Orbital Neurolymphomatosis in Patient with CNS Lymphoma
Abstract
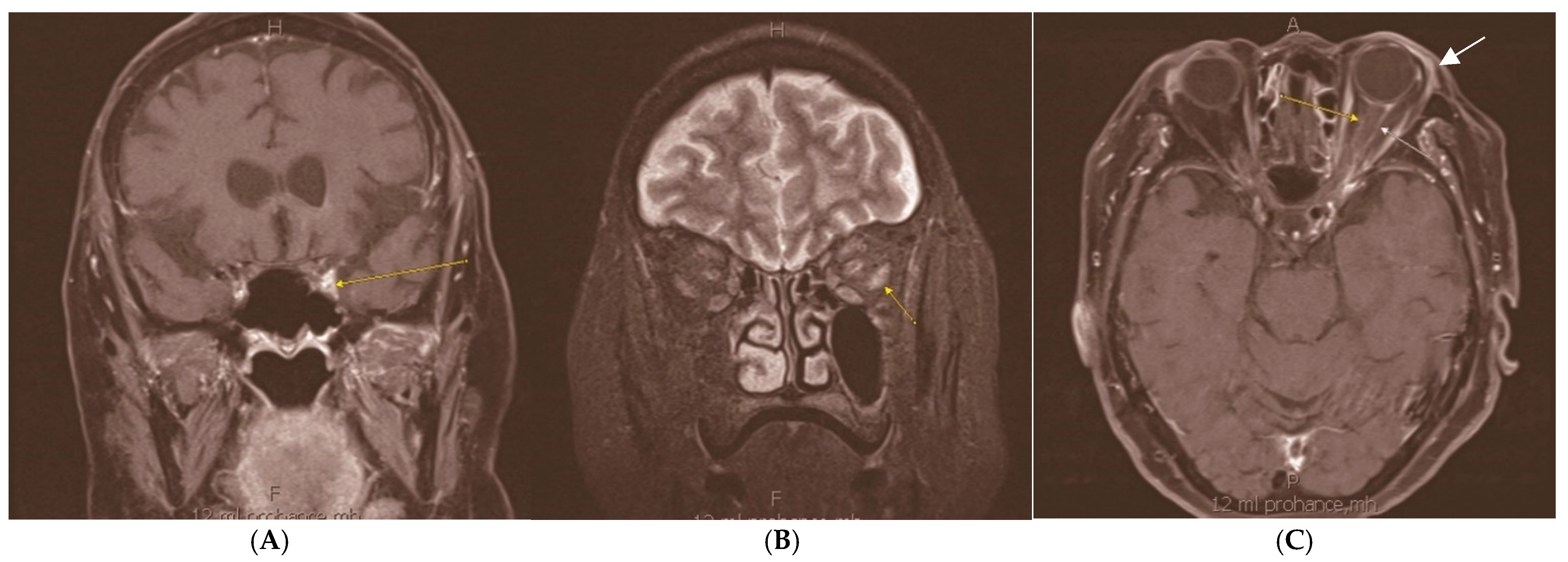
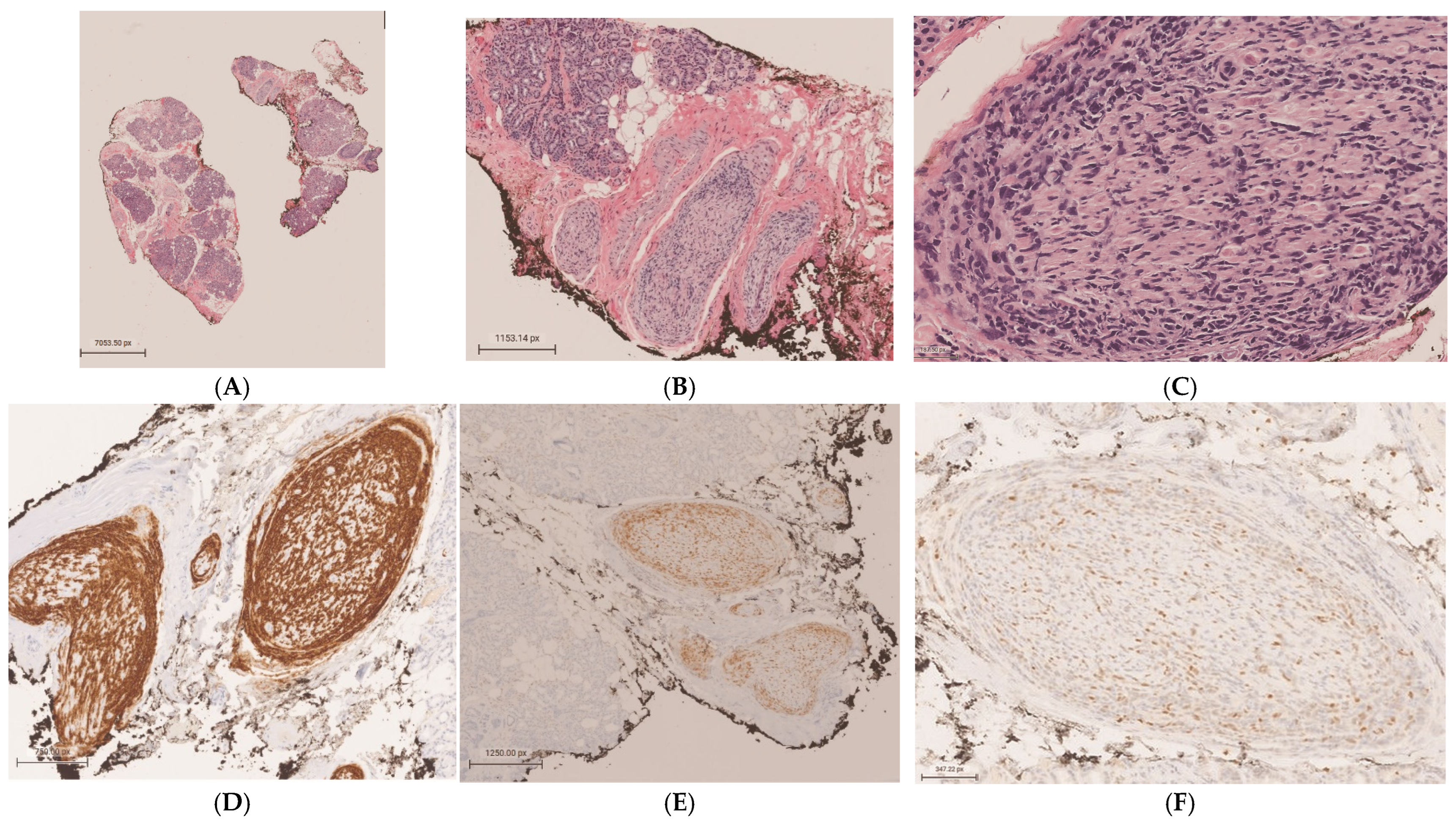
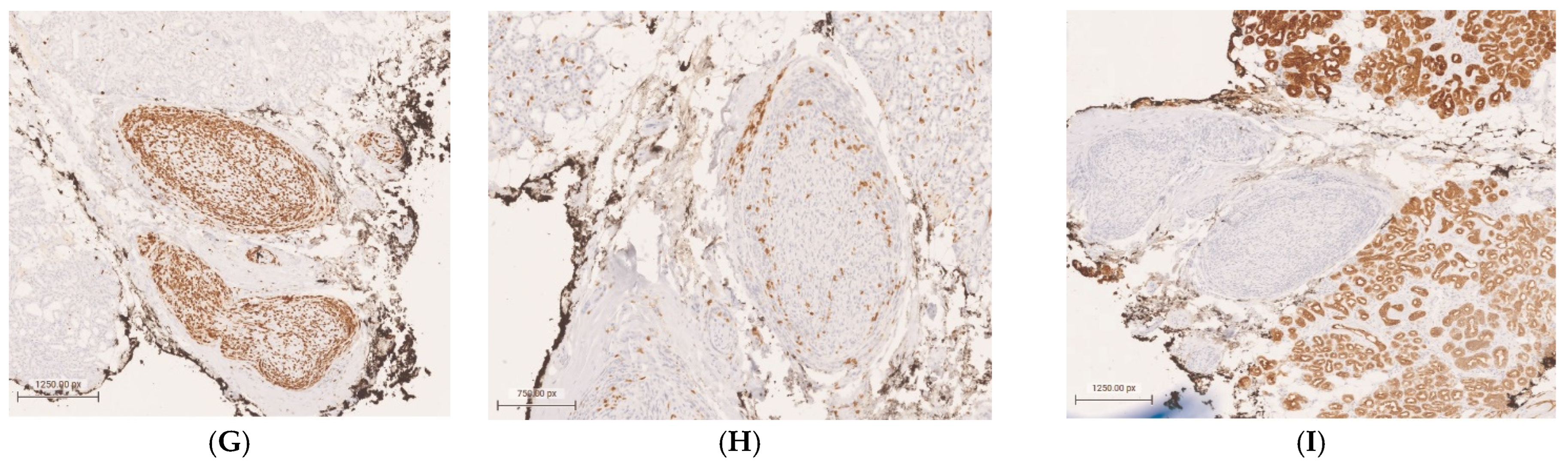
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NL | Neurolymphomatosis |
| CN | Cranial nerve |
| CNS | Central nervous system |
| DLBCL | Diffuse large B-cell lymphoma |
| MRI | Magnetic resonance imaging |
| CSF | Cerebrospinal fluid |
| FCM | Flow cytometry |
| CLIA 88 | Clinical Laboratory Improvement Amendment of 1988 |
| CD20 | Cluster of differentiation 20 |
| PAX-5 | Paired box 5 |
| c-Myc | Cellular myelocytomatosis oncogene |
| CD3 | Cluster of differentiation 3 |
| AE1/AE3 | Anti-cytokeratin cocktail |
| PET-CT | Positron emission tomography–computer tomography |
References
- Avila, J.D.; Vivar, C. Neurolymphomatosis: A review of 82 cases. In Neuromuscular and Clinical Neurophysiology (EMG): Peripheral Neuropathy II; Wiley: Hoboken, NJ, USA, 2017; Volume 88. [Google Scholar]
