della Morte, D.; Mercuri, N.B.; Schirinzi, T. Constipation distinguishes different clinical-biochemical patterns in de novo Parkinson’s disease. Park. Relat. Disord. 2022, 102, 64–67. [Google Scholar] [CrossRef]
- Mari, Z.; Mestre, T.A. The Disease Modification Conundrum in Parkinson’s Disease: Failures and Hopes. Front. Aging Neurosci. 2022, 14, 810860. [Google Scholar] [CrossRef]
- Quarantelli, M.; Quattrone, A.; Sarica, A.; Cicone, F.; Cascini, G.L.; Quattrone, A. Functional connectivity of the cortico-subcortical sensorimotor loop is modulated by the severity of nigrostriatal dopaminergic denervation in Parkinson’s Disease. NPJ Parkinsons Dis. 2022, 8, 122. [Google Scholar] [CrossRef]
- Cilia, R.; Cereda, E.; Akpalu, A.; Sarfo, F.S.; Cham, M.; Laryea, R.; Obese, V.; Oppon, K.; Del Sorbo, F.; Bonvegna, S.; et al. Natural history of motor symptoms in Parkinson’s disease and the long-duration response to levodopa. Brain 2020, 143, 2490–2501. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Owens-Walton, C.; Nir, T.M.; Al-Bachari, S.; Ambrogi, S.; Anderson, T.J.; Aventurato, Í.K.; Cendes, F.; Chen, Y.-L.; Ciullo, V.; Cook, P.; et al. A worldwide study of white matter microstructural alterations in people living with Parkinson’s disease. NPJ Parkinsons Dis. 2024, 10, 151. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, I.-W.; Tosun, D.; Foster, E.; Schuff, N. Progression of Regional Microstructural Degeneration in Parkinson’s Disease: A Multicenter Diffusion Tensor Imaging Study. PLoS ONE 2016, 11, e0165540. [Google Scholar] [CrossRef]
- Shih, Y.-C.; Ooi, L.Q.R.; Li, H.-H.; Allen, J.C.; Hartono, S.; Welton, T.; Tan, E.-K.; Chan, L.L. Serial deep gray nuclear DTI changes in Parkinson’s disease over twelve years. Front. Aging Neurosci. 2023, 15, 1169254. [Google Scholar] [CrossRef]
- Mohsen, M.; Mohamed, N.A.E.; Bedir, A.E.-T.M.; Razek, A.A.K.A.; Saied, A.E.A.M. Advanced MRI-based evaluation of gray and white matter changes in Parkinson’s disease. Egypt. J. Radiol. Nucl. Med. 2024, 55, 165. [Google Scholar] [CrossRef]
- Laansma, M.A.; Bright, J.K.; Al-Bachari, S.; Anderson, T.J.; Ard, T.; Assogna, F.; Baquero, K.A.; Berendse, H.W.; Blair, J.; Cendes, F.; et al. International Multicenter Analysis of Brain Structure Across Clinical Stages of Parkinson’s Disease. Mov. Disord. 2021, 36, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Patriat, R.; Niederer, J.; Kaplan, J.; Huffmaster, S.A.; Petrucci, M.; Eberly, L.; Harel, N.; MacKinnon, C. Morphological changes in the subthalamic nucleus of people with mild-to-moderate Parkinson’s disease: A 7T MRI study. Sci. Rep. 2020, 10, 8785. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Skidmore, F.; Liu, Y.; Tian, J.; Li, K. White Matter Microstructure Changes in the Thalamus in Parkinson Disease with Depression: A Diffusion Tensor MR Imaging Study. Am. J. Neuroradiol. 2010, 31, 1861–1866. [Google Scholar] [CrossRef]
- Zhan, W.; Kang, G.A.; Glass, G.A.; Zhang, Y.; Shirley, C.; Millin, R.; Possin, K.L.; Nezamzadeh, M.; Weiner, M.W.; Marks, W.J.; et al. Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Mov. Disord. 2012, 27, 90–97. [Google Scholar] [CrossRef]
- Hemmerle, A.M.; Herman, J.P.; Seroogy, K.B. Stress, depression and Parkinson’s disease. Exp. Neurol. 2012, 233, 79–86. [Google Scholar] [CrossRef]
- de Oliveira, R.V.; Pereira, J.S. Utility of manual fractional anisotropy measurements in the management of patients with Parkinson disease: A feasibility study with a 1.5-T magnetic resonance imaging system. Acta Radiol. Open 2021, 10. [Google Scholar] [CrossRef]
- Bove, F.; Angeloni, B.; Sanginario, P.; Rossini, P.M.; Calabresi, P.; Di Iorio, R. Neuroplasticity in levodopa-induced dyskinesias: An overview on pathophysiology and therapeutic targets. Prog. Neurobiol. 2024, 232, 102548. [Google Scholar] [CrossRef]
