Comparative Analysis of Perivascular Adipose Tissue Attenuation on Chest Computed Tomography Angiography in Patients with Marfan Syndrome: A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. High-Resolution Computed Tomography (HRCT)
2.3. Peri-Coronary Adipose Tissue Attenuation
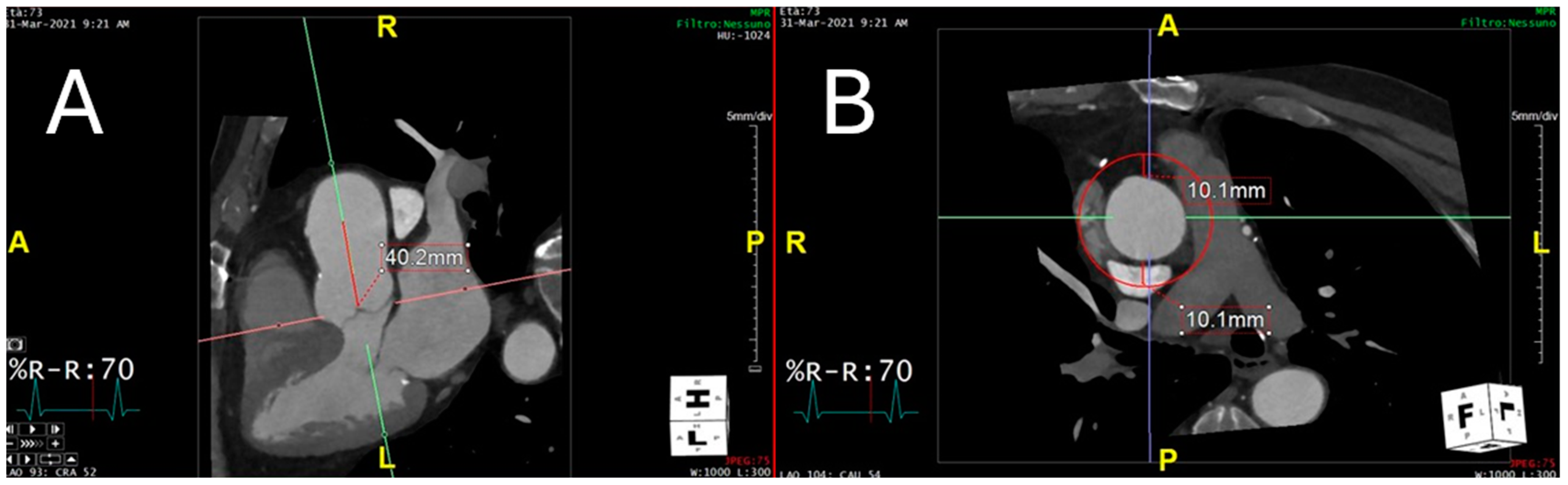
2.4. Technical Steps Required to Measure Perivascular Adipose Tissue Attenuation of the Proximal Ascending Thoracic Aorta on Chest Computed Tomography Angiograms
2.5. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, L.; Andrikopoulos, K.; Tian, J.; Lee, S.Y.; Keene, D.R.; Ono, R.; Reinhardt, D.P.; Sakai, L.Y.; Biery, N.J.; Bunton, T.; et al. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat. Genet. 1997, 17, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Collod-Béroud, G.; Le Bourdelles, S.; Ades, L.; Ala-Kokko, L.; Booms, P.; Boxer, M.; Child, A.; Comeglio, P.; De Paepe, A.; Hyland, J.C.; et al. Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum. Mutat. 2003, 22, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Marelli, S.; Micaglio, E.; Taurino, J.; Salvi, P.; Rurali, E.; Perrucci, G.L.; Dolci, C.; Udugampolage, N.S.; Caruso, R.; Gentilini, D.; et al. Marfan Syndrome: Enhanced Diagnostic Tools and Follow-up Management Strategies. Diagnostics 2023, 13, 2284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grewal, N.; Gittenberger-de Groot, A.C. Pathogenesis of aortic wall complications in Marfan syndrome. Cardiovasc. Pathol. 2018, 33, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wanga, S.; Fellows, A.L.; Barallobre-Barreiro, J.; Lu, R.; Davaapil, H.; Franken, R.; Fava, M.; Baig, F.; Skroblin, P.; et al. Glycoproteomic Analysis of the Aortic Extracellular Matrix in Marfan Patients. Arter. Thromb. Vasc. Biol. 2019, 39, 1859–1873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monda, E.; Caiazza, M.; Limongelli, G. The role of genetic testing in Marfan syndrome. Curr. Opin. Cardiol. 2024, 39, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Phornphutkul, C.; Rosenthal, A.; Nadas, A.S. Cardiac manifestations of Marfan syndrome in infancy and childhood. Circulation 1973, 47, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.I.; Burton, K.J.; Gray, J.; Bosner, M.S.; Kouchoukos, N.T.; Roman, M.J.; Boxer, M.; Devereux, R.B.; Tsipouras, P. Life expectancy in the Marfan syndrome. Am. J. Cardiol. 1995, 75, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Vanem, T.T.; Geiran, O.R.; Krohg-Sørensen, K.; Røe, C.; Paus, B.; Rand-Hendriksen, S. Survival, causes of death, and cardiovascular events in patients with Marfan syndrome. Mol. Genet. Genom. Med. 2018, 6, 1114–1123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur. Heart J. 2014, 35, 2779. [Google Scholar] [CrossRef] [PubMed]
