Abstract
Introduction: Liposclerosing myxofibrous tumors (LSMFTs) have been described as an infrequent and peculiar fibrous dysplasia variant with a predilection for the intertrochanteric femoral region and are not globally considered a distinct tumor. Given their features, they may be confused with a variety of entities. Our aim is to analyze the clinical, radiological, histopathological and molecular features of LSMFTs. Material and Methods: We report 15 new LSMFT cases managed in our tertiary referral hospital and compare our findings with those of the 241 previous LSMFT cases published in the English medical literature. Results: In plain radiography and computerized tomography, LSMFTs are well-defined intraosseous lytic masses with peripheral sclerotic rims and variable amounts of internal calcifications. Histopathologically, LSFMTs consist of variable amounts of spindle cells, bone matrix, adipose tissue, and cystic spaces embedded in a predominantly fibromyxoid stroma. Molecular tests reveal GNAS and TP53 mutations. Conclusions: Knowledge of LSMFT and its typical radiological appearance with heterogeneous histopathological findings—especially in small biopsies—are key to preventing the misdiagnosis and overtreatment of affected patients.
1. Introduction
Liposclerosing myxofibrous tumors (LSMFTs) are known as a benign fibro-osseous lesion with a predilection for the femoral intertrochanteric region [1,2]. They typically affect adults during the fourth decade of life [1]. They have distinctive radiological features [2] and a variety of histopathological findings [1] and may present malignant transformation in some cases [3,4,5]. LSMFT is not globally considered a distinct tumor and is a controversial entity [1,6,7,8]. In the latest WHO classification of Bone and Soft Tissue Tumors (2020), LSMFTs do not have their own chapter and are only mentioned in the fibrous dysplasia chapter [8]. The objective of this article is to present the clinical, radiological, histopathological, and molecular features of LSMFTs; describe their management; and present a review of the related literature.
2. Materials and Methods
La Paz University Hospital is a tertiary hospital and one of the referral hospitals for bone and soft tissue tumor management in Spain. Patients with histological diagnosis of fibrous dysplasia or bone infarct of proximal femur, as well as LSFMT of any other bone location during the period from January 1966 to December 2024, were included in this retrospective study. Clinical information and radiological images were obtained from their medical records. All available materials from the pathology files were reviewed, including hematoxylin- and eosin-stained slides, as well as immunohistochemical stains. Tissues were fixed in 10% formalin (24–48 h), embedded in paraffin, and serially sectioned (5 μm thick). Slides were individually evaluated by two expert pathologists in the field, and the final diagnoses were in agreement.
Molecular testing in the most recent non-decalcified cases was completed retrospectively via Next-Generation Sequencing (NGS). Genomic DNA was extracted from fixed and paraffin-embedded tumor tissue (FFPE), according to the manufacturer’s instructions (Qiagen, Hilden, Germany), using the Gene Read DNA FFPE KIT. The quantity and quality of DNA were assessed using a NanoDrop One spectrophotometer (Thermo Scientific, Waltham, MA, USA) and a Qubit 3.0 fluorometer (Thermo Fisher, Waltham, MA, USA). Hybrid-captured targeted libraries were prepared from 50 ng of genomic DNA with a Sophia Custom Solid Tumor Solution gene panel (Sophia Genetics, Lausanne, Switzerland), according to the manufacturer’s protocol. This panel includes relevant regions of 50 genes involved in solid tumor development. Libraries were denatured and paired-end (2 × 150 bp) sequenced on the Illumina MiSeq instrument (Illumina, San Diego, CA, USA). We used the Sophia DDM platform (SOPHIA GENETICS, Lausanne, Switzerland) to analyze single nucleotide variants and small insertions and deletions. FASTQ files were uploaded to the data portal and aligned with the human reference genome (GRCh37/hg19). After annotation in DDM, non-synonymous variants located in exonic or ±1.2 intronic splice regions were retained, and variants with a minor allele frequency < 0.01 (based on ExAC, GnomAD, and 1000 Genomes databases) were selected for the downstream analysis. At present, there is no standardized method to establish the best cut-off point for variant allele frequency. In this sense, we decided to set the percentage at 5% in the FFPE, as there was a high percentage of tumor in these samples, in an attempt to avoid false positives. We used an Integrative Genomics Viewer (Broad Institute, San Diego, CA, USA) to visualize the variants aligned against the reference genome in order to confirm the accuracy of the variant calls by checking for possible strand biases and sequencing errors. Identified variants were annotated using population databases (The Exome Aggregation Consortium (ExAC) and 1000 Genomes) and specific databases (Ensembl; PubMed; ClinVar; Catalogue of Somatic Mutations in Cancer, COSMIC; and The Cancer Genome Atlas, TCGA). Pathogenicity prediction was established with in silico prediction software: Sorting Intolerant from Tolerant (SIFT), Polyphen 2.0, and Mutation Taster (V6.8.0, Sophia DDM platform). Finally, the pathogenicity of variants was designated according to the recommendations of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [9].
