A Novel Biplex Onchocerca volvulus Rapid Diagnostic Test Evaluated Among 3- to 9-Year-Old Children in Maridi, South Sudan
Abstract
1. Introduction
2. Materials and Methods
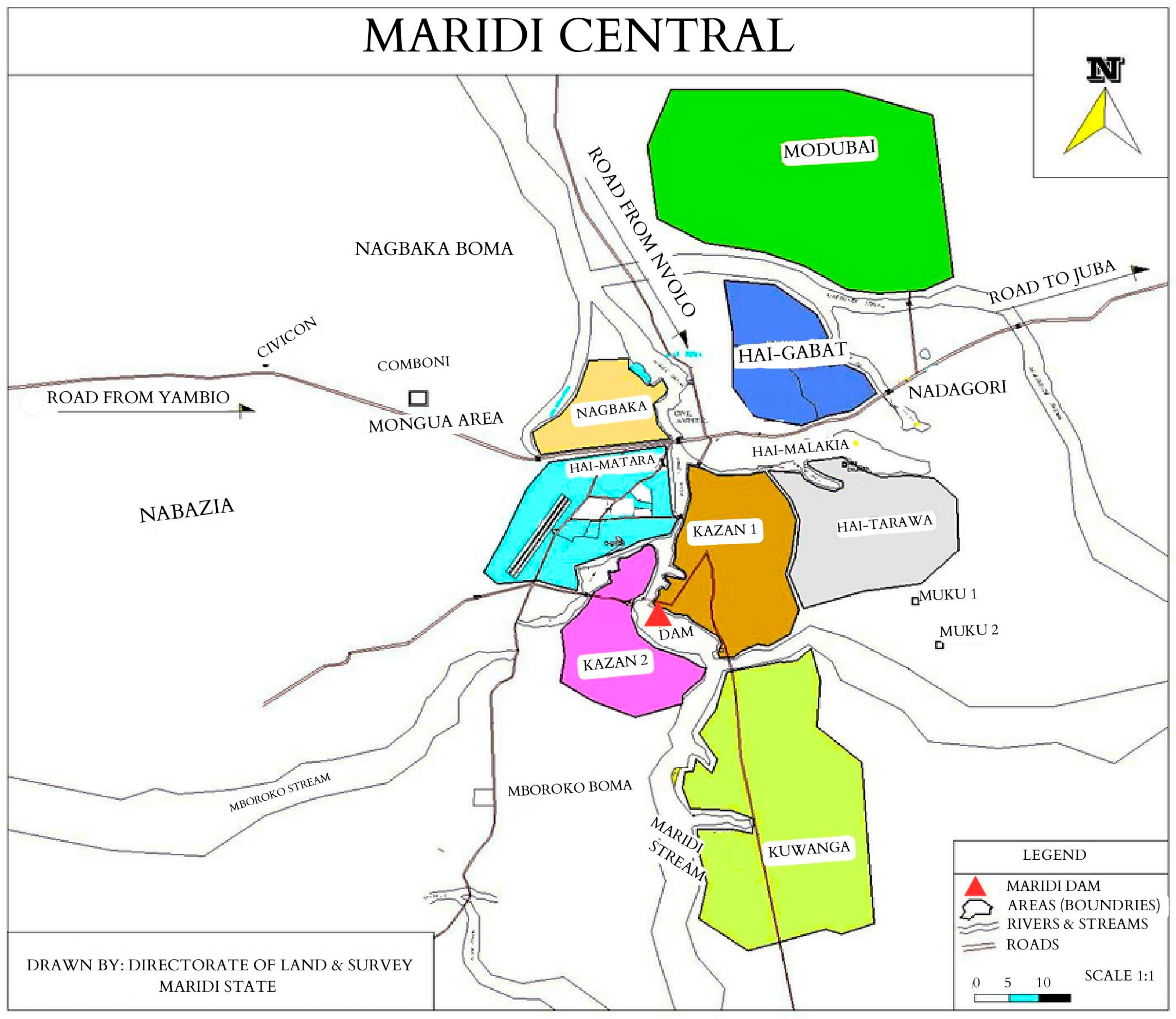
2.1. Study Setting
2.2. Study Procedures
2.3. Data Analysis
3. Results
3.1. Description of Study Participants
3.2. Comparison Between the Prototype DDTD Biplex A RDT and the Ov16 SD Bioline RDT Results
3.3. DDTD Biplex A RDT: Analysis of Test Lines
3.4. Effect of Timing on the Test Results
3.5. Feasibility and Acceptability of the Prototype DDTD Biplex A RDT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| CDTi | Community-directed treatment with ivermectin |
| DBS | Dried blood spot |
| DDTD | Drugs & Diagnostics for Tropical Diseases |
| ELISA | Enzyme-linked immunosorbent assay |
| HCW | Healthcare worker |
| LFA | Lateral flow assay |
| MDA | Mass drug administration |
| OEPA | Onchocerciasis Elimination Program for the Americas |
| O. volvulus | Onchocerca volvulus |
| RDT | Rapid diagnostic test |
| TPP | Target product profile |
| WHO | World Health Organization |
References
- Brattig, N.W.; Cheke, R.A.; Garms, R. Onchocerciasis (river blindness)–more than a century of research and control. Acta Trop. 2021, 218, 105677. [Google Scholar] [CrossRef] [PubMed]
- Hadermann, A.; Amaral, L.J.; Van Cutsem, G.; Siewe Fodjo, J.N.; Colebunders, R. Onchocerciasis-associated epilepsy: An update and future perspectives. Trends Parasitol. 2023, 39, 126–138. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Perneel, J.; Vieri, M.K.; Colebunders, R.; Kumar-Singh, S. The Secretome of Filarial Nematodes and Its Role in Host-Parasite Interactions and Pathogenicity in Onchocerciasis-Associated Epilepsy. Front. Cell Infect. Microbiol. 2021, 11, 662766. [Google Scholar] [CrossRef] [PubMed]
