Pathogen Detection and Diagnostic Scenarios in Chronic Prostatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Clinical Assessments
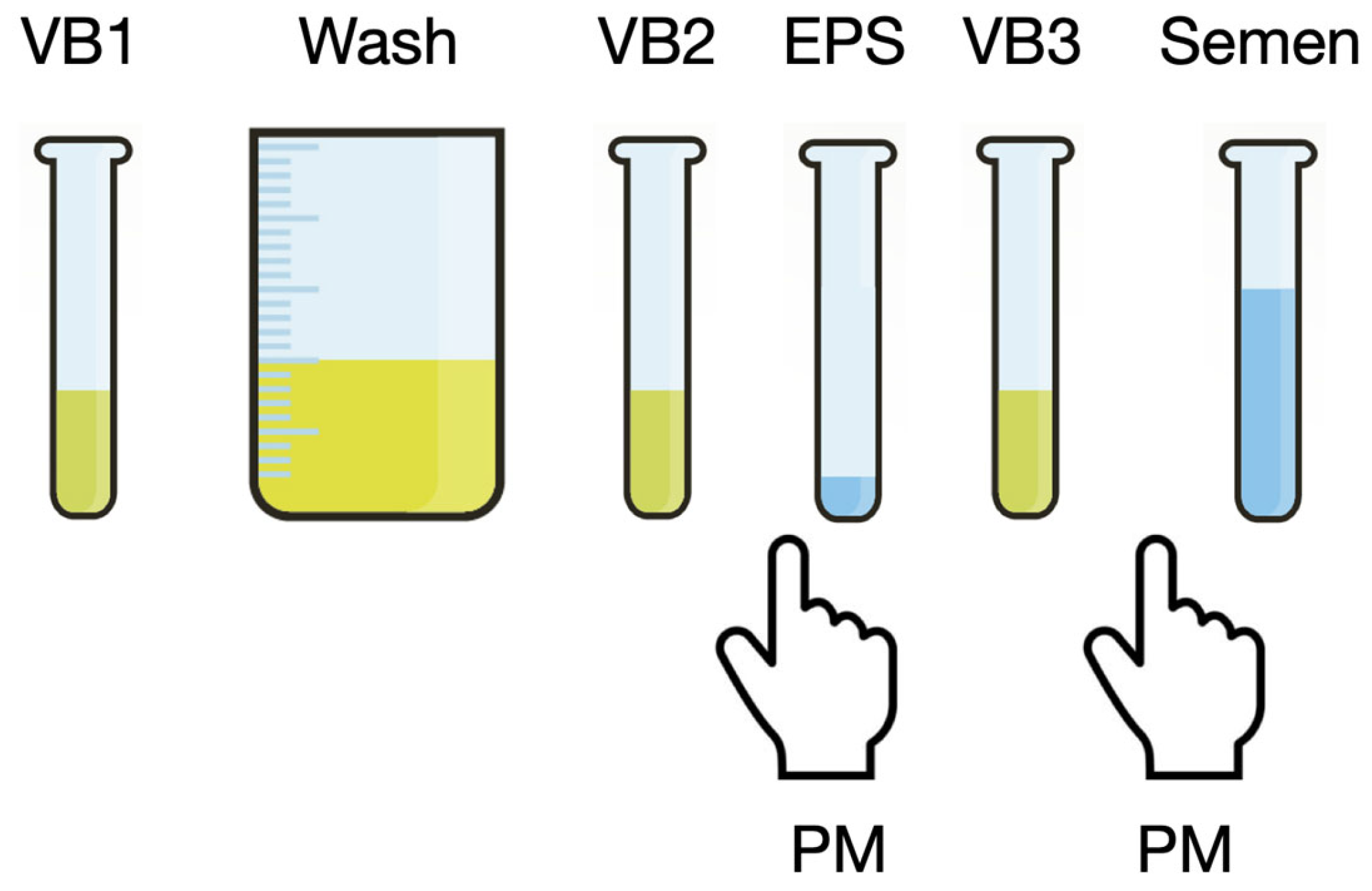
2.3. Microbiological Assessments
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical Assessments
3.2. General Microbiological Assessments
3.3. Traditional Uropathogens
3.3.1. Enterobacteriaceae
3.3.2. Enterococci
3.4. Sexually Transmitted Pathogens
3.5. Other Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | Chronic prostatitis |
| CP/CPPS | Chronic prostatitis/chronic pelvic pain syndrome |
| NIH-CPSI | NIH-Chronic Prostatitis Symptom Index |
| PPMT | Pre- and post-massage test |
| IPSS | International Prostate Symptom Score |
| PSA | Prostate-specific antigen |
| EPS | Expressed prostatic secretion |
References
- Propert, K.J.; Litwin, M.S.; Wang, Y.; Alexander, R.B.; Calhoun, E.; Nickel, J.C.; O’Leary, M.P.; Pontari, M.; McNaughton-Collins, M. Chronic Prostatitis Collaborative Research Network (CPCRN). Responsiveness of the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI). Qual. Life Res. 2006, 15, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.N.; Nyberg, L.; Nickel, J.C. NIH consensus definition and classification of prostatitis. JAMA 1999, 282, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.A.; Nickel, J.C.; Dolinga, R.; Prots, D. Clinical phenotyping of patients with chronic prostatitis/chronic pelvic pain syndrome and correlation with symptom severity. Urology 2009, 73, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Magri, V.; Wagenlehner, F.; Perletti, G.; Schneider, S.; Marras, E.; Naber, K.G.; Weidner, W. Use of the UPOINT chronic prostatitis/chronic pelvic pain syndrome classification in European patient cohorts: Sexual function domain improves correlations. J. Urol. 2010, 184, 2339–2345. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Nickel, J.C.; Kattan, M.W. Phenotypically directed multimodal therapy for chronic prostatitis/chronic pelvic pain syndrome: A prospective study using UPOINT. Urology 2010, 75, 1249–1253. [Google Scholar] [CrossRef]
- Meares, E.M.; Stamey, T.A. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Investig. Urol. 1968, 5, 492–518. [Google Scholar]
- Nickel, J.C. The pre and post massage test (PPMT): A simple screen for prostatitis. Tech. Urol. 1997, 3, 38–43. [Google Scholar]
- Stamatiou, K.; Magri, V.; Perletti, G.; Samara, E.; Christopoulos, G.; Trinchieri, A. How urologists deal with chronic prostatitis? The preliminary results of a Mediterranean survey. Arch. Ital. Urol. Androl. 2020, 92. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Attia, J.; Nickel, J.C.; Thammakraisorn, S.; Numthavaj, P.; McEvoy, M.; Thakkinstian, A. Management of chronic prostatitis/chronic pelvic pain syndrome: A systematic review and network meta-analysis. JAMA 2011, 305, 78–86. [Google Scholar] [CrossRef]