Previous medical literature case reports containing the terms “liposclerosing myxofibrous tumor” and “polymorphic fibro-osseous lesions of bone” in the PubMed database were reviewed and analyzed. Finally, the findings of the cases from our hospital and those described in the literature were compared.
3. Results
A total of 12 LSMFTs, 5 bone infarcts, and 26 fibrous dysplasia cases were found in our files. One of the LSMFT cases was excluded, given that it was an external consultation case, and no radiological information was available. Four fibrous dysplasia cases were morphologically re-classified to LSMFT upon histopathological evaluation. No bone infarct cases were re-classified. As a result, 15 cases of LSMFT were finally included in this study (Table 1). Ethics committee approval was not required, as this was a retrospective observational study. Our study population included eight males and seven females (ratio 1:0.9) aged 18 to 72 (mean age of 38). The clinical presentation consisted of local pain in seven cases, pathological fracture in two cases, and incidental detection in six patients. Prior local trauma history was registered in one case. All the tumors appeared in the femur, with 14 cases occurring in the intertrochanteric region and 1 in the distal diaphysis. In all cases, a single lesion was detected: eight on the right side and seven on the left.

Table 1.
Main characteristics of our LSFMT cases.
Plain radiography and computerized tomography (CT) images were available in nine cases. All of these cases presented a similar image, consisting of a well-defined intraosseous lytic mass with a peripheral sclerotic rim. Gross internal calcification was evident in three cases. In two cases, cystic areas with internal trabeculae were observed. No ground-glass matrix was detected. No periosteal reaction, infiltrative pattern, or soft tissue extension were seen. Magnetic resonance images (MRIs) were available in six cases. These images revealed intramedullary lesions, isointense or hypointense to skeletal muscle on T1 weighted images (WI) and hyperintense on T2WI. Peripheral fat areas were found in three lesions. Single-photon emission computed tomography (SPECT) was performed in three cases, displaying variable uptake. In one instance, no uptake was observed; in another, peripheral uptake was noted, and, in another case, moderate and uniform uptake was seen. The considered radiological differential diagnosis included fibrous dysplasia, LSMFT, intraosseous lipoma, aneurysmal bone cyst (ABC), unicameral bone cyst, enchondroma, osteoblastoma, intraosseous ganglion cyst, and chondromyxoid fibroma. Exemplary radiological findings are shown in Figure 1.
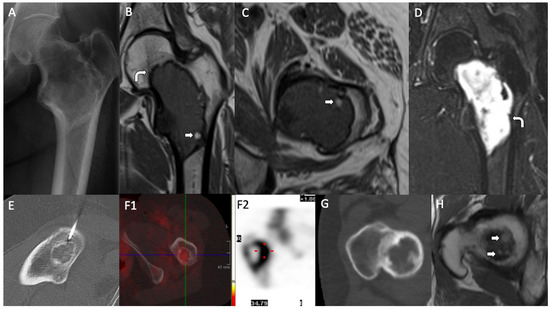
Figure 1.
Radiological findings. LSMFTs of three patients. (A–D): (A) Anteroposterior radiography: geographic lucent lesion with a sclerotic margin and foci of matrix calcification, centered in the intertrochanteric region. (B) MIR, coronal and (C) MRI, axial T1WI: central medullary lesion that elicits low signal and peripheral hyperintense foci compatible with fat (white arrows). (D) MRI, coronal T2WI: high signal. Low-signal sclerotic rim, hypointense on T1WI and T2WI (curve arrows). (E,F): (E) Axial image, CT-guided biopsy: lytic lesion with internal calcified foci. (F1) (SPECT) and (F2) (bone scintigraphy): moderate and heterogeneous uptake of the lesion (red dot: intense uptake area). (G,H): (G) Axial CT: lytic lesion with a sclerotic rim. (H) MRI, axial T1WI: small foci of T1 high signal at the superior aspect of the lesion, suggestive of fat (white arrows).
