Evolving Transplant Oncology: Evolving Criteria for Better Decision-Making
Abstract
1. Introduction
1.1. Defining Transplant Oncology
1.2. Milan Criteria for Hepatocellular Carcinoma and Other Criteria
1.3. Scope of This Review
2. Methodology
3. From Milan Criteria Towards the Future
3.1. Hepatocellular Carcinoma
| Name | Criteria | Cancer Type | Source |
|---|---|---|---|
| Milan Criteria |
| HCC | [7] |
| UCSF Criteria |
| HCC | [14,15] |
| Kyoto Criteria |
| HCC | [16] |
| Fukuoka Criteria |
| HCC | [25] |
| Up-to-seven Criteria |
| HCC | [17] |
| Spanish Criteria |
| HCC | [18] |
| AFP model or French Criteria |
| HCC | [19] |
| Metroticket 2.0 Model |
| HCC | [20] |
| Toronto expanded Criteria |
| HCC | [21] |
| 5-5-500 |
| HCC | [22] |
3.2. Other Liver-Related Malignancies
| Name | Criteria | Cancer Type | Source |
|---|---|---|---|
| Milan-NET (2007, revised in 2016) |
| NET | [26] |
| Mayo Clinic protocol for hilar cholangiocarcinoma |
| PHC | [27] |
| Selection criteria for CRLM |
| CRLM | [29] |
3.3. Hepatoblastoma
3.4. Recurrence After LT
4. Liquid Biopsy-cfDNA/ctDNA-CTC
Liquid Biopsy-cfDNA-ctDNA-CTC
5. Immunotherapy
6. Other Aspects of Transplant Oncology
6.1. Surgical Techniques
6.2. Perfusion Pumps
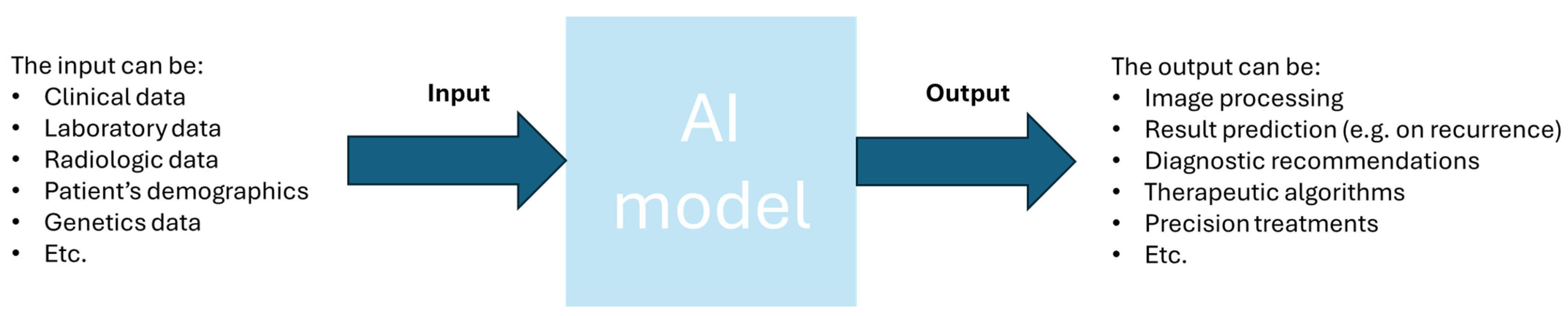
6.3. Artificial Intelligence
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fung, J.; DiSabato, D.; Liao, C.Y.; Ahmed, O.; Pillai, A. Perspective: Advances in Liver Transplantation for Hepatocellular Carcinoma—A Prototype for Transplant Oncology. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, S.; Melandro, F.; Giovanardi, F.; Shah, K.; De Peppo, V.; Mennini, G.; Ghinolfi, D.; Limkemann, A.; Pawlik, T.M.; Lai, Q. New Insights in the Setting of Transplant Oncology. Medicina 2023, 59, 568. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Sandri, G.B.L.; Lerut, J. Selection Tool Alpha-Fetoprotein for Patients Waiting for Liver Transplantation: How to Easily Manage a Fractal Algorithm. World J. Hepatol. 2015, 7, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Itano, O.; Shinoda, M.; Kitagawa, Y. Liver Transplantation for Hepatobiliary Malignancies: A New Era of “Transplant Oncology” Has Begun. Surg. Today 2017, 47, 403–415. [Google Scholar] [CrossRef]
- Hibi, T.; Sapisochin, G. What Is Transplant Oncology? Surgery 2019, 165, 281–285. [Google Scholar] [CrossRef]
- Karageorgos, F.F.; Karakasi, K.-E.; Vasileiadou, S.; Kofinas, A.; Antoniadis, N.; Katsanos, G.; Tsoulfas, G. The Evolving Role of Transplant Oncology. In Interdisciplinary Cancer Research; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Karageorgos, F.F.; Alexiou, M.; Tsoulfas, G.; Alexopoulos, A.H. Hydrogel-Based Vascularized Organ Tissue Engineering: A Systematized Review on Abdominal Organs. Gels 2024, 10, 653. [Google Scholar] [CrossRef]
- Karageorgos, F.F.; Neiros, S.; Karakasi, K.-E.; Vasileiadou, S.; Katsanos, G.; Antoniadis, N.; Tsoulfas, G. Artificial Kidney: Challenges and Opportunities. World J. Transpl. 2024, 14, 89025. [Google Scholar] [CrossRef]