- Domingue, G.J., Sr.; Hellstrom, W.J. Prostatitis. Clin. Microbiol. Rev. 1998, 11, 604–613. [Google Scholar] [CrossRef]
- Magri, V.; Wagenlehner, F.M.; Montanari, E.; Marras, E.; Orlandi, V.; Restelli, A.; Torresani, E.; Naber, K.G.; Perletti, G. Semen analysis in chronic bacterial prostatitis: Diagnostic and therapeutic implications. Asian J. Androl. 2009, 11, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.; European Lomefloxacin Prostatitis Study Group. Lomefloxacin versus ciprofloxacin in the treatment of chronic bacterial prostatitis. Int. J. Antimicrob. Agents 2002, 20, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Iovene, M.R.; Martora, F.; Mallardo, E.; De Sio, M.; Arcaniolo, D.; Del Vecchio, C.; Pagliuca, C.; Signoriello, G.; Vitiello, M. Enrichment of semen culture in the diagnosis of bacterial prostatitis. J. Microbiol. Methods 2018, 154, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, C.; Cariati, F.; Bagnulo, F.; Scaglione, E.; Carotenuto, C.; Farina, F.; D’Argenio, V.; Carraturo, F.; D’Aprile, P.; Vitiello, M.; et al. Microbiological Evaluation and Sperm DNA Fragmentation in Semen Samples of Patients Undergoing Fertility Investigation. Genes 2021, 12, 654. [Google Scholar] [CrossRef]
- Türk, S.; Korrovits, P.; Punab, M.; Mändar, R. Coryneform bacteria in semen of chronic prostatitis patients. Int. J. Androl. 2007, 30, 123–128. [Google Scholar] [CrossRef]
- Ch’ng, J.H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-associated infection by enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- Bartoletti, R.; Cai, T.; Nesi, G.; Albanese, S.; Meacci, F.; Mazzoli, S.; Naber, K. The impact of biofilm-producing bacteria on chronic bacterial prostatitis treatment: Results from a longitudinal cohort study. World J. Urol. 2014, 32, 737–742. [Google Scholar] [CrossRef]
- Trinchieri, A.; Abdelrahman, K.M.; Bhatti, K.H.; Bello, J.O.; Das, K.; Gatsev, O.; Gergova, I.; Magri, V.; Mourmouras, N.; Mourmouris, P.; et al. Spectrum of Causative Pathogens and Resistance Rates to Antibacterial Agents in Bacterial Prostatitis. Diagnostics 2021, 11, 1333. [Google Scholar] [CrossRef]
- Magri, V.; Marras, E.; Perletti, G. Chronic bacterial prostatitis: Enterococcal disease? Clin. Infect. Dis. 2011, 53, 1306–1307. [Google Scholar] [CrossRef]
- Cai, T.; Mazzoli, S.; Meacci, F.; Boddi, V.; Mondaini, N.; Malossini, G.; Bartoletti, R. Epidemiological features and resistance pattern in uropathogens isolated from chronic bacterial prostatitis. J. Microbiol. 2011, 49, 448–454. [Google Scholar] [CrossRef]