Intralesional resection (curettage) and reconstruction with bone grafting were the selected treatments in 14 cases, with reinforcement via osteosynthesis with a nail plate in 3 of these cases. Wait-and-see management was decided for one patient, due to her poor general condition. The median size of the resected fragments received in the Pathology Department measured 4.4 cm. A pre-surgical percutaneous echo-guided biopsy was performed in two cases with non-typical radiological findings. Histologically, the analyzed tissue was polymorphic. The fibromyxoid stroma was the predominant component in thirteen cases, being more myxoid in two of them. The stroma was loose in one case and more fibrous in another. Dense collagen bands were observed in one case. Variable dense and scarce cellular areas were present in all cases. They consisted of stellated or spindled cells with scant cytoplasm and round nuclei without nucleoli. These cells displayed focal immunoreactivity to smooth muscle actin (Clone 1A4, RTU, Agilent-Dako) and SATB2 (Clone EP281, RTU, Agilent-Dako (Agilent, Frederick, Colorado)), consistent with a bone-forming tumor, with no CD34 (Clone QBEnd-10, RTU, Agilent-Dako) and CD68 (Clone, PG-M1 RTU, Agilent-Dako) positivity. No atypical or pleomorphic cells were observed, and no giant osteoclast-like cells were seen. In two cases, woven bone with no osteoblastic rimming was the predominant component. Newly formed immature woven trabeculae were present in eight cases, pseudopagetoid bone (woven bone with irregular mosaic lines of calcification) was observed in two cases, and a psammomatoid pattern appeared in seven cases. More than one pattern was present in two cases. No cartilaginous areas were seen in our series. Adipose tissue was present in seven cases, found in the periphery in one case and intermixed with other components in the rest of the cases. Two of our three fat signal-detected tumors on MRI were histologically confirmed as adipose tissue. Xantomized cells were detected in seven cases. Microcystic irregular spaces “lymphangioma-like” were observed in six cases and macrocystic spaces in four cases. One case presented ABC-like areas. Macrocystic spaces and ABC-like changes were found in two of our cases where internal septation was detected radiologically. Small hyalinized vessels were seen in 11 cases and large hyalinized vessels in 1 case. Lymphoplasmacytic inflammatory cells were found in seven cases, either forming small groups or dispersed as isolated cells, one with a perivascular pattern. Hemosiderophages were found in two cases. Ischemic necrosis was present in two cases and, in one case, there were bone infarct areas. No malignant transformation was registered in our series. Exemplary histopathological findings are shown in Figure 2 and Figure 3.
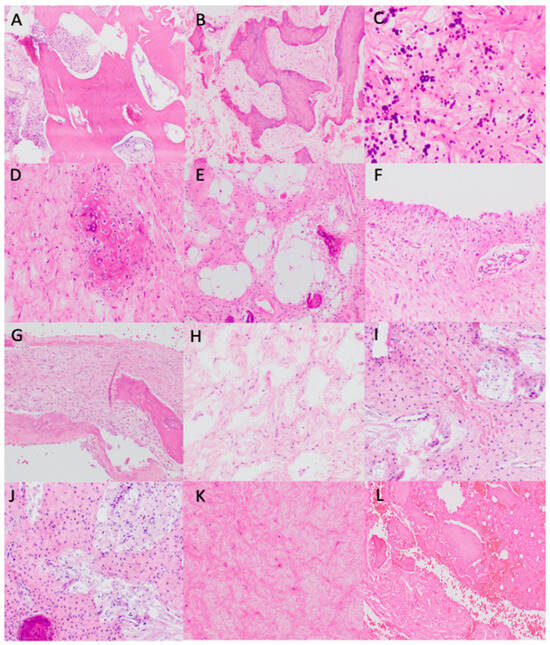
Figure 2.
Histopathological findings. (A) Sclerotic bone trabeculae (H&E, ×100). (B) Pseudopagetoid bone (H&E, ×100). (C) Psammomatoid bone (H&E, ×400). (D) Immature bone closely related to non-atypical spindle cell in a fibromyxoid stroma (H&E, ×200). (E) Adipose tissue intermixed with other components, especially bone-related (H&E, ×200). (F) Macrocystic wall without endothelial lining (H&E, ×200). (G) ABC-like changes: parallel bone trabeculae to the pseudovascular spaces without endothelial lining (H&E, ×200). (H) Microcystic spaces. (I) Dense collagen bands in a fibromyxoid stroma (H&E, ×200). (J) Xantomized cells intermixed with fibromyxoid stroma and bone (H&E, ×200). (K) Hyalinized and fibromyxoid stroma (H&E, ×200). (L) Bone infarct area (H&E, ×200).