- Bennuru, S.; Cotton, J.A.; Ribeiro, J.M.; Grote, A.; Harsha, B.; Holroyd, N.; Mhashilkar, A.; Molina, D.M.; Randall, A.Z.; Shandling, A.D.; et al. Stage-Specific Transcriptome and Proteome Analyses of the Filarial Parasite Onchocerca volvulus and Its Wolbachia Endosymbiont. mBio 2016, 7, e02028-16. [Google Scholar] [CrossRef] [PubMed]
- Bennuru, S.; Oduro-Boateng, G.; Osigwe, C.; Del Valle, P.; Golden, A.; Ogawa, G.M.; Cama, V.; Lustigman, S.; Nutman, T.B. Integrating Multiple Biomarkers to Increase Sensitivity for the Detection of Onchocerca volvulus Infection. J. Infect. Dis. 2020, 221, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Weil, G.J.; Ogunrinade, A.F.; Chandrashekar, R.; Kale, O.O. IgG4 subclass antibody serology for onchocerciasis. J. Infect. Dis. 1990, 161, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Hotterbeekx, A.; Perneel, J.; Mandro, M.; Abhafule, G.; Siewe Fodjo, J.N.; Dusabimana, A.; Abrams, S.; Kumar-Singh, S.; Colebunders, R. Comparison of Diagnostic Tests for Onchocerca volvulus in the Democratic Republic of Congo. Pathogens 2020, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Ekanya, R.; Beng, A.A.; Anim, M.A.; Pangwoh, Y.Z.; Dibando, O.E.; Gandjui, N.V.T.; Awah, A.R.; Amambo, G.N.; Nchanji, G.T.; Ndzeshang, B.L.; et al. Concordance between Ov16 Rapid Diagnostic Test(RDT) and Ov16 Enzyme-Linked Immunosorbent Assay (ELISA) for the Diagnosis of Onchocerciasis in Areas of Contrasting Endemicity in Cameroon. Parasite Epidemiol. Control 2023, 21, e00290. [Google Scholar] [CrossRef]
- WHO. Onchocerciasis. Guidelines for Stopping Mass Drug Administration and Verifying Elimination of Human Onchocerciasis. Available online: https://www.who.int/publications/i/item/9789241510011 (accessed on 16 February 2025).
- WHO. Onchocerciasis: Diagnostic Target Product Profile to Support Preventive Chemotherapy. Available online: https://www.who.int/publications/i/item/9789240024496 (accessed on 16 February 2025).