- Chen, V.L.; Xu, D.; Wicha, M.S.; Lok, A.S.; Parikh, N.D. Utility of Liquid Biopsy Analysis in Detection of Hepatocellular Carcinoma, Determination of Prognosis, and Disease Monitoring: A Systematic Review. Clin. Gastroenterol. Hepatol. 2020, 18, 2879–2902.e9. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular Carcinoma (HCC): Epidemiology, Etiology and Molecular Classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar]
- Ye, Q.; Ling, S.; Zheng, S.; Xu, X. Liquid Biopsy in Hepatocellular Carcinoma: Circulating Tumor Cells and Circulating Tumor DNA. Mol. Cancer 2019, 18, 114. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Harricharran, T.; Huaman, J.; Galuza, A.; Odumuwagun, O.; Tan, Y.; Ma, G.X.; Nguyen, M.T. Mechanisms of Hepatocellular Carcinoma Progression. World J. Gastroenterol. 2019, 25, 2279–2293. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Expansion of the Tumor Size Limits Does Not Adversely Impact Survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Bacchetti, P.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Comparison of the Proposed UCSF Criteria with the Milan Criteria and the Pittsburgh Modified TNM Criteria. Liver Transpl. 2002, 8, 765–774. [Google Scholar] [CrossRef]
- Takada, Y.; Ito, T.; Ueda, M.; Sakamoto, S.; Haga, H.; Maetani, Y.; Ogawa, K.; Ogura, Y.; Oike, F.; Egawa, H.; et al. Living Donor Liver Transplantation for Patients with HCC Exceeding the Milan Criteria: A Proposal of Expanded Criteria. Dig. Dis. 2007, 25, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting Survival after Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Silva, M.; Moya, A.; Berenguer, M.; Sanjuan, F.; López-Andujar, R.; Pareja, E.; Torres-Quevedo, R.; Aguilera, V.; Montalva, E.; De Juan, M.; et al. Expanded Criteria for Liver Transplantation in Patients with Cirrhosis and Hepatocellular Carcinoma. Liver Transplant. 2008, 14, 1449–1460. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot–Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- Sapisochin, G.; Goldaracena, N.; Laurence, J.M.; Dib, M.; Barbas, A.; Ghanekar, A.; Cleary, S.P.; Lilly, L.; Cattral, M.S.; Marquez, M.; et al. The Extended Toronto Criteria for Liver Transplantation in Patients with Hepatocellular Carcinoma: A Prospective Validation Study. Hepatology 2016, 64, 2077–2088. [Google Scholar] [CrossRef]
- Shimamura, T.; Akamatsu, N.; Fujiyoshi, M.; Kawaguchi, A.; Morita, S.; Kawasaki, S.; Uemoto, S.; Kokudo, N.; Hasegawa, K.; Ohdan, H.; et al. Expanded Living-donor Liver Transplantation Criteria for Patients with Hepatocellular Carcinoma Based on the Japanese Nationwide Survey: The 5-5-500 Rule—A Retrospective Study. Transpl. Int. 2019, 32, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Moeckli, B.; Ivanics, T.; Claasen, M.; Toso, C.; Sapisochin, G. Recent Developments and Ongoing Trials in Transplant Oncology. Liver Int. 2020, 40, 2326–2344. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.P.; Venook, A.; Kerlan, R.; Yao, F. Hepatocellular Carcinoma: Ablate and Wait versus Rapid Transplantation. Liver Transplant. 2010, 16, 925–929. [Google Scholar] [CrossRef]
