Evaluation of the HIV-1 Rapid Recency Assay and Limiting Antigen Avidity Enzyme Immunoassay for HIV Infection Status Interpretation in Long-Term Diagnosed Individuals in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Review
Data Transparency and Reproducibility
2.2. Specimens
2.3. Laboratory Methods
2.3.1. HIV-1 Test Samples
2.3.2. Performance of HIV-1 Limiting Antigen (LAg)-Avidity Enzyme Immunoassay (LAg-Avidity EIA)
2.3.3. Performance of Asanté™ HIV-1 Rapid Recency® Assay (ARRA) Procedure
Interpretation of Results of ARRA
Visual Interpretation
Strip Reader Interpretation
2.4. Data Analysis and Statistical Methods
2.4.1. False Recent Rates (FRRs)
2.4.2. Agreement Assessment
2.4.3. Correlation Analysis
3. Results
3.1. Performance Evaluation of LAg-Avidity EIA and ARRA for Long-Term HIV Infection Specimens
3.2. False Recent Rate (FRR) Analysis
3.3. Agreement Between LAg-Avidity EIA and ARRA
3.4. Performance of ARRA Reader Versus LAg-Avidity EIA for Recent HIV Infection Detection
3.5. Evaluation of ARRA Performance in High-Risk Populations with Unknown Infection Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Fact Sheet—Latest Global and Regional Statistics on the Status of the AIDS Epidemic. Available online: https://www.unaids.org/en/resources/documents/2024/UNAIDS_FactSheet (accessed on 22 January 2025).
- Manopaiboon, C.; Prybylski, D.; Subhachaturas, W.; Tanpradech, S.; Suksripanich, O.; Siangphoe, U.; Johnston, L.G.; Akarasewi, P.; Anand, A.; Fox, K.K.; et al. Unexpectedly high HIV prevalence among female sex workers in Bangkok, Thailand in a respondent-driven sampling survey. Int. J. STD AIDS 2013, 24, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Kakchapati, S.; Singh, D.R.; Rawal, B.B.; Lim, A. Sexual risk behaviors, HIV, and syphilis among female sex workers in Nepal. HIV 2017, 9, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Biomedical Maxim. HIV-1 Limiting Antigen Avidity EIA: Single Well Avidity Enzyme Immunoassay for Detection of Recent HIV-1 Infection; Biomedical Maxim: Rockville, MD, USA, 2013. [Google Scholar]
- UNAIDS. Joint United Nations Programme on HIV/AIDS. Global AIDS Update 2024: Fact Sheet. Available online: www.unaids.org (accessed on 22 January 2025).
- Arons, M.M.; Curran, K.G.; Msukwa, M.; Theu, J.; O’Malley, G.; Ernst, A.; Namakhoma, I.; Bello, G.; Telford, C.; Shanmugam, V. Acceptability and Feasibility of HIV Recent Infection Surveillance by Healthcare Workers Using a Rapid Test for Recent Infection at HIV Testing Sites-Malawi, 2019. BMC Health Serv. Res. 2022, 22, 341. [Google Scholar]