- Biamonte, M.A.; Cantey, P.T.; Coulibaly, Y.I.; Gass, K.M.; Hamill, L.C.; Hanna, C.; Lammie, P.J.; Kamgno, J.; Nutman, T.B.; Oguttu, D.W.; et al. Onchocerciasis: Target product profiles of in vitro diagnostics to support onchocerciasis elimination mapping and mass drug administration stopping decisions. PLoS Negl. Trop. Dis. 2022, 16, e0010682. [Google Scholar] [CrossRef] [PubMed]
- Feeser, K.R.; Cama, V.; Priest, J.W.; Thiele, E.A.; Wiegand, R.E.; Lakwo, T.; Feleke, S.M.; Cantey, P.T. Characterizing Reactivity to Onchocerca volvulus Antigens in Multiplex Bead Assays. Am. J. Trop. Med. Hyg. 2017, 97, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Cama, V.A.; McDonald, C.; Arcury-Quandt, A.; Eberhard, M.; Jenks, M.H.; Smith, J.; Feleke, S.M.; Abanyie, F.; Thomson, L.; Wiegand, R.E.; et al. Evaluation of an OV-16 IgG4 Enzyme-Linked Immunosorbent Assay in Humans and Its Application to Determine the Dynamics of Antibody Responses in a Non-Human Primate Model of Onchocerca volvulus Infection. Am. J. Trop. Med. Hyg. 2018, 99, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, M.L.; Dickerson, J.W.; Tsang, V.C.; Walker, E.M.; Ottesen, E.A.; Chandrashekar, R.; Weil, G.J.; Trpis, M.; Strobert, E.; Constantinidis, I.; et al. Onchocerca volvulus: Parasitologic and serologic responses in experimentally infected chimpanzees and mangabey monkeys. Exp. Parasitol. 1995, 80, 454–462. [Google Scholar] [CrossRef]
- South Sudan–County Population Estimates–2015–2020–Humanitarian Data Exchange. Available online: https://data.humdata.org/dataset/south-sudan-county-population-estimates-2015-2020 (accessed on 11 March 2023).
- Lakwo, T.L.; Raimon, S.; Tionga, M.; Siewe Fodjo, J.N.; Alinda, P.; Sebit, W.J.; Carter, J.Y.; Colebunders, R. The Role of the Maridi Dam in Causing an Onchocerciasis-Associated Epilepsy Epidemic in Maridi, South Sudan: An Epidemiological, Sociological, and Entomological Study. Pathogens 2020, 9, 315. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, E.; Mandro, M.; Mukendi, D.; Suykerbuyk, P.; Dolo, H.; Wonya’Rossi, D.; Ngave, F.; Ensoy-Musoro, C.; Laudisoit, A.; Hotterbeekx, A.; et al. High prevalence of epilepsy in onchocerciasis endemic health areas in Democratic Republic of the Congo. Infect. Dis. Poverty 2018, 7, 68. [Google Scholar] [CrossRef]
- Tumwine, J.K.; Vandemaele, K.; Chungong, S.; Richer, M.; Anker, M.; Ayana, Y.; Opoka, M.L.; Klaucke, D.N.; Quarello, A.; Spencer, P.S. Clinical and epidemiologic characteristics of nodding syndrome in Mundri County, southern Sudan. Afr. Health Sci. 2012, 12, 242–248. [Google Scholar] [CrossRef]
- Colebunders, R.; Carter, J.Y.; Olore, P.C.; Puok, K.; Bhattacharyya, S.; Menon, S.; Abd-Elfarag, G.; Ojok, M.; Ensoy-Musoro, C.; Lako, R.; et al. High prevalence of onchocerciasis-associated epilepsy in villages in Maridi County, Republic of South Sudan: A community-based survey. Seizure 2018, 63, 93–101. [Google Scholar]

| Factor | Level | Ov16 SD Bioline RDT Prevalence of Positive Test Result (%; 95%CI) | DDTD Biplex A RDT Prevalence of Positive T1 and T2 Lines Test Result (%; 95%CI) |
|---|---|---|---|