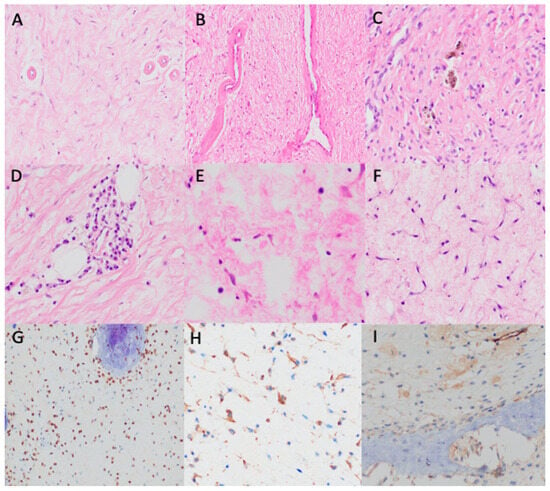
Figure 3.
Histopathological findings. (A,B) Hyalinized vessels (H&E, ×200). (C) Hemosiderophages (H&E, ×400). (D) Perivascular lymphoplasmacytic infiltrate (H&E, ×400). (E) Scattered mast cells (H&E, ×400). (F) Stellated and spindled non-atypical cells with scanty cytoplasm and round nuclei (H&E, ×400). (G) SATB2 positivity (×400). (H) SMA patchy positivity (×400). (I) Negative CD34 (×400).
Targeted next-generation sequencing was performed on the tumors of two patients, which revealed the presence of a pathogenic missense somatic variant in the GNAS oncogene (NM_080425.3) in hotspot position c.2530C > T, p.Arg844Cys (Location 20:57484420, COSM27887; also described as c.601C > T, p.Arg201Cys in the transcript NM_000516.7), with a variant allele frequency of 26% (average coverage in the sample 2556×) and a pathogenic missense variant in the TP53 tumor suppressor gene (NM_000546.5): c.520A > T, p.Arg174Trp (Location 17:7578410, COSM44782) with a variant allele frequency of 64% (average coverage in the sample 2484×).
The median follow-up spanned 176.4 months (13–312 months). There was no evidence of local recurrence or metastases. Three patients died from other causes (natural death, ovarian tumor, and multi-organ failure). No malignant transformation was detected.
4. Discussion
LSMFT was first described by Ragsdale et al. in 1986 [10]. Since then, 241 cases have been reported in the English language literature [2,3,4,5,6,7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. LSMFT was not mentioned in the third edition (2002) of the WHO [29] classification of Bone and Soft Tissue Tumors. In the fourth edition (2013), it appears as an osteosarcoma-associated condition, similarly to fibrous dysplasia or bone infarct [30]. In the fifth edition (2020), it does not have its own chapter and is only mentioned as a non-recommended related terminology to fibrous dysplasia [8].
The main characteristics of the previous published cases are summarized in Table 2. The published cases refer to 56 males and 59 females (ratio 1:1.05) with a mean age of 45.8 (from 14 to 84 years old), from the studies where this information was available [2,3,4,5,6,7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. In our series, there was a slight predominance in females and a younger mean age.

Table 2.
Summarized characteristics of previous published cases of LSFMT.
It was incidentally detected in 47 of the previous published cases [2,3,4,5,6,7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28], with 39 patients initially referred due to local pain and 11 having a pathological fracture. Three patients were referred due to prior local traumatism [15,20]. Most of the cases (149) appeared in the femur, 94.5% of which were located in the intertrochanteric region of the femur [2,3,4,5,6,7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Only four cases were in the distal femur [10,20,24]. Other affected bones included the humerus [2,10] (6), tibia [10,20,26] (4), iliac bone [2,12,20] (5), rib [2] (1), and cranial vault [10,22] (3). All of the cases were solitary lesions (54% of them on the left side), except for two that presented multiple bone lesions [4,14]. In our series, all cases were located in the femur.
