Useful Biomarkers for Preeclampsia: Evaluating the Diagnostic Potential of FIB-4 and FIB-5 Indices
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Design, Patient Selection, and Diagnosis
2.3. Statistical Analyses
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartal, M.F.; Sibai, B.M. Gestational Hypertension, Preeclampsia, and Eclampsia. In Queenan’s Management of High-Risk Pregnancy: An Evidence-Based Approach; Wiley Online Library: Hoboken, NJ, USA, 2024; pp. 281–287. [Google Scholar]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Vidaeff, A.; Pettker, C.M.; Simhan, H. Gestational hypertension and preeclampsia. Obstet. Gynecol. 2020, 135, E237–E260. [Google Scholar]
- Hammoud, G.M.; Ibdah, J.A. Preeclampsia-induced liver dysfunction, HELLP syndrome, and acute fatty liver of pregnancy. Clin. Liver Dis. 2014, 4, 69–73. [Google Scholar] [CrossRef]
- Cemortan, M.; Iliadi-Tulbure, C.; Sagaidac, I.; Cernetchi, O. Assessment of aspartate aminotransferase to Platelet Ratio Index and Fibrosis-4 Index score on women with intrahepatic cholestasis of pregnancy. AJOG Glob. Rep. 2024, 4, 100337. [Google Scholar] [CrossRef]
- Sapmaz, F.P.; Büyükturan, G.; Sakin, Y.S.; Kalkan, İ.H.; Atasoy, P. How effective are APRI, FIB-4, FIB-5 scores in predicting liver fibrosis in chronic hepatitis B patients? Medicine 2022, 101, e30488. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, C.; Cariello, M.; Graziano, G.; Battaglia, S.; Suppressa, P.; Piazzolla, G.; Sabbà, C.; Moschetta, A. AST to Platelet Ratio Index (APRI) is an easy-to-use predictor score for cardiovascular risk in metabolic subjects. Sci. Rep. 2021, 11, 14834. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, B.; Luo, Z.; Wang, Y.; Hu, Q.; Zhou, L. The fibrosis-5 index predicts major adverse cardiovascular events in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. Angiology 2024. online ahead of print. [Google Scholar] [CrossRef]
- Şaşmaz, M.İ.; Ayvaz, M.A.; Dülger, A.C.; Kaykısız, E.K.K.; Güven, R. Aspartate-aminotransferase to platelet ratio index score for predicting HELLP syndrome. Am. J. Emerg. Med. 2020, 38, 459–462. [Google Scholar] [CrossRef]
- Tolunay, H.E.; Kahraman, N.C.; Varli, E.N.; Reis, Y.A.; Celen, S.; Caglar, A.T. Can first-trimester AST to platelet ratio index scores predict HELLP syndrome. J. Coll. Physicians Surg. Pak. 2021, 31, 188–192. [Google Scholar]
- Gök, K.; Takmaz, T.; Köse, O.; Tüten, N.; Bostancı, M.S.; Özden, S. Effectiveness of the Fibrosis-4 Score in Predicting Intrahepatic Cholestasis of Pregnancy. Eur. Arch. Med. Res. 2022, 38, 4. [Google Scholar] [CrossRef]
- Özer, S.; Güneş, H.; Özer, A. FIB4 score is increased in severe preeclampsia. Turk. J. Biochem. 2024, 49, 56–63. [Google Scholar] [CrossRef]