| Village | Hai-Gabat | 2/50 (4.0; 0.7–14.9) | 0/50 (0; 0–8.9) |
| Kazana 1 | 22/50 (44.0; 30.3–58.7) | 11/50 (22.0; 12.0–36.3) | |
| Kazana 2 | 20/38 (52.6; 36.0–68.7) | 14/38 (36.8; 22.3–54.0) | |
| Hai-Matara | 17/51 (33.3; 21.2–48.0) | 9/51 (17.7; 8.9–31.4) | |
| Hai-Tarawa | 11/50 (22.0; 12.0–36.3) | 3/50 (6.0; 1.6–17.5) | |
| Sex | Female | 40/129 (31.0; 23.3–39.8) | 21/129 (16.3; 10.6–24.0) |
| Male | 32/110 (29.1; 21.0–38.7) | 16/110 (14.6; 8.8–22.9) | |
| Ivermectin Intake (2022) | No | 46/165 (27.9; 21.3–35.5) | 27/165 (16.4; 11.2–23.1) |
| Yes | 26/74 (35.1; 24.6–47.2) | 10/74 (13.5; 7.0–23.9) | |
| Dermatitis | No | 34/151 (22.5; 16.3–30.2) | 19/151 (12.6; 7.9–19.2) |
| Yes | 37/87 (42.5; 32.1–53.6) | 18/87 (20.7; 13.0–31.0) | |
| Epilepsy | No | 72/235 (30.6; 24.9–37.0) | 37/235 (15.7; 11.5–21.2) |
| Yes | 0/4 (0; 0–60.4) | 0/4 (0; 0–60.4) | |
| Age group (years) | Age 3–6 | 46/147 (31.3; 24.0–39.5) | 18/147 (12.2; 7.6–18.9) |
| Age 7–9 | 26/92 (28.3; 19.6–38.8) | 19/92 (20.7; 13.2–30.6) | |
| Total | 72/239 (30.1; 24.5-36.4) | 37/239 (15.5; 11.3–20.8) | |
| DDTD Biplex A Positivity Assumption | Seroprevalence (%; 95%CI) |
|---|---|
| Seroprevalence T1 and T2 | 37/239 (15.5; 11.3–20.8) |
| Seroprevalence T1 or T2 | 91/239 (38.1; 31.9–44.6) |
| Seroprevalence All T1 | 84/239 (35.2; 29.2–41.6) |
| Seroprevalence All T2 | 44/239 (18.4; 13.8–24.0) |
| Seroprevalence Only T1 | 47/239 (19.7; 14.9–25.4) |
| Seroprevalence Only T2 | 7/239 (2.9; 1.3–6.2) |
| DDTD Biplex A RDT | ||||||
|---|---|---|---|---|---|---|
| No line | Only T1 | Only T2 | T1 and T2 | TOTAL | ||
| Ov16 SD Bioline RDT | Negative | 147 | 7 | 7 | 6 | 167 |
| Positive | 1 | 40 | 0 | 31 | 72 | |
| TOTAL | 148 | 47 | 7 | 37 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadermann, A.; Jada, S.R.; Lubbers, C.; Amaral, L.-J.; Biamonte, M.; de Souza, D.K.; Bol, Y.Y.; Siewe Fodjo, J.N.; Colebunders, R. A Novel Biplex Onchocerca volvulus Rapid Diagnostic Test Evaluated Among 3- to 9-Year-Old Children in Maridi, South Sudan. Diagnostics 2025, 15, 563. https://doi.org/10.3390/diagnostics15050563
Hadermann A, Jada SR, Lubbers C, Amaral L-J, Biamonte M, de Souza DK, Bol YY, Siewe Fodjo JN, Colebunders R. A Novel Biplex Onchocerca volvulus Rapid Diagnostic Test Evaluated Among 3- to 9-Year-Old Children in Maridi, South Sudan. Diagnostics. 2025; 15(5):563. https://doi.org/10.3390/diagnostics15050563
Chicago/Turabian StyleHadermann, Amber, Stephen Raimon Jada, Charlotte Lubbers, Luís-Jorge Amaral, Marco Biamonte, Dziedzom Komi de Souza, Yak Yak Bol, Joseph Nelson Siewe Fodjo, and Robert Colebunders. 2025. "A Novel Biplex Onchocerca volvulus Rapid Diagnostic Test Evaluated Among 3- to 9-Year-Old Children in Maridi, South Sudan" Diagnostics 15, no. 5: 563. https://doi.org/10.3390/diagnostics15050563
APA StyleHadermann, A., Jada, S. R., Lubbers, C., Amaral, L.-J., Biamonte, M., de Souza, D. K., Bol, Y. Y., Siewe Fodjo, J. N., & Colebunders, R. (2025). A Novel Biplex Onchocerca volvulus Rapid Diagnostic Test Evaluated Among 3- to 9-Year-Old Children in Maridi, South Sudan. Diagnostics, 15(5), 563. https://doi.org/10.3390/diagnostics15050563