- Soejima, Y.; Taketomi, A.; Yoshizumi, T.; Uchiyama, H.; Aishima, S.; Terashi, T.; Shimada, M.; Maehara, Y. Extended Indication for Living Donor Liver Transplantation in Patients with Hepatocellular Carcinoma. Transplantation 2007, 83, 893–899. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Coppa, J.; Miceli, R.; Bhoori, S.; Bongini, M.; Camerini, T.; Milione, M.; Regalia, E.; Spreafico, C.; et al. The Long-Term Benefit of Liver Transplantation for Hepatic Metastases from Neuroendocrine Tumors. Am. J. Transplant. 2016, 16, 2892–2902. [Google Scholar] [CrossRef]
- Darwish Murad, S.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of Neoadjuvant Chemoradiation, Followed by Liver Transplantation, for Perihilar Cholangiocarcinoma at 12 US Centers. Gastroenterology 2012, 143, 88–98.e3. [Google Scholar] [CrossRef]
- Krendl, F.J.; Bellotti, R.; Sapisochin, G.; Schaefer, B.; Tilg, H.; Scheidl, S.; Margreiter, C.; Schneeberger, S.; Oberhuber, R.; Maglione, M. Transplant Oncology—Current Indications and Strategies to Advance the Field. JHEP Rep. 2024, 6, 100965. [Google Scholar] [CrossRef]
- Puia-Negulescu, S.; Lebossé, F.; Mabrut, J.-Y.; Muller, X.; Rossignol, G.; Antonini, T.; Erard, D.; Radenne, S.; Guillet, M.; Souquet, J.-C.; et al. Liver Transplantation for Colorectal Liver Metastases: Current Management and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3093. [Google Scholar] [CrossRef]
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal Liver Metastases: Current Management and Future Perspectives. World J. Clin. Oncol. 2020, 11, 761–808. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Robinson, T.J.; Cummins, K.; Tsung, A. Liver Transplantation for Unresectable Colorectal Liver Metastasis: Perspective and Review of Current Literature. Curr. Oncol. 2024, 31, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Piedvache, C.; Chiche, L.; Adam, J.P.; Salamé, E.; Bucur, P.; Cherqui, D.; Scatton, O.; Granger, V.; Ducreux, M.; et al. Liver Transplantation plus Chemotherapy versus Chemotherapy Alone in Patients with Permanently Unresectable Colorectal Liver Metastases (TransMet): Results from a Multicentre, Open-Label, Prospective, Randomised Controlled Trial. Lancet 2024, 404, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Ruffolo, L.I.; Kim, M.H.; Fujiki, M.; Hashimoto, K.; Imaoka, Y.; Melcher, M.L.; Aucejo, F.N.; Tomiyama, K.; Hernandez-Alejandro, R. The Current State of Liver Transplantation for Colorectal Liver Metastases in the United States: A Call for Standardized Reporting. Ann. Surg. Oncol. 2023, 30, 2769–2777. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Rela, M.; Eason, J.D.; Line, P.-D.; Fung, J.; Sakamoto, S.; Selzner, N.; Man, K.; Ghobrial, R.M.; Sapisochin, G. Liver Transplantation for Colorectal and Neuroendocrine Liver Metastases and Hepatoblastoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1131–1135. [Google Scholar] [CrossRef]
- Ilmer, M.; Guba, M. Liver Transplant Oncology: Towards Dynamic Tumor-Biology-Oriented Patient Selection. Cancers 2022, 14, 2662. [Google Scholar] [CrossRef]
- Straś, W.A.; Wasiak, D.; Łągiewska, B.; Tronina, O.; Hreńczuk, M.; Gotlib, J.; Lisik, W.; Małkowski, P. Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Risk Factors and Predictive Models. Ann. Transplant. 2022, 27. [Google Scholar] [CrossRef]