- Abbara, S.; Kalva, S.; Cury, R.C.; Isselbacher, E.M. Thoracic aortic disease: Spectrum of multidetector computed tomography imaging findings. J. Cardiovasc. Comput. Tomogr. 2007, 1, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black J 3rd Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; Collins, T.J.; et al. Peer Review Committee Members. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, K.; Anmin, R.; Cai, J.; Qi, Y.; Han, W.; Li, M.; Zhang, G.; Zhang, S.; Fu, L.; Han, W.; et al. Ascending aortic perivascular adipose tissue inflammation associates with aortic valve disease. J. Cardiol. 2022, 80, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.K.; Zhou, Z.; Zhang, J.; Zeng, R.; Wu, J.; Eitzman, D.T.; Chen, Y.E.; Chang, L. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1621–1630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, K.; Wahome, E.; Tsiachristas, A.; Antonopoulos, A.S.; Patel, P.; Lyasheva, M.; Kingham, L.; West, H.; Oikonomou, E.K.; Volpe, L.; et al. ORFAN Consortium. Inflammatory risk and cardiovascular events in patients without obstructive coronary artery disease: The ORFAN multicentre, longitudinal cohort study. Lancet 2024, 403, 2606–2618. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasqualetto, M.C.; Tuttolomondo, D.; Cutruzzolà, A.; Niccoli, G.; Dey, D.; Greco, A.; Martini, C.; Irace, C.; Rigo, F.; Gaibazzi, N. Human coronary inflammation by computed tomography: Relationship with coronary microvascular dysfunction. Int. J. Cardiol. 2021, 336, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, D.; Ticinesi, A.; Dey, D.; Martini, C.; Nouvenne, A.; Nicastro, M.; De Filippo, M.; Sverzellati, N.; Nicolini, F.; Meschi, T.; et al. Coronary inflammation on chest computed tomography and COVID-19 mortality. Eur. Radiol. 2024, 34, 5153–5163. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, D.; Martini, C.; Nicolini, F.; Formica, F.; Pini, A.; Secchi, F.; Volpi, R.; De Filippo, M.; Gaibazzi, N. Perivascular Adipose Tissue Attenuation on Computed Tomography beyond the Coronary Arteries. A Systematic Review. Diagnostics 2021, 11, 1495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaibazzi, N.; Martini, C.; Benatti, G.; Palumbo, A.A.; Cacciola, G.; Tuttolomondo, D. Atrial Fibrillation and Peri-Atrial Inflammation Measured through Adipose Tissue Attenuation on Cardiac Computed Tomography. Diagnostics 2021, 11, 2087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, A.; Belmonte, M.; Masetti, M.; Bergamaschi, L.; Paolisso, P.; Borgese, L.; Angeli, F.; Armillotta, M.; Dierckx, R.; Verstreken, S.; et al. CTA-Derived Pericoronary Fat Attenuation Index Predicts Allograft Rejection and Cardiovascular Events in Heart Transplant Recipients. JACC Cardiovasc. Imaging 2024, 18, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Nogic, J.; Kim, J.; Layland, J.; Cheng, K.; Dey, D.; Wong, D.T.; Cameron, J.D.; Brown, A.J. Peri-Coronary Adipose Tissue Is a Predictor of Stent Failure in Patients Undergoing Percutaneous Coronary Intervention. Cardiovasc. Revasc. Med. 2023, 53, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Sartorio, D.; Tuttolomondo, D.; Napolitano, F.; Siniscalchi, C.; Borrello, B.; Palumbo, A.A.; Nicolini, F. Attenuation of peri-vascular fat at computed tomography to measure inflammation in ascending aorta aneurysms. Eur. J. Prev. Cardiol. 2021, 28, e23–e25. [Google Scholar] [CrossRef] [PubMed]
- Gaibazzi, N.; Tuttolomondo, D.; Nicolini, F.; Tafuni, A.; Sartorio, D.; Martini, C.; Maestri, F.; Gallingani, A.; De Filippo, M.; Corradi, D. The Histopathological Correlate of Peri-Vascular Adipose Tissue Attenuation on Computed Tomography in Surgical Ascending Aorta Aneurysms: Is This a Measure of Tissue Inflammation? Diagnostics 2021, 11, 1799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuttolomondo, D.; De Filippo, M.; Sartorio, D.; Nicolini, F.; Niccoli, G.; Gaibazzi, N. Peri-vascular adipose tissue attenuation on chest computed tomography in patients with Marfan Syndrome: A case series. Acta Biomed. 2021, 92, e2021468. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Jeon, J.S.; Noh, H.; Lee, E.J.; Kwon, S.H. Association between abdominal perivascular adipose tissue quantity and quality assessed by CT and cardiometabolic risk. Clin. Nutr. 2023, 42, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, F.; Jia, X.; Qiao, H.; Liu, Y.; Yang, X.; Li, Y.; Zhang, M.; Yang, Q. Pericarotid adipose tissue computed tomography attenuation distinguishes different stages of carotid atherosclerotic disease: A cross-sectional study. Quant. Imaging Med. Surg. 2023, 13, 8247–8258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dias-Neto, M.; Meekel, J.P.; van Schaik, T.G.; Hoozemans, J.; Sousa-Nunes, F.; Henriques-Coelho, T.; Lely, R.J.; Wisselink, W.; Blankensteijn, J.D.; Yeung, K.K. High Density of Periaortic Adipose Tissue in Abdominal Aortic Aneurysm. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, H.; Myneni, P.K.; Patel, P.; Askin, G.; Gialdini, G.; Al-Dasuqi, K.; Kamel, H.; Gupta, A. Association Between Carotid Artery Perivascular Fat Density and Cerebrovascular Ischemic Events. J. Am. Heart Assoc. 2018, 7, e010383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antoniades, C.; Tousoulis, D.; Vavlukis, M.; Fleming, I.; Duncker, D.J.; Eringa, E.; Manfrini, O.; Antonopoulos, A.S.; Oikonomou, E.; Padró, T.; et al. Perivascular adipose tissue as a source of therapeutic targets and clinical biomarkers. Eur. Heart J. 2023, 44, 3827–3844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.; Gu, H.; Chang, N.; Li, S.; Xu, T.; Liu, M.; Wang, X. Assessing Abdominal Aortic Aneurysm Progression by Using Perivascular Adipose Tissue Attenuation on Computed Tomography Angiography. Korean J. Radiol. 