- Papeš, D.; Pasini, M.; Jerončić, A.; Vargović, M.; Kotarski, V.; Markotić, A.; Škerk, V. Detection of sexually transmitted pathogens in patients with chronic prostatitis/chronic pelvic pain: A prospective clinical study. Int. J. STD AIDS 2017, 28, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Ouzounova-Raykova, V.; Ouzounova, I.; Mitov, I.G. May Chlamydia trachomatis be an aetiological agent of chronic prostatic infection? Andrologia 2010, 42, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Mändar, R.; Raukas, E.; Türk, S.; Korrovits, P.; Punab, M. Mycoplasmas in semen of chronic prostatitis patients. Scand. J. Urol. Nephrol. 2005, 39, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Weidner, W.; Krause, W.; Schiefer, H.G.; Brunner, H.; Friedrich, H.J. Ureaplasmal infections of the male urogenital tract, in particular prostatitis, and semen quality. Urol. Int. 1985, 40, 5–9. [Google Scholar] [CrossRef]
- Puerta Suárez, J.; Hernandez, J.C.; Cardona Maya, W.D. Molecular analysis of microorganisms in the semen and their impact on semen parameters. Arch. Ital. Urol. Androl. 2022, 94, 199–205. [Google Scholar] [CrossRef]
- Kogan, M.; Naboka, Y.; Ferzauli, A.; Ibishev, K.; Gudima, I.; Ismailov, R. Does the microbiota spectrum of prostate secretion affect the clinical status of patients with chronic bacterial prostatitis? Int. J. Urol. 2021, 28, 1254–1259. [Google Scholar] [CrossRef]
- Mändar, R.; Punab, M.; Korrovits, P.; Türk, S.; Ausmees, K.; Lapp, E.; Preem, J.K.; Oopkaup, K.; Salumets, A.; Truu, J. Seminal microbiome in men with and without prostatitis. Int. J. Urol. 2017, 24, 211–216. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Altemus, J.; Polackwich, A.S.; Tucky, B.; Wang, H.; Eng, C. The Urinary Microbiome Differs Significantly Between Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Controls as Well as Between Patients with Different Clinical Phenotypes. Urology 2016, 92, 26–32. [Google Scholar] [CrossRef]
- Weidner, W. Treating chronic prostatitis: Antibiotics no, alpha-blockers maybe. Ann. Intern. Med. 2004, 141, 639–640. [Google Scholar] [CrossRef]
- Doiron, R.C.; Shoskes, D.A.; Nickel, J.C. Male CP/CPPS: Where do we stand? World J. Urol. 2019, 37, 1015–1022. [Google Scholar] [CrossRef]
- Murphy, A.B.; Macejko, A.; Taylor, A.; Nadler, R.B. Chronic prostatitis: Management strategies. Drugs 2009, 69, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Tamanini, I.; Odorizzi, K.; Gallelli, L.; Lanzafame, M.; Mazzoli, S.; Lanzafame, P.; Massidda, O.; Palmieri, A.; Wagenlehner, F.M.E.; et al. The diagnostic yield of the Meares & Stamey test can be significantly improved by symptom-based patient selection and the experience of the test performer. Prostate Cancer Prostatic Dis. 2024, 27, 300–304. [Google Scholar] [PubMed]