- Hahn, M.; Herber, A.; Berg, T.; Seehofer, D.; van Bömmel, F. Enhancing HCC Recurrence Prediction after Liver Transplantation: From DCP plus AFP-L3 Model to GALAD Score. J. Hepatol. 2024, 80, e271–e272. [Google Scholar] [CrossRef]
- Halazun, K.J.; Rosenblatt, R.E.; Mehta, N.; Lai, Q.; Hajifathalian, K.; Gorgen, A.; Brar, G.; Sasaki, K.; Doyle, M.B.M.; Tabrizian, P.; et al. Dynamic α-Fetoprotein Response and Outcomes After Liver Transplant for Hepatocellular Carcinoma. JAMA Surg. 2021, 156, 559. [Google Scholar] [CrossRef]
- Choi, J.; Bano, A.; Azzi, J. Biomarkers in Solid Organ Transplantation. Clin. Lab. Med. 2019, 39, 73–85. [Google Scholar] [CrossRef]
- Berenguer, M.; de Martin, E.; Hessheimer, A.J.; Levitsky, J.; Maluf, D.G.; Mas, V.R.; Selzner, N.; Hernàndez-Èvole, H.; Lutu, A.; Wahid, N.; et al. European Society for Organ Transplantation Consensus Statement on Biomarkers in Liver Transplantation. Transpl. Int. 2023, 36, 11358. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-Free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.; Esmail, A.; Chang, J.C.; Ghobrial, R.M.; Abdelrahim, M. Utility of Cell-Free DNA Detection in Transplant Oncology. Cancers 2022, 14, 743. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef]
- Dao, J.; Conway, P.J.; Subramani, B.; Meyyappan, D.; Russell, S.; Mahadevan, D. Using CfDNA and CtDNA as Oncologic Markers: A Path to Clinical Validation. Int. J. Mol. Sci. 2023, 24, 13219. [Google Scholar] [CrossRef]
- Xu, S.-J.; Wei, Q.; Hu, X.; Li, C.-B.; Yang, Z.; Zheng, S.-S.; Xu, X. No-Touch Recipient Hepatectomy in Liver Transplantation for Liver Malignancies: A State-of-the-Art Review. Hepatobiliary Pancreat. Dis. Int. 2025, 24, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Amado, V.; González-Rubio, S.; Zamora, J.; Alejandre, R.; Espejo-Cruz, M.L.; Linares, C.; Sánchez-Frías, M.; García-Jurado, G.; Montero, J.L.; Ciria, R.; et al. Clearance of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Undergoing Surgical Resection or Liver Transplantation. Cancers 2021, 13, 2476. [Google Scholar] [CrossRef]
- Xie, Y.-L.; Yang, Z.; Feng, X.; Yang, Q.; Ye, L.-S.; Li, X.-B.; Tang, H.; Zhang, Y.-C.; Liu, W.; Zhang, T.; et al. Association of Phenotypic Transformation of Circulating Tumor Cells and Early Recurrence in Patients with Hepatocellular Carcinoma Following Liver Transplantation. Asian J. Surg. 2022, 45, 435–440. [Google Scholar] [CrossRef]
- Hwang, H.S.; Yoo, J.E.; Han, D.H.; Choi, J.S.; Lee, J.G.; Joo, D.J.; Kim, M.S.; Kim, S.I.; Choi, G.H.; Park, Y.N. Circulating Cancer Stem Cells Expressing EpCAM/CD90 in Hepatocellular Carcinoma: A Pilot Study for Predicting Tumor Recurrence after Living Donor Liver Transplantation. Gut Liver 2022, 16, 443–455. [Google Scholar] [CrossRef]
- Abboud, K.; Umoru, G.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Al-Shamsi, H.O.; Javle, M.; Saharia, A.; Connor, A.A.; Kodali, S.; et al. Immune Checkpoint Inhibitors for Solid Tumors in the Adjuvant Setting: Current Progress, Future Directions, and Role in Transplant Oncology. Cancers 2023, 15, 1433. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; Abudayyeh, A.; Abdel-Wahab, N.; Diab, A.; Murakami, N.; Kaseb, A.O.; Chang, J.C.; Gaber, A.O.; et al. Utilization of Immunotherapy for the Treatment of Hepatocellular Carcinoma in the Peri-Transplant Setting: Transplant Oncology View. Cancers 2022, 14, 1760. [Google Scholar] [CrossRef] [PubMed]
