A State-of-the-Art Review of Ophthalmological Indications for a Cesarean Section: Is There a Patient for Whom a Cesarean Section Is Really Indicated?
Abstract
1. Introduction
2. Materials and Methods
3. Results
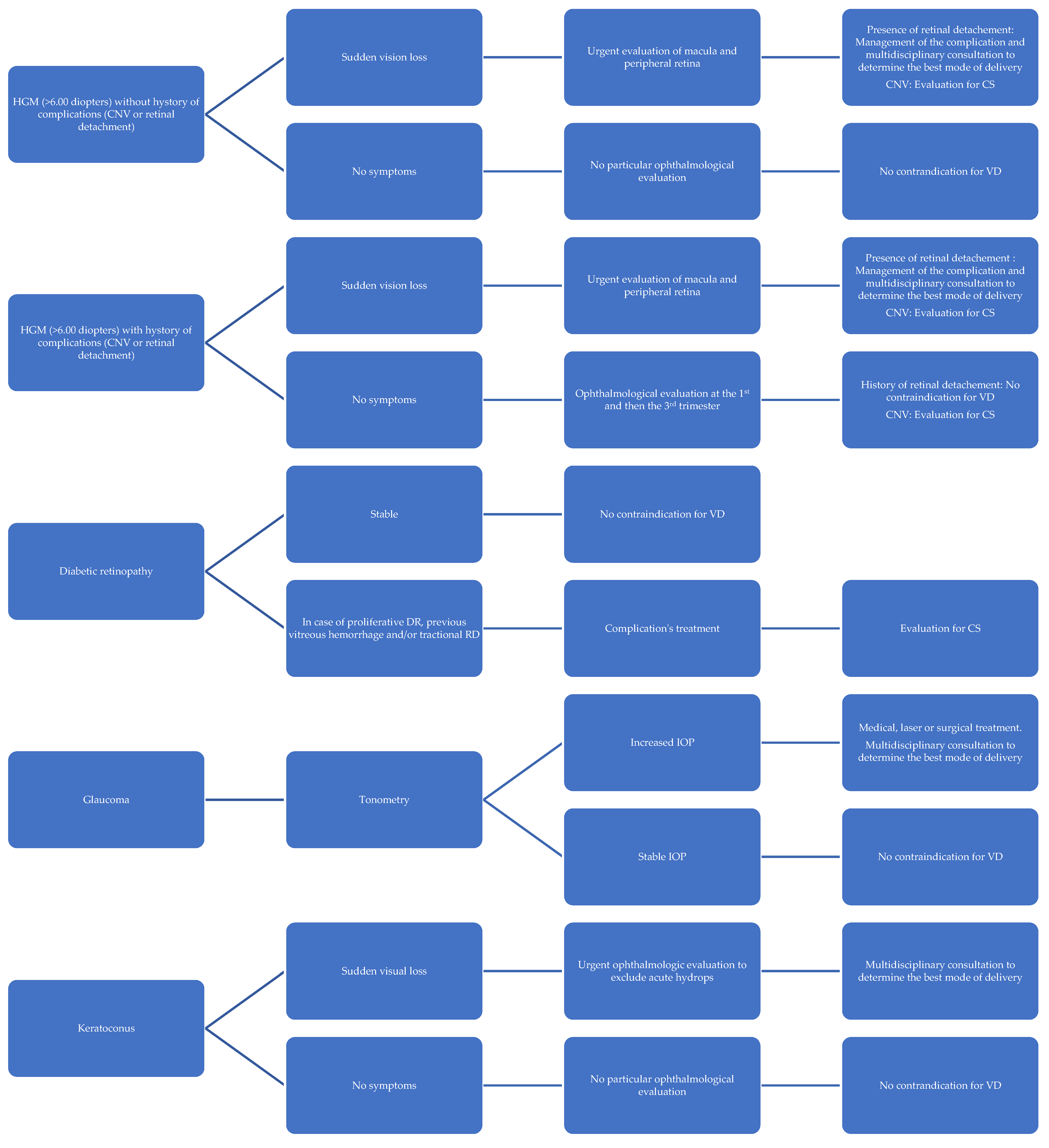
3.1. Myopia
3.2. Retinal Detachment
3.3. Diabetic Retinopathy
3.4. Glaucoma
3.5. Keratoconus
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- WHO. Caesarean Section Rates Continue to Rise, Amid Growing Inequalities in Access. 2021. Available online: https://www.who.int/news/item/16-06-2021-Caesarean-section-rates-continue-to-rise-amid-growing-inequalities-in-access (accessed on 1 January 2025).
- WHO. Statement on Caesarean Section Rates. 2015. Available online: https://www.who.int/publications/i/item/WHO-RHR-15.02 (accessed on 5 December 2024).