- Fritzenwanker, M.; Imirzalioglu, C.; Chakraborty, T.; Wagenlehner, F.M. Modern diagnostic methods for urinary tract infections. Expert. Rev. Anti Infect. Ther. 2016, 14, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ren, L.; Lv, H.; Ding, Q.; Lou, S.; Zhang, W.; Dong, Z. Atypical microorganisms in expressed prostatic secretion from patients with chronic prostatitis/chronic pelvic pain syndrome: Microbiological results from a case-control study. Urol. Int. 2013, 91, 410–416. [Google Scholar] [CrossRef]
- Arora, H.C.; Eng, C.; Shoskes, D.A. Gut microbiome and chronic prostatitis/chronic pelvic pain syndrome. Ann. Transl. Med. 2017, 5, 30. [Google Scholar] [CrossRef]
- Hashemi, N.; Tondro Anamag, F.; Javan Balegh Marand, A.; Rahnama’I, M.S.; Herizchi Ghadim, H.; Salehi-Pourmehr, H.; Hajebrahimi, S. A systematic and comprehensive review of the role of microbiota in urinary chronic pelvic pain syndrome. Neurourol. Urodyn. 2024, 43, 1859–1882. [Google Scholar] [CrossRef]
- Wang, S.; Zang, M.; Yang, X.; Lv, L.; Chen, L.; Cui, J.; Liu, Y.; Xia, Y.; Zhou, N.; Yang, Z.; et al. Gut microbiome in men with chronic prostatitis/chronic pelvic pain syndrome: Profiling and its predictive significance. World J. Urol. 2023, 41, 3019–3026. [Google Scholar] [CrossRef]
- Mjaess, G.; Karam, A.; Roumeguère, T.; Diamand, R.; Aoun, F.; McVary, K.; Moul, J.W.; De Nunzio, C.; Albisinni, S. Urinary microbiota and prostatic diseases: The key for the lock? A systematic review. Prostate Cancer Prostatic Dis. 2023, 26, 451–460. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H. Cross-sectional study on the etiological diagnosis of the patients with chronic prostatitis-like symptoms by application of the urine-prostate-semen test. Health Sci. Rep. 2022, 5, e574. [Google Scholar] [CrossRef]
- Heras-Cañas, V.; Gutiérrez-Soto, B.; Serrano-García, M.L.; Vázquez-Alonso, F.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Chronic bacterial prostatitis. Clinical and microbiological study of 332 cases. Med. Clin. 2016, 147, 144–147. [Google Scholar] [CrossRef]