- Grisariu, S.; Avni, B.; Batchelor, T.T.; van den Bent, M.J.; Bokstein, F.; Schiff, D.; Kuittinen, O.; Chamberlain, M.C.; Roth, P.; Nemets, A.; et al. Neurolymphomatosis: An International Primary CNS Lymphoma Collaborative Group Report. Blood 2010, 115, 5005–5011. [Google Scholar] [PubMed]
- Liu, K.C.; Hennessey, M.A.; McCall, C.M.; Proia, P.D. Ocular involvement in neurolymphomatosis. Am. J. Ophthalmol. Case Rep. 2018, 10, 148–151. [Google Scholar] [PubMed]
- Fritzhand, S.J.; Esmaeli, B.; Sun, J.; Debnam, J.M. Primary disease sites and patterns of spread of neurolymphomatosis in the orbit associated with lymphoma. Cancer Imaging 2021, 21, 39. [Google Scholar] [PubMed]
- Kim, J.L.; Mendoza, P.R.; Rashid, A.; Hayek, B.; Grossniklaus, H.E. Optic nerve lymphoma: Report of two cases and review of the literature. Surv. Ophthalmol. 2015, 60, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Novo, M.; Nowakowski, G.S.; Ristow, K.M.; Spinner, R.J.; Hunt, C.H.; King, R.L.; Lachance, D.H.; Habermann, T.M.; Micallef, I.N.; et al. Clinical manifestations of, diagnostic approach to, and treatment of neurolymphomatosis in the rituximab era. Blood Adv. 2021, 5, 1379–1387. [Google Scholar] [PubMed]