- Granade, T.C.; Nguyen, S.; Kuehl, D.S.; Parekh, B.S. Development of a Novel Rapid HIV Test for Simultaneous Detection of Recent or Long-Term HIV Type 1 Infection Using a Single Testing Device. AIDS Res. Hum. Retroviruses 2013, 29, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, E.A.; Kim, A.A.; Dobbs, T.; Zungu, I.; Payne, D.; Maher, A.D.; Curran, K.; Kim, E.; Kwalira, H.; Limula, H. Performance of a Novel Rapid Test for Recent HIV Infection among Newly-Diagnosed Pregnant Adolescent Girls and Young Women in Four High-HIV-Prevalence Districts-Malawi, 2017–2018. PLoS ONE 2022, 17, e0262071. [Google Scholar] [CrossRef] [PubMed]
- Duong, Y.T.; Qiu, M.; De, A.K.; Jackson, K.; Dobbs, T.; Kim, A.A.; Nkengasong, J.N.; Parekh, B.S. Detection of Recent HIV-1 Infection Using a New Limiting-Antigen Avidity Assay: Potential for HIV-1 Incidence Estimates and Avidity Maturation Studies. PLoS ONE 2012, 7, e33328. [Google Scholar]
- Singh, B.; Mthombeni, J.; Olorunfemi, G.; Goosen, M.; Cutler, E.; Julius, H.; Brukwe, Z.; Puren, A. Evaluation of the accuracy of the Asanté assay as a point-of-care rapid test for HIV-1 recent infections using serum bank specimens from blood donors in South Africa, July 2018–August 2021. S. Afr. Med. J. 2023, 113, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rwibasira, G.N.; Malamba, S.S.; Musengimana, G.; Nkunda, R.C.; Omolo, J.; Remera, E.; Masengesho, V.; Mbonitegeka, V.; Dzinamarira, T.; Kayirangwa, E. Recent Infections among Individuals with a New HIV Diagnosis in Rwanda, 2018–2020. PLoS ONE 2021, 16, e0259708. [Google Scholar] [CrossRef] [PubMed]
- Pattanasin, S.; van Griensven, F.; Mock, P.A.; Sukwicha, W.; Kongpechsatit, O.; Krasan, C.; O’Connor, S.; Hickey, A.C.; Ungsedhapand, C.; Woodring, J.V. HIV and Syphilis Prevalence among Transgender Women and Men Who Have Sex with Men, Silom Community Clinic, Bangkok, Thailand, 2017–2019. AIDS Care 2022, 34, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Sedia Biosciences Corparation. Asanté™ HIV-1 Rapid Recency® Assay; Sedia Biosciences Corparation: Beaverton, OR, USA, 2019. [Google Scholar]