- Sankar, K.; Gong, J.; Osipov, A.; Miles, S.A.; Kosari, K.; Nissen, N.N.; Hendifar, A.E.; Koltsova, E.K.; Yang, J.D. Recent Advances in the Management of Hepatocellular Carcinoma. Clin. Mol. Hepatol. 2023, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; He, A.R.; Khushman, M.; Rayyan, Y. Advances in Immunotherapy for Transplant Oncology. Cancers 2024, 16, 2369. [Google Scholar] [CrossRef]
- Alghamdi, S.; Al-Hamoudi, W. Hepatocellular Carcinoma: The Role of Immunotherapy and Transplantation in the Era of Transplant Oncology. Cancers 2023, 15, 5115. [Google Scholar] [CrossRef]
- Pettas, T.; Lachanoudi, S.; Karageorgos, F.F.; Ziogas, I.A.; Fylaktou, A.; Papalois, V.; Katsanos, G.; Antoniadis, N.; Tsoulfas, G. Immunotherapy and Liver Transplantation for Hepatocellular Carcinoma: Current and Future Challenges. World J. Transplant. 2025, 15, 100256. [Google Scholar] [CrossRef]
- Xiang, Z.; He, C.; Lu, D.; Zheng, S.; Xu, X. Editorial: Liver Transplantation for Liver Cancer in the Era of Transplant Oncology: Accurate Diagnosis and Treatment. Front. Immunol. 2023, 14, 1276566. [Google Scholar] [CrossRef]
- Line, P.-D.; Hagness, M.; Berstad, A.E.; Foss, A.; Dueland, S. A Novel Concept for Partial Liver Transplantation in Nonresectable Colorectal Liver Metastases. Ann. Surg. 2015, 262, e5–e9. [Google Scholar] [CrossRef]
- Königsrainer, A.; Templin, S.; Capobianco, I.; Königsrainer, I.; Bitzer, M.; Zender, L.; Sipos, B.; Kanz, L.; Wagner, S.; Nadalin, S. Paradigm Shift in the Management of Irresectable Colorectal Liver Metastases. Ann. Surg. 2019, 270, 327–332. [Google Scholar] [CrossRef]
- Soubrane, O.; Scatton, O. The Development of Transplant Oncology May Worsen the Liver Gap and Needs New Technical Options in Liver Transplantation. Ann. Surg. 2023, 279, 226–227. [Google Scholar] [CrossRef]
- Lin, X.; Xiao, M.; Gu, Y.-J.; Zhu, H.-K.; Li, M.-X.; Zhuang, L.; Zheng, S.-S.; Li, Q.-Y. The “No-Touch” Technique Improves the Survival of Patients with Advanced Hepatocellular Carcinomas Treated by Liver Transplantation: A Single-Center Prospective Randomized Controlled Trial. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 253–262. [Google Scholar] [CrossRef]
- Moon, D.-B.; Lee, S.-G.; Hwang, S.; Kim, K.-H.; Ahn, C.-S.; Ha, T.-Y.; Song, G.-W.; Jung, D.-H.; Park, G.-C.; Namkoong, J.-M.; et al. No-Touch En Bloc Right Lobe Living-Donor Liver Transplantation with Inferior Vena Cava Replacement for Hepatocellular Carcinoma Close to Retrohepatic Inferior Vena Cava: Case Report. Transpl. Proc. 2013, 45, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Finotti, M.; Romano, M.; Grossi, U.; Dalla Bona, E.; Pelizzo, P.; Piccino, M.; Scopelliti, M.; Zanatta, P.; Zanus, G. Innovations in Liver Preservation Techniques for Transplants from Donors after Circulatory Death: A Special Focus on Transplant Oncology. J. Clin. Med. 2024, 13, 5371. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadou, S.; Antoniadis, N.; Kofinas, A.; Karakasi, K.-E.; Katsanos, G.; Tsoulfas, G. Machine Perfusion Strategies in Liver and Renal Transplantation. In Current Challenges and Advances in Organ Donation and Transplantation; IntechOpen: London, UK, 2023. [Google Scholar]