- Venturella, R.; Quaresima, P.; Micieli, M.; Rania, E.; Palumbo, A.; Visconti, F.; Zullo, F.; Di Carlo, C. Non-obstetrical indications for Caesarean section: A state-of-the-art review. Arch. Gynecol. Obstet. 2018, 298, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Saccone, G.; Pellegrino, R.; Vaccarisi, S.; Taranto, L.; Mazzulla, R.; Bernardo, S.; Venturella, R.; Di Carlo, C.; Morelli, M. Incidental diagnosis of a pancreatic adenocarcinoma in a woman affected by gestational diabetes mellitus: Case report and literature review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100471. [Google Scholar] [CrossRef]
- Quaresima, P.; Angeletti, M.; Luziatelli, D.; Luziatelli, S.; Venturella, R.; Di Carlo, C.; Bernardo, S. Pregnancy associated transient osteoporosis of the hip (PR-TOH): A non-obstetric indication to Caesarean section. A case report with literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, B.; Özateş, S.; Coşkun, B.; Kıncı, M.F.; Şimşir, C. Retrospective evaluation of indications and birth results of Caesarean section due to ophthalmologic diseases. Eur. Res. J. 2020, 6, 287–291. [Google Scholar] [CrossRef]
- Eshraghi, N.; Ghaemi, M.; Shabannejad, Z.; Bazmi, E.; Foroozesh, M.; Haddadi, M.; Azizi, S.; Mansouri, Z.; Hantoushzadeh, S. Analysis of medico-legal claims related to deliveries: Caesarean section vs. vaginal delivery. PLoS ONE 2024, 19, e0312614. [Google Scholar] [CrossRef] [PubMed]
- Zwecker, P.; Azoulay, L.; Abenhaim, H. Effect of fear of litigation on obstetric care: A nationwide analysis on obstetric practice. Am. J. Perinatol. 2011, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Khong, E.W.C.; Chan, H.H.L.; Watson, S.L.; Lim, L.L. Pregnancy and the eye. Curr. Opin. Ophthalmol. 2021, 32, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, D.; Sung, V.C.; Paschopoulos, M.; Moschos, M.M.; Panidis, P.; Kalogeropoulos, C. The physiologic and pathologic effects of pregnancy on the human visual system. J. Obstet. Gynaecol. 2019, 39, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Karska-Basta, I.; Tarasiewicz, M.; Kubicka-Trząska, A.; Miniewicz, J.; Romanowska-Dixon, B. Cięcie cesarskie a zaburzenia związane z narządem wzroku [Caesarean section and eye disorders]. Ginekol. Pol. 2016, 87, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Atassi, A.R. Intraokulare Druckschwankungen unter der Geburt [Intraocular pressure variations during delivery]. Geburtshilfe Frauenheilkd. 1972, 32, 832–834. [Google Scholar]
- Neri, A.; Grausbord, R.; Kremer, I.; Ovadia, J.; Treister, G. The management of labor in high myopic patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 1985, 19, 277–279. [Google Scholar] [CrossRef]
- Prost, M. Wysoka krótkowzroczność a poród [Severe myopia and delivery]. Klin. Oczna 1996, 98, 129–130. [Google Scholar] [PubMed]
- Landau, D.; Seelenfreund, M.H.; Tadmor, O.; Silverstone, B.-Z.; Diamant, Y.; Service, R. The effect of normal childbirth on eyes with abnormalities predisposing to rhegmatogenous retinal detachment. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995, 233, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Kuba, G.B.; Kroll, P. Geburtsleitung und Indikationen zur Interruptio und Sectio caesarea bei Augenerkrankungen—Eine Ubersicht [Labor monitoring and indications for abortion and Caesarean section in eye diseases—An overview]. Klin. Monbl. Augenheilkd. 1997, 211, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Sapuła-Grabowska, M.; Ciszewska, J.; Brydak-Godowska, J.; Sawa, A.; Laszewicz, P.; Bartha, E.; Pietrzak, B. Delivery in Myopic Women: A Comparison of Mode of Delivery in Years 1990, 2000, and 2010. Med. Sci. Monit. 2019, 25, 7715–7719. [Google Scholar] [CrossRef]
- Bitton, K.; Bacquet, J.-L.; Amoroso, F.; Mrejen, S.; Paques, M.; Souied, E.H. Immediate post partum macular subretinal bleeding in a highly myopic patient: A case report. BMC Ophthalmol. 2021, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Wielgos, M.; Bomba-Opoń, D.; Breborowicz, G.H.; Czajkowski, K.; Debski, R.; Leszczynska-Gorzelak, B.; Oszukowski, P.; Radowicki, S.; Zimmer, M. Recommendations of the Polish Society of Gynecologists and Obstetricians Regarding Caesarean Sections. Ginekologia Polska. 2018. Available online: https://journals.viamedica.pl/ginekologia_polska/article/download/GP.a2018.0110/46969 (accessed on 1 January 2025).