- Yufenyuy, E.L.; Detorio, M.; Dobbs, T.; Patel, H.K.; Jackson, K.; Vedapuri, S.; Parekh, B.S. Performance Evaluation of the Asante Rapid Recency Assay for Verification of HIV Diagnosis and Detection of Recent HIV-1 Infections: Implications for Epidemic Control. PLoS Glob. Public Health 2022, 2, e0000316. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.A.; Rehle, T. Short Communication: Assessing Estimates of HIV Incidence with a Recent Infection Testing Algorithm That Includes Viral Load Testing and Exposure to Antiretroviral Therapy. AIDS Res. Hum. Retroviruses 2018, 34, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Brookmeyer, R. Measuring the HIV/AIDS epidemic: Approaches and challenges. Epidemiol. Rev. 2010, 32, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Sanders, G.D.; Bayoumi, A.M.; Sundaram, V.; Bilir, S.P.; Neukermans, C.P.; Rydzak, C.E.; Douglass, L.R.; Lazzeroni, L.C.; Holodniy, M.; Owens, D.K. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N. Engl. J. Med. 2005, 352, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Facente, S.N.; Grebe, E.; Maher, A.D.; Fox, D.; Scheer, S.; Mahy, M.; Dalal, S.; Lowrance, D.; Marsh, K. Use of HIV Recency Assays for HIV Incidence Estimation and Other Surveillance Use Cases: Systematic Review. JMIR Public Health Surveill. 2022, 8, e34410. [Google Scholar] [CrossRef] [PubMed]
- Msukwa, M.T.; MacLachlan, E.W.; Gugsa, S.T.; Theu, J.; Namakhoma, I.; Bangara, F.; Blair, C.L.; Payne, D.; Curran, K.G.; Arons, M.; et al. Characterising persons diagnosed with HIV as either recent or long-term using a cross-sectional analysis of recent infection surveillance data collected in Malawi from September 2019 to March 2020. BMJ Open 2022, 12, e064707. [Google Scholar] [CrossRef] [PubMed]
- Glass, T.; Myer, L.; Lesosky, M. The role of HIV viral load in mathematical models of HIV transmission and treatment: A review. BMJ Glob. Health 2020, 5, e001800. [Google Scholar] [CrossRef] [PubMed]
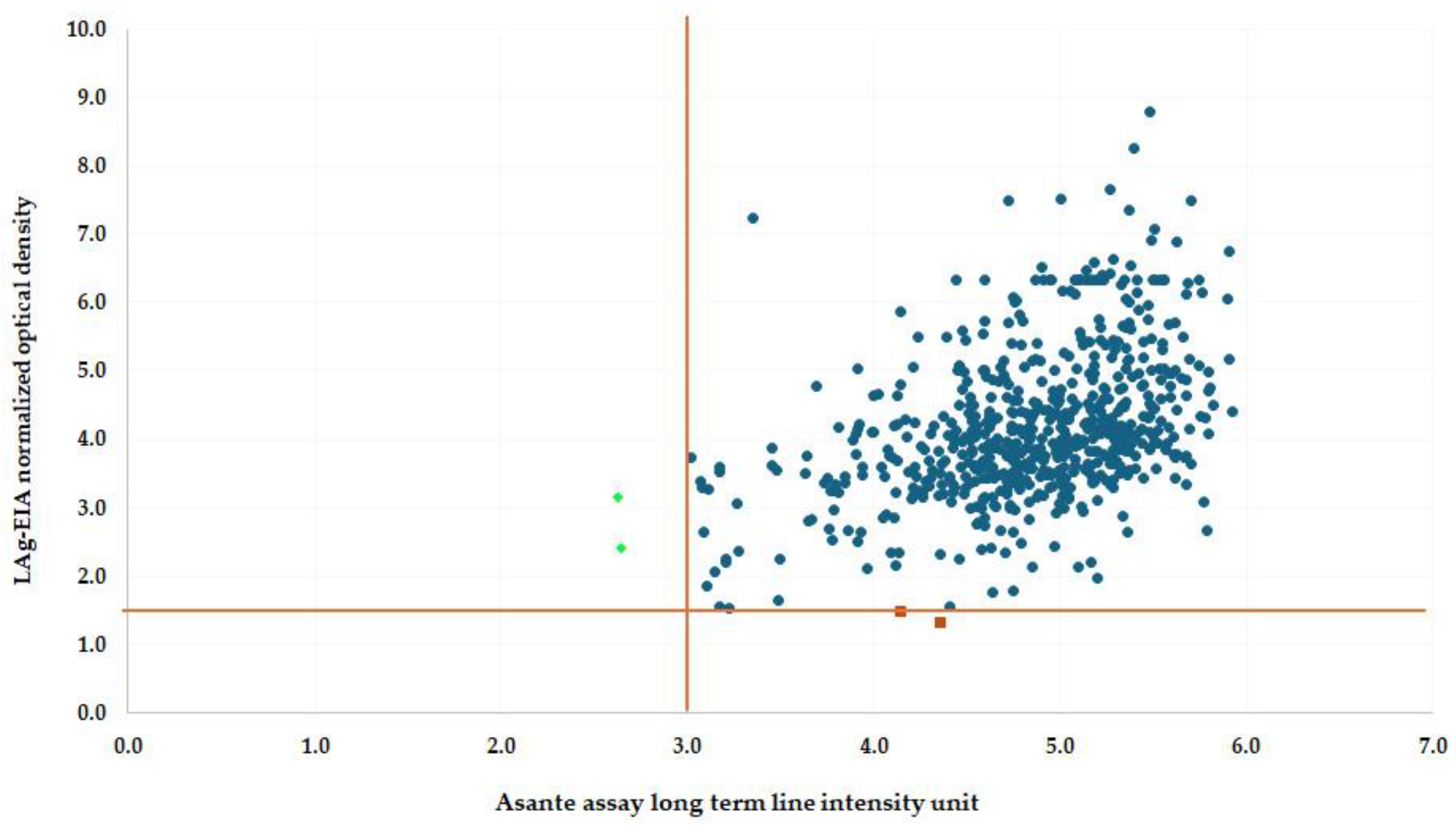
| Assay | Recent Infections | Long-Term Infections | Total (N = 634) | False Recent Rate (FRR) |
|---|---|---|---|---|
| LAg-Avidity EIA | 2 * | 632 | 634 | 0.32% |
| ARRA Visual | 0 | 634 | 634 | 0.00% |
| ARRA Reader-Based | 2 * | 632 | 634 | 0.32% |
| Specimen ID | LAg-Avidity EIA Classification (ODn) | ARRA Visual Interpretation | ARRA Reader-Based Interpretation (PVL, LTL Intensity) | Comments |
|---|---|---|---|---|
| 80-8001-027 | Long-term Infection (3.163) | Long-term Infection | * Recent Infection (5.19, 2.63) | Misclassified by ARRA reader |
| 90-9010-143 | Long-term Infection (2.419) | Long-term Infection | * Recent Infection (4.17, 2.64) | Misclassified by ARRA reader |