- Briceño, J. Artificial Intelligence and Organ Transplantation: Challenges and Expectations. Curr. Opin. Organ Transplant. 2020, 25, 393–398. [Google Scholar] [CrossRef]
- Ivanics, T.; Patel, M.S.; Erdman, L.; Sapisochin, G. Artificial Intelligence in Transplantation (Machine-Learning Classifiers and Transplant Oncology). Curr. Opin. Organ Transplant. 2020, 25, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.; Moeckli, B.; Delaune, V.; Oldani, G.; Andres, A.; Compagnon, P. Artificial Intelligence: Present and Future Potential for Solid Organ Transplantation. Transpl. Int. 2022, 35, 10640. [Google Scholar] [CrossRef]
- Nam, J.Y.; Lee, J.-H.; Bae, J.; Chang, Y.; Cho, Y.; Sinn, D.H.; Kim, B.H.; Kim, S.H.; Yi, N.-J.; Lee, K.-W.; et al. Novel Model to Predict HCC Recurrence after Liver Transplantation Obtained Using Deep Learning: A Multicenter Study. Cancers 2020, 12, 2791. [Google Scholar] [CrossRef]
- Lai, Q.; De Stefano, C.; Emond, J.; Bhangui, P.; Ikegami, T.; Schaefer, B.; Hoppe-Lotichius, M.; Mrzljak, A.; Ito, T.; Vivarelli, M.; et al. Development and Validation of an Artificial Intelligence Model for Predicting Post-transplant Hepatocellular Cancer Recurrence. Cancer Commun. 2023, 43, 1381–1385. [Google Scholar] [CrossRef]
- Kamaleswaran, R.; Sataphaty, S.K.; Mas, V.R.; Eason, J.D.; Maluf, D.G. Artificial Intelligence May Predict Early Sepsis After Liver Transplantation. Front. Physiol. 2021, 12, 692667. [Google Scholar] [CrossRef]
- Ershoff, B.D.; Lee, C.K.; Wray, C.L.; Agopian, V.G.; Urban, G.; Baldi, P.; Cannesson, M. Training and Validation of Deep Neural Networks for the Prediction of 90-Day Post-Liver Transplant Mortality Using UNOS Registry Data. Transplant. Proc. 2020, 52, 246–258. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karageorgos, F.F.; Karakasi, K.-E.; Kofinas, A.; Antoniadis, N.; Katsanos, G.; Tsoulfas, G. Evolving Transplant Oncology: Evolving Criteria for Better Decision-Making. Diagnostics 2025, 15, 820. https://doi.org/10.3390/diagnostics15070820
Karageorgos FF, Karakasi K-E, Kofinas A, Antoniadis N, Katsanos G, Tsoulfas G. Evolving Transplant Oncology: Evolving Criteria for Better Decision-Making. Diagnostics. 2025; 15(7):820. https://doi.org/10.3390/diagnostics15070820
Chicago/Turabian StyleKarageorgos, Filippos F., Konstantina-Eleni Karakasi, Athanasios Kofinas, Nikolaos Antoniadis, Georgios Katsanos, and Georgios Tsoulfas. 2025. "Evolving Transplant Oncology: Evolving Criteria for Better Decision-Making" Diagnostics 15, no. 7: 820. https://doi.org/10.3390/diagnostics15070820
APA StyleKarageorgos, F. F., Karakasi, K.-E., Kofinas, A., Antoniadis, N., Katsanos, G., & Tsoulfas, G. (2025). Evolving Transplant Oncology: Evolving Criteria for Better Decision-Making. Diagnostics, 15(7), 820. https://doi.org/10.3390/diagnostics15070820