In previously reported LSMFTs, plain radiography and CT displayed well-defined, lytic, expansile, and geographic lesions with a sclerotic rim. Internal calcifications were seen in six cases and internal septations in two cases [2,16,17,18,22,23,25,27]. Ground-glass areas appeared in 20 cases [2,3,4,5,6,7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Cystic lesion was observed in one case [6]. Scalloped endosteum was evidenced in two cases [6,15]. There were heterogeneous well-defined masses, hypointense to the skeletal muscle on T1WI and hyperintense on T2WI. They showed heterogeneous enhancement after paramagnetic contrast administration. No distinct fatty components were seen. PET demonstrated intense uptake in the cases where it was performed [16,19,25]. The radiological findings of LSMFT in our patient cohort presented similarities, when compared to previously reported cases. As in prior reports, our plain radiography and CT findings showed LSMFT’s characteristic well-defined lytic, expansile, and geographic lesions with a sclerotic rim. These lesions often presented internal septations and areas of calcification, which were also observed in previous studies. Our MRI findings also presented parallels with those from the medical literature. A fatty component was seen in two cases, as typically described in the literature [2].
The previously reported LSMFTs were histologically heterogeneous lesions, with different components, including bone, adipose tissue, xantomized cells, fibromyxoid stroma, and cyst formations [2,3,4,5,6,7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Fibromyxoid stroma was present in all cases, except in three (in which it was fibrous or collagenoid). Woven bone with pagetoid, curvilinear, and trabecular (fibrous dysplasia-like) patterns was mostly present [2,3,4,5,6,7,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Fat necrosis was evident in four cases [6,18,24]. Dystrophic calcifications were observed in three cases [13,18,22]. ABC-like areas were found in one case [6]. Scattered lymphocytes were described in one case [15]. Our cases also presented similar histopathological findings as those described in the literature. The most frequent component of our series was a fibromyxoid stroma followed by woven bone and adipose tissue. All components were intermixed. Histological components not previously reported in the literature included microcystic spaces, hyalinized stroma, small hyalinized vessels, and the presence of hemosiderophages and mast cells. Macrocystic spaces and ABC-like changes were found in two of our cases where internal septation was radiologically detected. In two of our three tumors with fat signal on MRI, the adipose tissue was histologically confirmed.
The immunohistochemical profile of LSMFT has not been previously reported in the medical literature. In our series, consistent with a bone-forming tumor, cells adjacent to woven bone—as well as those distanced from it—revealed SATB2 positivity. Smooth muscle actin was positive in a patchy manner with CD34 negative immunoreactivity. GNAS p.Arg844 pathogenic variants have been only studied in four of the published cases, being present only in three of them [12,14].
Malignant transformation occurred in 24 of the published cases (9.9%) [2,3,4,5,10,11,20], mainly to osteosarcoma or high-grade sarcoma. It either existed at the time of diagnosis or developed during the follow-up period. No molecular studies were performed in any of these cases. In cases with a malignant component at initial presentation, a cystic osteosarcoma with fibromyxoid areas should be ruled out.
Curettage and bone grafting were the treatments of choice in most published cases, as in our series. Conservative management was preferable in two published cases, and a transtibial amputation and hemiarthroplasty were performed in two cases with malignant transformation [4,20]. The median follow-up time of the non-malignant published cases was 29.3 months, and no evidence of relapse was seen. Two cases with malignant transformation died due to metastases. No malignant transformation nor local relapses occurred in our series.
LSMFT presents a diagnostic challenge due to its clinical, radiological, and histopathological features overlapping with those of several bone tumors. The most subtle differential diagnosis should be made with conventional fibrous dysplasia, as they present overlapping radiological and pathological features. Monostotic fibrous dysplasia is a benign tumor that affects children and young- and middle-aged adults [18]. They are not usually painful. They arise in meta-diaphysis of long bones or the cranio-facial area and are lytic and geographic non-aggressive lesions with a “ground-glass” appearance on radiological images but lack the fatty and myxoid components of LSMFTs [18]. They may also present a sclerotic rim.
Histologically, fibrous dysplasia consists of a fibrous stroma with bland fibroblastic cells and irregular woven bone trabeculae lacking conspicuous osteoblastic rimming [8]. The percentage of the bone component of reported LSMFT cases is not clearly described. Bone areas were present in less than 10% of the tumor in more than half of our cases. Diagnostic difficulty could arise if the heterogenicity of LSMFT is not known. On rare occasions, fibrous dysplasia may contain multi-nucleated osteoclast-like giant cells, myxoid areas, metaplastic fat areas, or xantomized cells. No small hyalinized vessels have been described in fibrous dysplasia [8]. Some authors have proposed that LSMFT may be a traumatized fibrous dysplasia variant based on the fact that the proximal femur is more susceptible to fatigue fracture [6]. While this is an interesting point of view, prior local traumatism was present only in 0.02% of all cases, which leads us to believe that it is probably a non-related condition. Four of our cases were initially diagnosed as fibrous dysplasia and re-classified as LSMFT based on the presence of any of the following: little bone areas, large adipocytic areas, large fibromyxoid areas, and/or small hyalinized vessels.