- Mendoza-Rodríguez, R.; Hernández-Chico, I.; Gutiérrez-Soto, B.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Microbial etiology of bacterial chronic prostatitis: Systematic review. Rev. Esp. Quimioter. 2023, 36, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Budía, A.; Luis Palmero, J.; Broseta, E.; Tejadillos, S.; Benedicto, A.; Queipo, J.A.; Gobernado, M.; Fernando Jiménez Cruz, J. Value of semen culture in the diagnosis of chronic bacterial prostatitis: A simplified method. Scand. J. Urol. Nephrol. 2006, 40, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Zegarra Montes, L.Z.; Sanchez Mejia, A.A.; Loza Munarriz, C.A.; Gutierrez, E.C. Semen and urine culture in the diagnosis of chronic bacterial prostatitis. Int. Braz. J. Urol. 2008, 34, 30–37. [Google Scholar] [CrossRef] [PubMed]

| Test | 5-Glass | 4-Glass | 2-Glass | VB2-Semen | One-Way ANOVA | Tukey’s HSD Post Hoc Test |
|---|---|---|---|---|---|---|
| Mean age (±SD) | 46.51 ± 13.42 | 47.34 ± 14.55 | 47.42 ± 15.01 | 45.39 ± 12.96 | p = 0.06 | p > 0.05, all comparisons |
| Mean PSA, ng/mL (±SD) | 2.50 ± 3.068 | 2.67 ± 3.47 | 2.58 ± 2.87 | 2.35 ± 2.75 | p = 0.42 | p > 0.05, all comparisons |
| Mean Qmax, mL/S (±SD) | 16.19 ± 9.41 | 16.22 ± 10.15 | 16.09 ± 10.41 | 16.07 ± 8.67 | p = 0.99 | p > 0.05, all comparisons |
| Post-void residual bladder urine, mL | 40.05 ± 55.11 | 42.38 ± 58.31 | 45.04 ± 59.37 | 38.73 ± 52.72 | p = 0.42 | p > 0.05, all comparisons |
| NIH-CPSI total score (mean ± SD) | 21.83 ± 7.19 | 22.14 ± 6.98 | 22.56 ± 7.12 | 21.98 ± 7.27 | p = 0.55 | p > 0.05, all comparisons |
| NIH pain score (mean ± SD) | 10.12 ± 5.48 | 10.13 ± 7.62 | 10.73 ± 9.59 | 10.10 ± 6.81 | p = 0.61 | p > 0.05, all comparisons |
| NIH voiding symptom score (mean ± SD) | 4.61 ± 2.68 | 4.89 ± 2.61 | 5.05 ± 2.57 | 4.43 ± 2.71 | p = 0.047 | p > 0.05, for all comparisons, except: 2-glass vs. VB2-semen, p = 0.0081 |
| NIH QoL impact score (mean ± SD) | 7.48 ± 2.71 | 7.68 ± 2.70 | 7.69 ± 2.63 | 7.60 ± 2.71 | p = 0.58 | p > 0.05, all comparisons |
| IPSS (mean ± SD) | 11.93 ± 6.85 | 12.56 ± 6.81 | 13.56 ± 7.04 | 11.45 ± 7.02 | p = 0.0003 | p > 0.05, for all comparisons, except: 2-glass vs. VB2-semen, p = 0.0002 |
| Pathogen Load, cfu/mL | EPS, % | VB3, % | Semen, % |
|---|---|---|---|
| 103 | 43.62 | 32.58 | 30.29 |
| 104 | 29.53 | 17.98 | 23.72 |
| 105 | 20.81 | 26.22 | 30.93 |
| 106 | 6.04 | 23.22 | 15.06 |
| Total | 100 | 100 | 100 |
| Diagnostic Test | Significance of Difference Between Diagnostic Tests (Pearson’s χ2 or Fisher’s Exact Test) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-Glass | 4-Glass | 2-Glass | VB2-S | 5G vs. 4G | 5G vs. 2G | 5G vs. VB2-S | 4G vs. 2G | 4G vs. VB2-S | 2G vs. VB2-S | |||||
| Species | n | % | n | % | n | % | n | % | p | p | p | p | p | p |
| Escherichia coli | 251 | 46.22 | 123 | 62.76 | 71 | 61.74 | 159 | 35.10 | 0.034 | 0.098 | 0.033 | 0.94 | 0.0002 | 0.0023 |
| Enterococcus faecalis | 172 | 31.68 | 37 | 18.88 | 19 | 16.52 | 182 | 40.18 | 0.007 | 0.011 | 0.038 | 0.67 | <0.0001 | 0.0003 |