- Shiha, G.; Seif, S.; Eldesoky, A.; Elbasiony, M.; Soliman, R.; Metwally, A.; Zalata, K.; Mikhail, N. A simple bedside blood test (Fibrofast; FIB-5) is superior to FIB-4 index for the differentiation between non-significant and significant fibrosis in patients with chronic hepatitis C. Hepatol. Int. 2017, 11, 286–291. [Google Scholar] [CrossRef]
- Chang, K.-J.; Seow, K.-M.; Chen, K.-H. Preeclampsia: Recent advances in predicting, preventing, and managing the maternal and fetal life-threatening condition. Int. J. Environ. Res. Public Health 2023, 20, 2994. [Google Scholar] [CrossRef]
- Minakami, H.; Oka, N.; Sato, T.; Tamada, T.; Yasuda, Y.; Hirota, N. Preeclampsia: A microvesicular fat disease of the liver? Am. J. Obstet. Gynecol. 1988, 159, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Alese, M.O.; Moodley, J.; Naicker, T. Preeclampsia and HELLP syndrome, the role of the liver. J. Matern.-Fetal Neonatal Med. 2021, 34, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chaiworapongsa, T.; Romero, R.; Savasan, Z.A.; Kusanovic, J.P.; Ogge, G.; Soto, E.; Dong, Z.; Tarca, A.; Gaurav, B.; Hassan, S.S. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J. Matern.-Fetal Neonatal Med. 2011, 24, 1187–1207. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Wilson, S.; White, A.; Young, A.; Davies, M.; Pollard, S. The management of the surgical complications of HELLP syndrome. Ann. R. Coll. Surg. Engl. 2014, 96, 512–516. [Google Scholar] [CrossRef]
- Frank Wolf, M.; Peleg, D.; Kariv Silberstein, N.; Assy, N.; Djibre, A.; Ben-Shachar, I. Correlation between changes in liver stiffness and preeclampsia as shown by transient elastography. Hypertens. Pregnancy 2016, 35, 536–541. [Google Scholar] [CrossRef]
- Naruse, K. Liver biomarkers and preeclampsia: An easy-accessible prediction method of the disease. Hypertens. Res. 2024, 47, 2610–2611. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, L.; Cheng, H.; Chi, P.; Zhuang, Y.; Alifu, X.; Zhou, H.; Qiu, Y.; Huang, Y.; Ainiwan, D. The associations of maternal liver biomarkers in early pregnancy with the risk of gestational diabetes mellitus: A prospective cohort study and Mendelian randomization analysis. Front. Endocrinol. 2024, 15, 1396347. [Google Scholar] [CrossRef]
- Chen, Y.; Ou, W.; Lin, D.; Lin, M.; Huang, X.; Ni, S.; Chen, S.; Yong, J.; O’Gara, M.C.; Tan, X. Increased uric acid, gamma-glutamyl transpeptidase and alkaline phosphatase in early-pregnancy associated with the development of gestational hypertension and preeclampsia. Front. Cardiovasc. Med. 2021, 8, 756140. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, T.; Yuksel, B. Prognostic Value of Fibrosis 4 (FIB-4) Index in Sepsis Patients. J. Pers. Med. 2024, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Han, Y.; Zheng, L.; Liu, J.; Wu, Y.; Bao, Z.; Liu, L.; Li, W. Association of non-invasive markers with significant fibrosis in patients with nonalcoholic fatty liver disease: A cross-sectional study. Diabetes Metab. Syndr. Obes. 2023, 16, 2255–2268. [Google Scholar] [CrossRef]