Fibrous dysplasia has been shown to harbor a missense mutation at codon 201 in exon 8 of GNAS [12]. GNAS p.Arg844 pathogenic variants have been previously described in LSMFT [12,14], but GNAS mutations have been studied only in 0.02% of all LSMFT cases. It is well known that the same genetic mutation may present different phenotypes. GNAS-activating mutations have been identified across many cancer types, such as pancreatic adenocarcinoma, squamous cell carcinoma of the head and neck, breast neoplasm, gastric adenocarcinoma, adrenal cortex carcinoma, and sex cord-stromal tumor, as well as in fibrous dysplasia and LSMFT. Nevertheless, the precise role of this mutation in tumor development or in malignant transformation in LSMFT cases remains unknown. The GNAS oncogene, which is known to be implicated in cell proliferation and tumoral invasiveness, could be considered as a new prognostic factor for these tumors. TP53—which encodes tumor suppressor p53—is the most frequently mutated gene in human cancers. TP53 mutations are also potentially prognostic and predictive markers, as well as targets for pharmacological intervention [31]. To date, however, no variants in this gene have been described in LSMFT, and no TP53 mutations have been described in fibrous dysplasia [8]. Molecular studies in large cohorts of patients are required to elucidate the roles of these two genes in LSMFT.
Other benign tumors are mistaken in the differential diagnosis of LSMFT due to its polymorphic appearance, although most of these tumors do not usually show radiological similarities. Chondromyxoid fibromas are benign tumors that can affect any bone of young adults [7]. They are eccentric, well-defined lytic lesions that may contain small calcifications on radiological studies. They consist of lobules with central chondromyxoid areas and peripheric multi-nucleated osteoclast-like giant cells. GMR1 mutations have been found in this type of tumor. Enchondromas are benign chondrogenic tumors that arise at any age in the meta-diaphysis of short and long tubular bones [18]. They are well-defined radiolucent and lytic neoplasms with central punctate “popcorn” calcifications. They do not, however, present the mixed lytic and sclerotic pattern of LSMFTs [18]. They are histologically composed of small and separated hypocellular hyaline cartilage nodules with a metaplastic bone rim and small chondrocytes in their lacunae [18]. Cartilage areas are exceptional in LSMFT, having only been described in a case series [28].
Osteoblastomas are locally aggressive bone formation tumors that tend to develop over the second and third decades of life [20]. Although any bone may be affected, they typically arise in posterior vertebrae elements. They measure over 2 cm and are clearly lytic masses with reactive peripheral sclerosis and central mineralization [20]. Anastomosing trabecular rimmed by osteoblasts and scattered multi-nucleated osteoclast-like giant cells are commonly histologically observed [20], and FOS translocations are usually present. Intraosseous lipomas may appear at any age and in any bone [2]. They are lytic masses with a sclerotic rim and evidence of homogeneous macroscopic fat through CT or MRI [1]. They consist of mature adipocytic tissue, and areas of fat necrosis may be present [1]. Bone infarcts may appear at any age and in any bone [18]. They are lytic lesions with central lucency surrounded by sclerosis with a serpiginous border imitating “smoke up the chimney” [18]. Necrotic bone areas, dystrophic calcifications, fat necrosis, or hyaline fibrous tissue may be histologically seen [18], as in LSMFT.
Other cystic bone lesions should be considered in those LSMFT cases with an evident cystic component. Simple bone cysts appear during the first decade of life. They may be asymptomatic or can cause pathological fractures [18]. They are more frequently found in humeral and femoral meta-diaphysis [18]. They are well-defined lytic masses with cortical thinning and a sclerotic rim. The “fallen fragment” radiological characteristic sign is seen in this lesion [18]. It consists of thin, unicystic walls filled with clear, serous liquid [18]. ABCs are usually eccentrically located expansile lesions with a multi-chamber appearance, with multiple septations and low signals on T1 and T2 that demarcate spaces with fluid–fluid levels and demonstrate enhancement after contrast administration, resulting in a “honeycomb appearance”, in contrast with LSMFTs [2]. In histological terms, they are blood-filled cystic spaces without an endothelial lining separated by thin septa. The fibrous septa contain a dense proliferation of fibroblasts, scattered multi-nucleated osteoclast-type giant cells, and strands of woven bone rimmed with osteoblasts [32]. Intraosseous ganglion cysts are usually found around the ankle [6]. Radiologically, they are lytic, multi-loculated lesions with a sclerotic rim and a fluid-like appearance, with fibrous tissue with no epithelial cell lining and translucent gelatinous material seen upon pathological examination.