2023, 24, 974–982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Memon, A.A.; Zarrouk, M.; Ågren-Witteschus, S.; Sundquist, J.; Gottsäter, A.; Sundquist, K. Identification of novel diagnostic and prognostic biomarkers for abdominal aortic aneurysm. Eur. J. Prev. Cardiol. 2020, 27, 132–142. [Google Scholar] [CrossRef] [PubMed]


| All Patients (n = 97) | MFS Group (n = 54) | Control Group (n = 43) | p Values | |
|---|---|---|---|---|
| Demography and personal history | ||||
| Age, years, median SD | 43.4 (11) | 41.5 (13) | 45.9 (8) | 0.058 |
| Males, % | 40 (41) | 21 (39) | 19 (44) | 0.599 |
| Hypertension, % | 0 (0) | 0 (0) | 0 (0) | - |
| Diabetes, % | 0 (0) | 0 (0) | 0 (0) | - |
| Obesity, % | 0 (0) | 0 (0) | 0 (0) | - |
| Dyslipidemia, % | 0 (0) | 0 (0) | 0 (0) | - |
| Smoking habit, % | 0 (0) | 0 (0) | 0 (0) | - |
| CCS, % | 0 (0) | 0 (0) | 0 (0) | - |
| Chest computed Tomography | ||||
| AA diameter, mm, median SD | 33.2 (4.9) | 33.4 (3.9) | 33 (3.1) | 0.695 |
| PVAT in the AA [lower–upper quartile], HU | −72.6 [−74.8 to −70.9] | −70.6 [−72.6 to −68.5] | −75.1 [−77.1 to −73.1] | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuttolomondo, D.; Secchi, F.; Gaibazzi, N.; Udugampolage, N.S.; Pini, A.; De Filippo, M.; Spagnolo, P.; Caruso, R.; Taurino, J. Comparative Analysis of Perivascular Adipose Tissue Attenuation on Chest Computed Tomography Angiography in Patients with Marfan Syndrome: A Case–Control Study. Diagnostics 2025, 15, 673. https://doi.org/10.3390/diagnostics15060673
Tuttolomondo D, Secchi F, Gaibazzi N, Udugampolage NS, Pini A, De Filippo M, Spagnolo P, Caruso R, Taurino J. Comparative Analysis of Perivascular Adipose Tissue Attenuation on Chest Computed Tomography Angiography in Patients with Marfan Syndrome: A Case–Control Study. Diagnostics. 2025; 15(6):673. https://doi.org/10.3390/diagnostics15060673
Chicago/Turabian StyleTuttolomondo, Domenico, Francesco Secchi, Nicola Gaibazzi, Nathasha Samali Udugampolage, Alessandro Pini, Massimo De Filippo, Pietro Spagnolo, Rosario Caruso, and Jacopo Taurino. 2025. "Comparative Analysis of Perivascular Adipose Tissue Attenuation on Chest Computed Tomography Angiography in Patients with Marfan Syndrome: A Case–Control Study" Diagnostics 15, no. 6: 673. https://doi.org/10.3390/diagnostics15060673
APA StyleTuttolomondo, D., Secchi, F., Gaibazzi, N., Udugampolage, N. S., Pini, A., De Filippo, M., Spagnolo, P., Caruso, R., & Taurino, J. (2025). Comparative Analysis of Perivascular Adipose Tissue Attenuation on Chest Computed Tomography Angiography in Patients with Marfan Syndrome: A Case–Control Study. Diagnostics, 15(6), 673. https://doi.org/10.3390/diagnostics15060673