- Ozkan, D.; Ibanoglu, M.C.; Adar, K.; Ozkan, M.; Lutfi Tapisiz, O.; Engin-Ustun, Y.; Iskender, C.T. Efficacy of blood parameters in predicting the severity of gestational hypertension and preeclampsia. J. Obstet. Gynaecol. 2023, 43, 2144175. [Google Scholar] [CrossRef]
- Li, Z.; Dai, Y.; Yun, L.; Guo, W. A prediction model for the progression from gestational hypertension to pre-eclampsia complicated with HELLP syndrome. Int. J. Gynecol. Obstet. 2024, 165, 1002–1012. [Google Scholar] [CrossRef]
- İpek, G.; Tanaçan, A.; Ağaoğlu, Z.; Baştemur, A.G.; Yıldız, E.G.; Şahin, D. The role of aspartate aminotransferase to platelet ratio index (APRI) in the first trimester for the prediction of superimposed preeclampsia: A case-control study from a tertiary center. Pregnancy Hypertens. 2024, 37, 101132. [Google Scholar] [CrossRef]
- Kolhe, K.M.; Amarapurkar, A.; Parikh, P.; Chaubal, A.; Chauhan, S.; Khairnar, H.; Walke, S.; Ingle, M.; Pandey, V.; Shukla, A. Aspartate transaminase to platelet ratio index (APRI) but not FIB-5 or FIB-4 is accurate in ruling out significant fibrosis in patients with non-alcoholic fatty liver disease (NAFLD) in an urban slum-dwelling population. BMJ Open Gastroenterol. 2019, 6, e000288. [Google Scholar] [CrossRef]
- Kumari, B.; Kumar, R.; Sharma, S.; Banerjee, A.; Kumar, V.; Kumar, P.; Chaudhary, N.; Kumar, S.; Raj, K. Diagnostic accuracy of FIB-4 and FIB-5 scores as compared to fibroscan for assessment of liver fibrosis in patients with non-alcoholic fatty liver disease. Cureus 2021, 13, e17622. [Google Scholar] [CrossRef]
- Eto, F.; Nezu, T.; Aoki, S.; Kuzume, D.; Hosomi, N.; Maruyama, H. Liver fibrosis index is associated with functional outcome among acute ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 2024, 33, 107537. [Google Scholar] [CrossRef] [PubMed]
- Metwally, K.; Elsabaawy, M.; Abdel-Samiee, M.; Morad, W.; Ehsan, N.; Abdelsameea, E. FIB-5 versus FIB-4 index for assessment of hepatic fibrosis in chronic hepatitis B affected patients. Clin. Exp. Hepatol. 2020, 6, 335–338. [Google Scholar] [CrossRef] [PubMed]
| Preeclampsia (n = 207) | Control (n = 205) | p-Value | |
|---|---|---|---|
| Maternal age | 31.2 ± 6.89 | 29.5 ± 5.13 | 0.067 a |
| Gravidity | 3.1 ± 1.9 | 2.9 ± 1.3 | 0.18 a |
| Parity | 1.8 ± 0.9 | 1.5 ± 1.1 | 0.36 a |
| Systolic blood pressure (mmHg) | 156.5 ± 9.2 | 110.6 ± 7.3 | <0.001 a |
| Diastolic blood pressure (mmHg) | 96.8 ± 7.5 | 63.5 ± 6.8 | <0.001 a |
| Cesarean delivery | 182 (87%) | 56 (27%) | <0.001 b |
| Vaginal delivery | 26 (13%) | 149 (73%) | <0.001 b |
| Gestational age at delivery (weeks) | 35.1 ± 1.7 | 38.2 ± 0.6 | 0.034 a |
| Birth weight (grams) | 2511 ± 593 | 3176 ± 684 | <0.001 a |
| Birth length (cm) | 44.16 ± 3.98 | 50.21 ± 4.79 | <0.001 a |
| First-minute Apgar score | 7.6 ± 1.2 | 8.1 ± 0.8 | 0.025 a |
| Fifth-minute Apgar score | 8.7 ± 0.9 | 9.2 ± 1.1 | 0.036 a |