- Planetta, P.; Schulze, E.; Geary, E.; Corcos, D.; Goldman, J.; Little, D.; Vaillancourt, D. Thalamic Projection Fiber Integrity in de novo Parkinson Disease. Am. J. Neuroradiol. 2013, 34, 74–79. [Google Scholar] [CrossRef]
- Chan, L.L.; Ng, K.M.; Yeoh, C.S.; Rumpel, H.; Li, H.H.; Tan, E.K. Putaminal Diffusivity Correlates with Disease Progression in Parkinson’s Disease. Medicine 2016, 95, e2594. [Google Scholar] [CrossRef] [PubMed]
- Surova, Y.; Nilsson, M.; Lampinen, B.; Lätt, J.; Hall, S.; Widner, H.; van Westen, D.; Hansson, O. Alteration of putaminal fractional anisotropy in Parkinson’s disease: A longitudinal diffusion kurtosis imaging study. Neuroradiology 2018, 60, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, E.; Agosta, F.; Piramide, N.; Filippi, M. Progression of grey and white matter brain damage in Parkinson’s disease: A critical review of structural MRI literature. J. Neurol. 2021, 268, 3144–3179. [Google Scholar] [CrossRef] [PubMed]

| De Novo PD (n = 31) | Control Subjects (n = 33) | |
|---|---|---|
| Male/Female | 22/9 | 16/17 |
| Age | 60.0 ± 11.4 | 56.6 ± 13.8 |
| BMI | 25.4 ± 3.0 | 24.2 ± 3.0 |
| Disease duration (months) | 18.0 ± 9.9 | - |
| H&Y | 1.8 ± 0.6 | - |
| MDS-UPDRS III | 27.1 ± 11.1 | - |
| MoCA | 25.4 ± 3.4 | - |
| MMSE | 27.9 ± 2.0 | - |
| NMSS | 32.1 ± 26.6 | - |
| Region | De Novo PD Patients | Control Subjects | p | |
|---|---|---|---|---|
| Right Putamen | Max | 0.711 ± 0.079 | 0.710 ± 0.067 | 0.985 |
| Min | 0.117 ± 0.038 | 0.113 ± 0.025 | 0.652 | |
| Mean | 0.366 ± 0.036 | 0.364 ± 0.033 | 0.839 | |
| Std | 0.121 ± 0.014 | 0.126 ± 0.012 | 0.185 | |
| Left Putamen | Max | 0.681 ± 0.084 | 0.689 ± 0.070 | 0.672 |
| Min | 0.115 ± 0.034 | 0.117 ± 0.033 | 0.868 | |
| Mean | 0.360 ± 0.035 | 0.362 ± 0.032 | 0.764 | |
| Std | 0.119 ± 0.013 | 0.011 ± 0.08 | 0.989 | |
| Right Thalamus | Max | 0.541 ± 0.077 | 0.586 ± 0.075 | 0.018 * |
| Min | 0.196 ± 0.061 | 0.213 ± 0.184 | 0.640 | |
| Mean | 0.359 ± 0.052 | 0.364 ± 0.033 | 0.045 * | |
| Std | 0.098 ± 0.024 | 0.114 ± 0.021 | 0.010 * | |
| Left Thalamus | Max | 0.541 ± 0.077 | 0.595 ± 0.085 | 0.012 * |
| Min | 0.207 ± 0.055 | 0.193 ± 0.049 | 0.295 | |
| Mean | 0.366 ± 0.040 | 0.374 ± 0.033 | 0.387 | |
| Std | 0.098 ± 0.024 | 0.114 ± 0.021 | 0.010 * |
| Regions | FA Measures | Clinical Variables | Rho | p Value |
|---|---|---|---|---|
| Left putamen | Mean FA | MMSE | 0.43 | 0.039 |
| Right putamen | Mean FA | H&Y | −0.52 | 0.007 |
| Left putamen | Mean FA | MDS-UPDRS III | −0.42 | 0.038 |
| Right putamen | Std FA | NMSS | −0.41 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giuliano, F.; Pucci, N.; Serio, M.L.; Picchi, E.; Minosse, S.; Ferrazzoli, V.; Da Ros, V.; Schirinzi, T.; Conti, M.; Bovenzi, R.; et al. Early Alterations in De Novo Parkinson’s Disease Revealed by Diffusion Tensor Imaging: Preliminary Study. Diagnostics 2025, 15, 841. https://doi.org/10.3390/diagnostics15070841
Di Giuliano F, Pucci N, Serio ML, Picchi E, Minosse S, Ferrazzoli V, Da Ros V, Schirinzi T, Conti M, Bovenzi R, et al. Early Alterations in De Novo Parkinson’s Disease Revealed by Diffusion Tensor Imaging: Preliminary Study. Diagnostics. 2025; 15(7):841. https://doi.org/10.3390/diagnostics15070841
Chicago/Turabian StyleDi Giuliano, Francesca, Noemi Pucci, Maria Lina Serio, Eliseo Picchi, Silvia Minosse, Valentina Ferrazzoli, Valerio Da Ros, Tommaso Schirinzi, Matteo Conti, Roberta Bovenzi, and et al. 2025. "Early Alterations in De Novo Parkinson’s Disease Revealed by Diffusion Tensor Imaging: Preliminary Study" Diagnostics 15, no. 7: 841. https://doi.org/10.3390/diagnostics15070841
APA StyleDi Giuliano, F., Pucci, N., Serio, M. L., Picchi, E., Minosse, S., Ferrazzoli, V., Da Ros, V., Schirinzi, T., Conti, M., Bovenzi, R., Mascioli, D., & Garaci, F. (2025). Early Alterations in De Novo Parkinson’s Disease Revealed by Diffusion Tensor Imaging: Preliminary Study. Diagnostics, 15(7), 841. https://doi.org/10.3390/diagnostics15070841