- Polish Ophthalmological Society. Konsensus Okulistyczno-Położniczy w Sprawie Wskazań do Rozwiązania Porodu Drogą Cięcia Cesarskiego z Powodu Zmian w Narządzie Wzroku. 2017. Available online: https://www.ptgin.pl/index.php/lekarze/rekomendacje2/rekomendacje (accessed on 1 January 2025).
- Moneta-Wielgos, J.; Lipa, M.; Brydak-Godowska, J.; Rekas, M.; Wielgos, M. Ophthalmological and obstetric management in pregnant women with retinal disorders. Ginekol. Pol. 2019, 90, 285–288. [Google Scholar] [CrossRef]
- Mohammadi, S.-F.; Letafat-Nejad, M.; Ashrafi, E.; Delshad-Aghdam, H. A survey of ophthalmologists and gynecologists regarding termination of pregnancy and choice of delivery mode in the presence of eye diseases. J. Curr. Ophthalmol. 2017, 29, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Montero, A.; Bes-Rastrollo, M.; Moreno-Montañés, J.; Moreno-Galarraga, L.; Martínez-González, M. Effect of pregnancy in myopia progression: The SUN cohort. Eye 2017, 31, 1085–1092. [Google Scholar] [CrossRef]
- Ghazi, N.G.; Green, W.R. Pathology and pathogenesis of retinal detachment. Eye 2002, 16, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Jünemann, A.G.; Sterk, N.; Rejdak, R. Einfluss des Geburtsmodus auf vorbestehende Augenerkrankungen [Influence of mode of delivery on pre-existing eye diseases]. Ophthalmologe 2012, 109, 229–234. [Google Scholar] [CrossRef]
- Mitry, D.; Charteris, D.G.; Fleck, B.W.; Campbell, H.; Singh, J. The epidemiology of rhegmatogenous retinal detachment: Geographical variation and clinical associations. Br. J. Ophthalmol. 2010, 94, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.; Steele, D.; McAlister, C.; Lam, W.-C. Delivery recommendations for pregnant females with risk factors for rhegmatogenous retinal detachment. Can. J. Ophthalmol. 2015, 50, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Zloto, K.; Peretz, Z.; Eting, E.; Or, L.; Pras, E.; Dubinsky-Pertzov, B. Rates of recurrent retinal detachment after vaginal versus Caesarean deliveries: A Retrospective Analysis and Review of the Literature. Retina 2024, 44, 78–82. [Google Scholar] [PubMed]
- Adler, G.; Key, D.; Schaap-Fogler, M.; Ehrlich, R.; Hadayer, A.; Axer-Siegel, R.; Dotan, A. Does the Method of Delivery Influence the Recurrence of Rhegmatogenous Retinal Detachment? Available online: https://openophthalmologyjournal.com/VOLUME/17/ELOCATOR/e187436412308180/FULLTEXT/ (accessed on 1 January 2025).
- Inglesby, D.V.; Little, B.C.; Chignell, A.H. Surgery for detachment of the retina should not affect a normal delivery. BMJ 1990, 300, 980. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Chaudhary, T.; Aggarwal, S.; Maiti, G.; Jaiswal, K.; Yadav, J. Ophthalmic considerations in pregnancy. Med. J. Armed Forces India 2013, 69, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Rosenn, B.; Miodovnik, M.; Kranias, G.; Khoury, J.; Combs, C.A.; Mimouni, F.; Siddiqi, T.A.; Lipman, M.J. Progression of diabetic retinopathy in pregnancy: Association with hypertension in pregnancy. Am. J. Obstet. Gynecol. 1992, 166, 1214–1218. [Google Scholar] [CrossRef]
- Wickremasinghe, S.S.; Tranos, P.G.; Davey, C. Valsalva haemorrhagic retinopathy in a pregnant woman: Implications for delivery. Acta Ophthalmol. Scand. 2003, 81, 420–422. [Google Scholar] [CrossRef]
- Ladjimi, A.; Zaouali, S.; Messaoud, R.; Ben Yahia, S.; Attia, S.; Jenzri, S.; Khairallah, M. Valsalva retinopathy induced by labour. Eur. J. Ophthalmol. 2002, 12, 336–338. [Google Scholar] [CrossRef]
- Abdelaal, A.M.; Alqahtani, A.S. Mode of Delivery in the Setting of Repeated Vitreous Hemorrhages in Proliferative Diabetic Retinopathy: A Case Report and Review of the Literature. Cureus 2020, 12, e11239. [Google Scholar] [CrossRef] [PubMed]
- Alnoman, A.; Peeva, M.; Badeghiesh, A.M.; Baghlaf, H.A.; Dahan, M.H. Pregnancy, delivery and neonatal outcomes among women with diabetic retinopathy. J. Matern. Fetal Neonatal Med. 2022, 35, 10621–10628. [Google Scholar] [CrossRef]
- NICE Guidelines. Diabetes in Pregnancy: Management from Preconception to the Postnatal Period. 2020. Available online: https://www.nice.org.uk/guidance/ng3 (accessed on 1 January 2025).