| Proteus Myrabilis | 28 | 5.16 | 12 | 6.12 | 8 | 6.96 | 23 | 5.08 | 0.65 | 0.48 | 0.98 | 0.78 | 0.67 | 0.49 |
| Klebsiella spp. | 22 | 4.05 | 3 | 1.53 | 3 | 2.61 | 22 | 4.86 | 0.11 | 0.59 | 0.51 | 0.67 | 0.046 | 0.44 |
| Morganella Morganii | 22 | 4.05 | 6 | 3.06 | 5 | 4.35 | 19 | 4.19 | 0.52 | 0.80 | 0.86 | 0.54 | 0.46 | 0.99 |
| Pseudomonas aeruginosa | 17 | 3.13 | 3 | 1.53 | 5 | 4.35 | 12 | 2.65 | 0.31 | 0.56 | 0.69 | 0.15 | 0.57 | 0.37 |
| Staphylococcus aureus | 14 | 2.58 | 9 | 4.59 | 2 | 1.74 | 12 | 2.65 | 0.19 | 0.99 | 0.91 | 0.34 | 0.24 | 0.74 |
| Citrobacter Koseri | 10 | 1.84 | 1 | 0.51 | 1 | 0.87 | 17 | 3.75 | 0.30 | 0.99 | 0.23 | 0.99 | 0.21 | 0.71 |
| Enterobacter cloacae | 5 | 0.92 | 1 | 0.51 | 1 | 0.87 | 5 | 1.10 | 0.99 | 0.99 | 0.76 | 0.99 | 0.67 | 0.99 |
| Enterococcus faecium | 2 | 0.37 | 1 | 0.51 | 0 | 0.00 | 2 | 0.44 | 0.99 | n.a. | 0.99 | n.a. | 0.99 | n.a. |
| Total Gram-negative | 355 | 65.38 | 149 | 76.02 | 94 | 81.74 | 257 | 56.73 | 0.23 | 0.15 | 0.17 | 0.68 | 0.028 | 0.021 |
| Total Gram-positive | 188 | 34.62 | 47 | 23.98 | 21 | 18.26 | 196 | 43.27 | 0.037 | 0.009 | 0.044 | 0.34 | 0.0006 | 0.0003 |
| Total traditional uropathogens | 543 | 100 | 196 | 100 | 115 | 100 | 453 | 100 | ||||||
| Traditional uropathogens/total tested population (n = 1047),% | 51.86 | 18.72 | 10.98 | 43.27 | ||||||||||
| Ureaplasma urealyticum | 118 | 67.43 | 21 | 55.26 | 12 | 54.55 | 124 | 71.26 | 0.51 | 0.57 | 0.74 | 0.97 | 0.39 | 0.47 |
| Chlamydia trachomatis | 40 | 22.86 | 16 | 42.11 | 7 | 31.82 | 36 | 20.69 | 0.074 | 0.47 | 0.69 | 0.59 | 0.039 | 0.35 |
| Mycoplasma hominis | 10 | 5.71 | 0 | 0.00 | 3 | 13.64 | 9 | 5.17 | n.a. | 0.19 | 0.83 | n.a. | n.a. | 0.16 |
| Neisseria gonorrheae | 4 | 2.29 | 1 | 2.63 | 0 | 0.00 | 2 | 1.15 | 0.99 | n.a. | 0.68 | n.a. | 0.45 | n.a. |
| Candida spp. | 2 | 1.14 | 0 | 0.00 | 0 | 0.00 | 2 | 1.15 | n.a. | n.a. | 0.99 | n.a. | n.a. | n.a. |
| Trichomonas Vaginalis | 1 | 0.57 | 0 | 0.00 | 0 | 0.00 | 1 | 0.57 | n.a. | n.a. | 0.99 | n.a. | n.a. | n.a. |
| Total sexually transmitted pathogens | 175 | 100 | 38 | 100 | 22 | 100 | 174 | 100 | ||||||
| Total ST pathogens/total tested population (n = 1047),% | 16.71 | 3.63 | 2.10 | 16.62 | ||||||||||
| Streptococcus beta-haemolyticus Gr. B | 55 | 35.71 | 14 | 31.82 | 11 | 39.29 | 63 | 37.72 | 0.73 | 0.81 | 0.79 | 0.65 | 0.61 | 0.91 |
| Haemophylus parainfluenzae | 32 | 20.78 | 5 | 11.36 | 5 | 17.86 | 38 | 22.75 | 0.23 | 0.99 | 0.73 | 0.51 | 0.21 | 0.81 |
| Staphylococcus spp. (coagulase-negative) | 28 | 18.18 | 11 | 25.00 | 5 | 17.86 | 33 | 19.76 | 0.41 | 0.99 | 0.76 | 0.77 | 0.54 | 0.99 |