| 77-7707-024 | * Recent Infection (1.326) | Long-term Infection | Long-term Infection (5.91, 4.35) | Discrepancy between assays |
| 77-7707-008 | * Recent Infection (1.492) | Long-term Infection | Long-term Infection (5.59, 4.14) | Discrepancy between assays |
| Tests | LAg-Avidity EIA (Recent) | LAg-Avidity EIA (Long-Term) | Total | Mcnemar’s Test p Value |
|---|---|---|---|---|
| ARRA Visual | 0 | 634 | 634 | 0.500 |
| ARRA Reader-Based | 2 * | 632 | 634 | 1.000 |
| Group | Total Samples Tested | LAg-Avidity EIA Long-Term Results | ARRA Long-Term Results | ARRA Recent Results | Inconsistency Rate (%) |
|---|---|---|---|---|---|
| Pregnant women | 130 | 130 | 129 | 1 | 0.77 |
| Female sex workers | 86 | 86 | 86 | - | 0.00 |
| MSM | 8 | 8 | 8 | - | 0.00 |
| Total | 224 | 224 | 223 | 1 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suparak, S.; Unpol, P.; Ngueanchanthong, K.; Jomjunyoung, S.; Thanyacharern, W.; Chisholm, S.P.; Smanthong, N.; Yingyong, T.; Okada, P.A. Evaluation of the HIV-1 Rapid Recency Assay and Limiting Antigen Avidity Enzyme Immunoassay for HIV Infection Status Interpretation in Long-Term Diagnosed Individuals in Thailand. Diagnostics 2025, 15, 444. https://doi.org/10.3390/diagnostics15040444
Suparak S, Unpol P, Ngueanchanthong K, Jomjunyoung S, Thanyacharern W, Chisholm SP, Smanthong N, Yingyong T, Okada PA. Evaluation of the HIV-1 Rapid Recency Assay and Limiting Antigen Avidity Enzyme Immunoassay for HIV Infection Status Interpretation in Long-Term Diagnosed Individuals in Thailand. Diagnostics. 2025; 15(4):444. https://doi.org/10.3390/diagnostics15040444
Chicago/Turabian StyleSuparak, Supaporn, Petai Unpol, Kanokwan Ngueanchanthong, Siriphailin Jomjunyoung, Wipawee Thanyacharern, Sirilada Pimpa Chisholm, Nitis Smanthong, Thitipong Yingyong, and Pilailuk Akkapaiboon Okada. 2025. "Evaluation of the HIV-1 Rapid Recency Assay and Limiting Antigen Avidity Enzyme Immunoassay for HIV Infection Status Interpretation in Long-Term Diagnosed Individuals in Thailand" Diagnostics 15, no. 4: 444. https://doi.org/10.3390/diagnostics15040444
APA StyleSuparak, S., Unpol, P., Ngueanchanthong, K., Jomjunyoung, S., Thanyacharern, W., Chisholm, S. P., Smanthong, N., Yingyong, T., & Okada, P. A. (2025). Evaluation of the HIV-1 Rapid Recency Assay and Limiting Antigen Avidity Enzyme Immunoassay for HIV Infection Status Interpretation in Long-Term Diagnosed Individuals in Thailand. Diagnostics, 15(4), 444. https://doi.org/10.3390/diagnostics15040444