- Feghali, M.; Khoury, J.C.; Shveiky, D.; Miodovnik, M. Association of vaginal delivery efforts with retinal disease in women with type I diabetes. J. Matern. Fetal Neonatal Med. 2012, 25, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Qureshi, I.A. Intraocular pressure: Association with menstrual cycle, pregnancy and menopause in apparently healthy women. Chin. J. Physiol. 1995, 38, 229–234. [Google Scholar]
- Banad, N.R.; Choudhari, N.; Dikshit, S.; Garudadri, C.; Senthil, S. Trabeculectomy in pregnancy: Case studies and literature review. Indian J. Ophthalmol. 2020, 68, 420–426. [Google Scholar]
- Naderan, M.; Jahanrad, A. Topographic, tomographic and biomechanical corneal changes during pregnancy in patients with keratoconus: A cohort study. Acta Ophthalmol. 2017, 95, e291–e296. [Google Scholar] [CrossRef] [PubMed]
- Stolp, W.; Kamin, W.; Liedtke, M.; Borgmann, H. Augenerkrankungen und Geburtsleitung. Untersuchungen über Vorgänge am Auge unter der Geburt am Beispiel von subkonjunktivalen Blutungen (Hyposphagmen) [Eye diseases and control of labor. Studies of changes in the eye in labor exemplified by subconjunctival hemorrhage (hyposphagmas)]. Geburtshilfe Frauenheilkd. 1989, 49, 357–362. [Google Scholar] [PubMed]
- Spaeth, G.L. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105 (Suppl. S1), 1–169. [Google Scholar]
- Qin, Q.; Chen, C.; Cugati, S. Ophthalmic associations in pregnancy. Aust. J. Gen. Pract. 2020, 49, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Saha, B.C.; Onkar, A.; Ambasta, A.; Kumari, A. Management of glaucoma in pregnancy—Balancing safety with efficacy. Ther. Adv. Ophthalmol. 2021, 13, 25158414211022876. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, P.; Sethi, P.; Mithal, S. Effect of pregnancy and labor on intraocular pressure. Int. Surg. 1976, 61, 82–84. [Google Scholar] [PubMed]
- Bilgihan, K.; Hondur, A.; Sul, S.; Ozturk, S. Pregnancy-induced progression of keratoconus. Cornea 2011, 30, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.A.; Sinnott, L.T.; Wagner, H.; Friedman, C.; Zadnik, K.; CLEK Study Group. The influence of gender and hormone status on the severity and progression of keratoconus. Cornea 2010, 29, 65–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaresima, P.; Covello, G.; Bitonti, G.; Di Carlo, C.; Morelli, M.; Guido, M. A State-of-the-Art Review of Ophthalmological Indications for a Cesarean Section: Is There a Patient for Whom a Cesarean Section Is Really Indicated? Diagnostics 2025, 15, 418. https://doi.org/10.3390/diagnostics15040418
Quaresima P, Covello G, Bitonti G, Di Carlo C, Morelli M, Guido M. A State-of-the-Art Review of Ophthalmological Indications for a Cesarean Section: Is There a Patient for Whom a Cesarean Section Is Really Indicated? Diagnostics. 2025; 15(4):418. https://doi.org/10.3390/diagnostics15040418
Chicago/Turabian StyleQuaresima, Paola, Giuseppe Covello, Giovanna Bitonti, Costantino Di Carlo, Michele Morelli, and Maurizio Guido. 2025. "A State-of-the-Art Review of Ophthalmological Indications for a Cesarean Section: Is There a Patient for Whom a Cesarean Section Is Really Indicated?" Diagnostics 15, no. 4: 418. https://doi.org/10.3390/diagnostics15040418
APA StyleQuaresima, P., Covello, G., Bitonti, G., Di Carlo, C., Morelli, M., & Guido, M. (2025). A State-of-the-Art Review of Ophthalmological Indications for a Cesarean Section: Is There a Patient for Whom a Cesarean Section Is Really Indicated? Diagnostics, 15(4), 418. https://doi.org/10.3390/diagnostics15040418