5. Conclusions
LSMFTs should be included in the differential diagnosis of solitary bone lesions in the femoral intertrochanteric region. It is important to recognize typical radiological findings and heterogeneous histopathological components, especially in small biopsies, in order to avoid the misdiagnosis or overtreatment of patients. Keeping in mind that up to approximately 10% of the considered cases had a reported malignant transformation is key to providing accurate monitoring of potentially affected patients.
Author Contributions
Conception and design of the study and acquisition and analysis of data: E.M.P.-B. and J.J.P.-K. Radiology images: G.S.d.C. and M.T.-V. Pathology figures: E.M.P.-B. and J.J.P.-K. Molecular studies: J.S.-G. and I.B. Drafting the manuscript: E.M.P.-B., G.S.d.C., M.T.-V., J.S.-G., I.B., E.O.-C. and J.J.P.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This is a retrospective observational study which did not involve performing complementary procedures directly on the patients so Ethics Committee approval was not necessary.
Informed Consent Statement
Verbal informed consent has been obtained from patients or families to publish this paper.
Data Availability Statement
Research data are available upon reasonable request to the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deel, C.; Hassell, L. Liposclerosing Myxofibrous Tumor: A Review. Arch. Pathol. Lab. Med. 2016, 140, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Kransdorf, M.J.; Murphey, M.D.; Sweet, D.E. Liposclerosing myxofibrous tumor: A radiologic-pathologic-distinct fibro-osseous lesion of bone with a marked predilection for the intertrochanteric region of the femur. Radiology 1999, 212, 693–698. [Google Scholar] [CrossRef]
- Campbell, K.; Wodajo, F. Two-step Malignant Transformation of a Liposclerosing Myxofibrous Tumor of Bone. Clin. Orthop. 2008, 466, 2873–2877. [Google Scholar] [CrossRef] [PubMed]
- Bahk, W.J.; Seo, K.J. Malignant transformation of liposclerosing myxofibrous tumour. Pathology 2021, 53, 660–663. [Google Scholar] [CrossRef]
- Illac, C.; Delisle, M.B.; Bonnevialle, P.; Chiavassa-Gandois, H.; de Pinieux, G.; Gomez-Brouchet, A. Telangiectatic osteosarcoma secondary to a liposclerosing myxofibrous tumor: A case report. Ann. Pathol. 2012, 32, 259–262. [Google Scholar] [CrossRef]
- Heim-Hall, J.M.; Williams, R.P. Liposclerosing myxofibrous tumour: A traumatized variant of fibrous dysplasia? Report of four cases and review of the literature. Histopathology 2004, 45, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, J.; McCarthy, E.F. Liposclerosing myxofibrous tumor (LSMFT), a study of 33 patients: Should it be a distinct entity? Iowa Orthop. J. 2012, 32, 35–39. [Google Scholar]
- Siegal, G.; Bloem, J.; Cates, J.; Hameed, M.; WHO Classification of Tumours Editorial Board. Fibrous Dysplasia. In Soft Tissue and Bone Tumours, 5th ed.; IARC Press: Geneva, Switzerland, 2020; pp. 472–474. [Google Scholar]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, B.D.; Sweet, D.E. Bone. In the Pathology of Incipient Neoplasia; Saunders: Philadelphia, PA, USA, 1986; pp. 381–423. [Google Scholar]
- Gilkey, F.W. Liposclerosing myxofibrous tumor of bone. Hum. Pathol. 1993, 24, 1264. [Google Scholar] [CrossRef] [PubMed]
- Matsuba, A.; Ogose, A.; Tokunaga, K.; Kawashima, H.; Hotta, T.; Urakawa, S.; Umezu, H.; Higuchi, T.; Endo, N. Activating Gs alpha mutation at the Arg201 codon in liposclerosing myxofibrous tumor. Hum. Pathol. 2003, 34, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, H.M.; Al-Nakshabandi, N.A.; Saliken, J.; Munk, P.L.; Nielsen, T.O.; Masri, B.; O’connell, J.X. Liposclerosing myxofibrous tumour. Eur. J. Radiol. Extra 2005, 55, 83–87. [Google Scholar] [CrossRef]