| Preeclampsia (n = 207) | Control (n = 205) | p-Value | |
|---|---|---|---|
| Hemoglobin (g/dL) | 11.50 (10.50–12.60) | 11.80 (11.01–12.75) | 0.024 a |
| Platelet (×103/mm3) | 220 (181.5–286.5) | 225 (194–269) | 0.643 a |
| Alanine aminotransferase (U/L) | 19.2 (8.4–29.7) | 10.9 (6.3–15.7) | <0.001 a |
| Aspartate aminotransferase (U/L) | 21.7 (14.2–33.5) | 16.1 (13.8–19.4) | <0.001 a |
| Alkaline phosphatase (U/L) | 138.6 (103.5–184.5) | 116.3 (91.1–137.8) | 0.037 a |
| Serum albumin (g/L) | 35.4 (32.3–38.7) | 37.3 (36.2–40.5) | 0.164 a |
| Serum creatinine (mg/dL) | 0.58 ± 0.19 | 0.53 ± 0.12 | 0.058 b |
| Spot urine protein/creatinine ratio | 0.72 ± 0.25 | 0.14 ± 0.07 | <0.001 b |
| FIB-4 score | 0.86 ± 0.39 | 0.63 ± 0.24 | <0.001 b |
| FIB-5 score | −3.34 (−7.33–1.43) | −1.92 (−4.13–0.79) | <0.006 a |
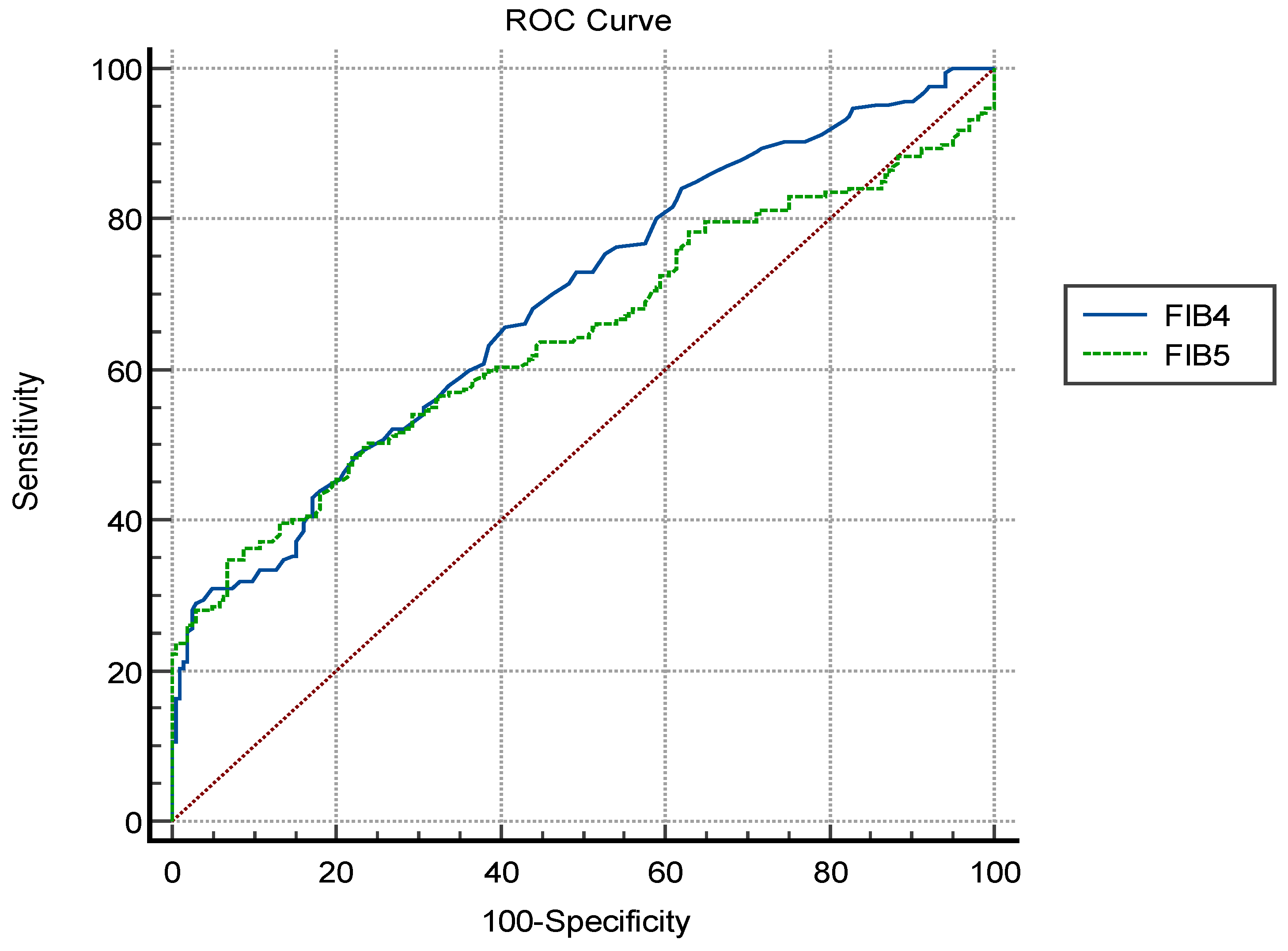
| AUC * | 95% Confidence Interval | Cut-Off Value | Sensitivity | Specificity | p Value | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| FIB-4 | 0.689 | (0.642–0.733) | >0.78 | 58.7 | 77.5 | <0.001 | |
| FIB-5 | 0.643 | (0.589–0.697) | <−3.13 | 54.1 | 69.2 | <0.001 | |
| Comparison of AUCs | AUC | ||||||
| Difference | 95% Confidence interval | ||||||
| FIB-4 and FIB-5 | 0.046 | (0.005–0.098) | 0.081 | ||||
| SPCR | ALT | AST | ALP | FIB-5 | FIB-4 | ||
|---|---|---|---|---|---|---|---|
| FIB-4 | r | 0.695 | −0.196 | 0.536 | 0.027 | −0.733 | |
| p | <0.001 | 0.042 | <0.001 | 0.588 | <0.001 | (–) | |
| FIB-5 | r | −0.542 | 0.274 | −0.246 | −0.190 | −0.733 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | (–) | <0.001 | |
| Univariate Regression (Unadjusted) | Multivariate Regression (Adjusted) | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | OR | %95 CI | p Value | Parameter | OR a | %95 CI | p Value |
| FIB-4 | 2.178 | (1.436–3.479) | <0.001 | FIB-4 | 1.936 | (1.367–3.015) | <0.001 |
| FIB-5 | 0.917 | (0.825–0.984) | <0.001 | FIB-5 | 0.724 | (0.665–0.813) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albayrak, M.; Arslan, H.F. Useful Biomarkers for Preeclampsia: Evaluating the Diagnostic Potential of FIB-4 and FIB-5 Indices. Diagnostics 2025, 15, 693. https://doi.org/10.3390/diagnostics15060693
Albayrak M, Arslan HF. Useful Biomarkers for Preeclampsia: Evaluating the Diagnostic Potential of FIB-4 and FIB-5 Indices. Diagnostics. 2025; 15(6):693. https://doi.org/10.3390/diagnostics15060693
Chicago/Turabian StyleAlbayrak, Mehmet, and Hilmi Furkan Arslan. 2025. "Useful Biomarkers for Preeclampsia: Evaluating the Diagnostic Potential of FIB-4 and FIB-5 Indices" Diagnostics 15, no. 6: 693. https://doi.org/10.3390/diagnostics15060693
APA StyleAlbayrak, M., & Arslan, H. F. (2025). Useful Biomarkers for Preeclampsia: Evaluating the Diagnostic Potential of FIB-4 and FIB-5 Indices. Diagnostics, 15(6), 693. https://doi.org/10.3390/diagnostics15060693