| Corynebacterium seminale | 12 | 7.79 | 4 | 9.09 | 4 | 14.29 | 6 | 3.59 | 0.76 | 0.29 | 0.12 | 0.71 | 0.22 | 0.052 |
| Streptococcus agalactiae | 9 | 5.84 | 9 | 20.45 | 2 | 7.14 | 3 | 1.80 | 0.018 | 0.68 | 0.081 | 0.31 | 0.0001 | 0.16 |
| Gardnerella Vaginalis | 5 | 3.25 | 0 | 0.00 | 0 | 0.00 | 6 | 3.59 | n.a. | n.a. | 0.99 | n.a. | n.a. | n.a. |
| Serratia mascescens | 4 | 2.60 | 0 | 0.00 | 0 | 0.00 | 4 | 2.40 | n.a. | n.a. | 0.99 | n.a. | n.a. | n.a. |
| Peptostreptococcus spp. (AC) | 3 | 1.95 | 1 | 2.27 | 0 | 0.00 | 3 | 1.80 | 0.99 | n.a. | 0.99 | n.a. | 0.99 | n.a. |
| Kocuria spp. | 2 | 1.30 | 0 | 0.00 | 1 | 3.57 | 6 | 3.59 | n.a. | 0.40 | 0.28 | n.a. | n.a. | 0.99 |
| Actinomyces spp. | 2 | 1.30 | 0 | 0.00 | 0 | 0.00 | 2 | 1.20 | n.a. | n.a. | 0.99 | n.a. | n.a. | n.a. |
| Clostridium spp. | 1 | 0.65 | 0 | 0.00 | 0 | 0.00 | 1 | 0.60 | n.a. | n.a. | 0.99 | n.a. | n.a. | n.a. |
| Acinetobacter spp. | 1 | 0.65 | 0 | 0.00 | 0 | 0.00 | 2 | 1.20 | n.a. | n.a. | 0.99 | n.a. | n.a. | n.a. |
| Total other pathogens | 154 | 100 | 44 | 100 | 28 | 100 | 167 | 100 | ||||||
| Total other pathogens/total tested population (n = 1047),% | 14.71 | 4.20 | 2.67 | 15.95 | ||||||||||
| Total pathogens detected, n | 872 | 278 | 165 | 794 | ||||||||||
| Total pathogens detected/total tested population (n = 1047),% | 83.29 | 26.55 | 15.76 | 75.84 | <0.0001 | <0.0001 | 0.15 | <0.0001 | <0.0001 | <0.0001 | ||||
| % Traditional uropathogens/total pathogens detected | 62.27 | 70.50 | 69.70 | 57.05 | 0.25 | 0.39 | 0.27 | 0.94 | 0.054 | 0.13 | ||||
| % ST pathogens/total pathogens detected | 20.07 | 13.67 | 13.33 | 21.91 | 0.044 | 0.088 | 0.45 | 0.93 | 0.013 | 0.038 | ||||
| % other pathogens/total pathogens detected | 17.66 | 15.83 | 16.97 | 21.03 | 0.55 | 0.85 | 0.15 | 0.78 | 0.12 | 0.33 | ||||
| Total% | 100 | 100 | 100 | 100 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magri, V.; Perletti, G.; Stamatiou, K. Pathogen Detection and Diagnostic Scenarios in Chronic Prostatitis. Diagnostics 2025, 15, 762. https://doi.org/10.3390/diagnostics15060762
Magri V, Perletti G, Stamatiou K. Pathogen Detection and Diagnostic Scenarios in Chronic Prostatitis. Diagnostics. 2025; 15(6):762. https://doi.org/10.3390/diagnostics15060762
Chicago/Turabian StyleMagri, Vittorio, Gianpaolo Perletti, and Konstantinos Stamatiou. 2025. "Pathogen Detection and Diagnostic Scenarios in Chronic Prostatitis" Diagnostics 15, no. 6: 762. https://doi.org/10.3390/diagnostics15060762
APA StyleMagri, V., Perletti, G., & Stamatiou, K. (2025). Pathogen Detection and Diagnostic Scenarios in Chronic Prostatitis. Diagnostics, 15(6), 762. https://doi.org/10.3390/diagnostics15060762