- Corsi, A.; De Maio, F.; Ippolito, E.; Cherman, N.; Robey, P.G.; Riminucci, M.; Bianco, P. Monostotic fibrous dysplasia of the proximal femur and liposclerosing myxofibrous tumor: Which one is which? J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006, 21, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.C.K.; Rao, C.G. Polymorphic fibro-osseous lesion of the bone: A case report with review of literature. Indian. J. Pathol. Microbiol. 2008, 51, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Pérez-Andrés, R.; Lorenzo, J.C.; Vilanova, J.C. Diagnostic imaging of liposclerosing myxofibrous tumor of bone. Radiologia 2010, 52, 251–254. [Google Scholar] [CrossRef] [PubMed]
- González Ortega, F.J.; Peñas García, J.; Ramírez Garrido, F. Tumor fibromixoide lipoesclerosante. Rev. Argent. Radiol. 2015, 79, 222–223. [Google Scholar] [CrossRef]
- Técualt-Gómez, R.; Atencio-Chan, A.; Cario-Méndez, A.G.; Amaya-Zepeda, R.A.; Balderas-Martinez, J.; González-Valladares, J.R. Bone liposclerosing myxofibrous tumor. Case presentation and literature review. Acta Ortop. Mex. 2015, 29, 191–195. [Google Scholar]
- Kim, J.; Chen, W.; Resnik, C.; Dilsizian, V.; Chen, Q.; Kimball, A.S. FDG uptake in liposclerosing myxofibrous tumor causes upstaging of Hodgkin lymphoma. Clin. Nucl. Med. 2015, 40, 325–327. [Google Scholar] [CrossRef]
- Regado, E.R.; Garcia, P.B.L.; Caruso, A.C.; de Almeida, A.L.B.; Aymoré, I.L.; Meohas, W.; Aguiar, D.P. Liposclerosing myxofibrous tumor: A series of 9 cases and review of the literature. J. Orthop. 2016, 13, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Kampen, W.U.; Antunovic, L.; Luebke, A.M.; Sauter, G.; Strobel, K.; Paycha, F. Bone SPECT/CT imaging of a liposclerosing myxofibroid tumor in an unexpected localization. Eur. J. Hybrid. Imaging 2018, 2, 29. [Google Scholar] [CrossRef]
- Ploof, J.; Shaikh, H.; Melli, J.; Jour, G.; Turtz, A. Liposclerosing Myxofibrous Tumor of the Cranial Vault: A Case Report. Neurosurgery 2019, 84, E207–E210. [Google Scholar] [CrossRef]
- Pai, S.N.; Kumar, M.M. Liposclerosing myxofibrous tumour. BMJ Case Rep. 2021, 14, e245487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, D.; Yu, W.; Wang, C. Liposclerosing myxofibrous tumor of the distal femur: A case report. Front. Surg. 2022, 9, 1009975. [Google Scholar] [CrossRef] [PubMed]
- Beytemür, O. Liposclerosing myxofibrous tumor: A rare tumor of proximal femur. Jt. Dis. Relat. Surg. 2017, 28, 210–213. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, Y.S.; Lee, J.H.; Kim, H.K.; Yeom, B.W.; Choi, J.S.; Lim, H.C.; Kim, C.H. Liposclerosing Myxofibrous Tumor in Tibia—A Case Report and Review of the Literature. Korean J. Pathol. 2005, 39, 207–210. [Google Scholar]
- Teruel-González, V.; Vicente-Zuloaga, M.; Oncalada-Calderón, E. Liposclerosing myxofi brous hip tumour. A case. Rev. Esp. Cir. Ortop. Traumatol. 2010, 54, 120–125. [Google Scholar]
- Ragsdale, B.D. Polymorphic fibro-osseous lesions of bone: An almost site-specific diagnostic problem of the proximal femur. Hum. Pathol. 1993, 24, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.M.; Unni, K.K.; Mertens, F.; WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, 3rd ed.; IARC Press: Geneva, Switzerland, 2002. [Google Scholar]
- Rosenberg, A.E.; Cleton-Jansen, A.M.; de Pinieux, G.; Deyrup, A.T.; Hauben, E.; Squire, J. Osteosarcoma. In WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, 4th ed.; IARC Press: Geneva, Switzerland, 2013. [Google Scholar]
- Nishikawa, S.; Iwakuma, T. Drugs Targeting p53 Mutations with FDA Approval and in Clinical Trials. Cancers 2023, 15, 429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agaram, N.P.; Bredella, M.A.; WHO Classification of Tumours Editorial Board. Aneurysmal bone cyst. In Soft Tissue and Bone Tumours, 5th ed.; Agency for Research on Cancer: Lyon, France, 2020; pp. 437–439. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).