- Gan, H.K.; Azad, A.; Cher, L.; Mitchell, P.L. Neurolymphomatosis: Diagnosis, management, and outcomes in patients treated with rituximab. Neuro Oncol. 2009, 12, 212–215. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shooshani, T.; Han, M.; Tao, J.P.; Spiegel, S.J.; Estopinal, M.D.V. Orbital Neurolymphomatosis in Patient with CNS Lymphoma. Diagnostics 2025, 15, 780. https://doi.org/10.3390/diagnostics15060780
Shooshani T, Han M, Tao JP, Spiegel SJ, Estopinal MDV. Orbital Neurolymphomatosis in Patient with CNS Lymphoma. Diagnostics. 2025; 15(6):780. https://doi.org/10.3390/diagnostics15060780
Chicago/Turabian StyleShooshani, Tara, Michael Han, Jeremiah P. Tao, Samuel J. Spiegel, and Maria Del Valle Estopinal. 2025. "Orbital Neurolymphomatosis in Patient with CNS Lymphoma" Diagnostics 15, no. 6: 780. https://doi.org/10.3390/diagnostics15060780
APA StyleShooshani, T., Han, M., Tao, J. P., Spiegel, S. J., & Estopinal, M. D. V. (2025). Orbital Neurolymphomatosis in Patient with CNS Lymphoma. Diagnostics, 15(6), 780. https://doi.org/10.3390/diagnostics15060780




