Ultra-High Contrast (UHC) MRI of the Brain, Spinal Cord and Optic Nerves in Multiple Sclerosis Using Directly Acquired and Synthetic Bipolar Filter (BLAIR) Images
Abstract
1. Introduction
2. Basic Physics
2.1. Tissue Property Filters (TP-Filters) and the Inversion Recovery (IR) Sequence
2.2. Contrast at Tissue Boundaries
2.3. T1 Maps and Qualitative—Quantitative MRI
2.4. Log then Subtracted Inversion Recovery (lSIR) Sequences
2.5. Composite (c) Bipolar Filters (T1 as well as T2, T2*, and/or D*)
3. Methods
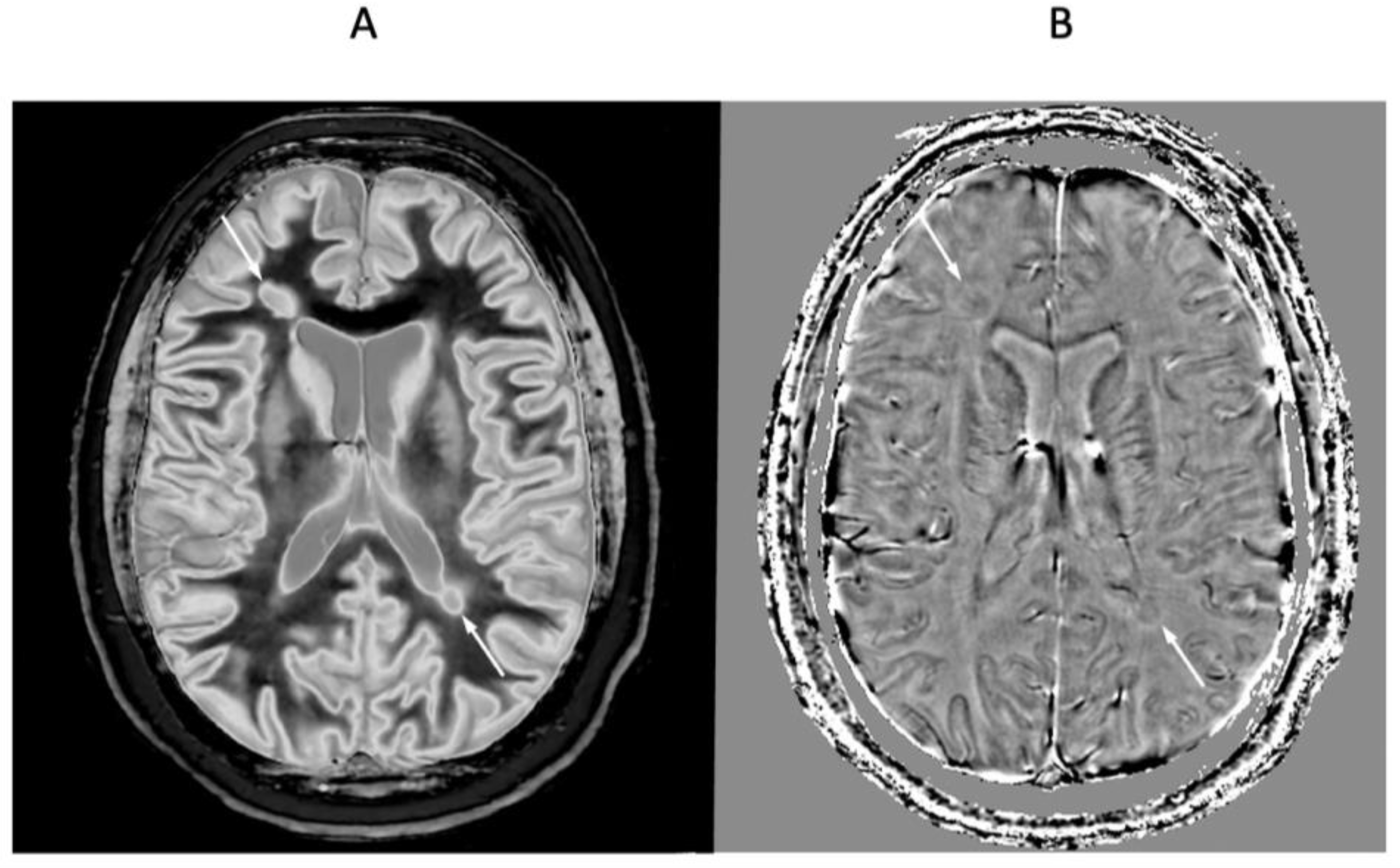
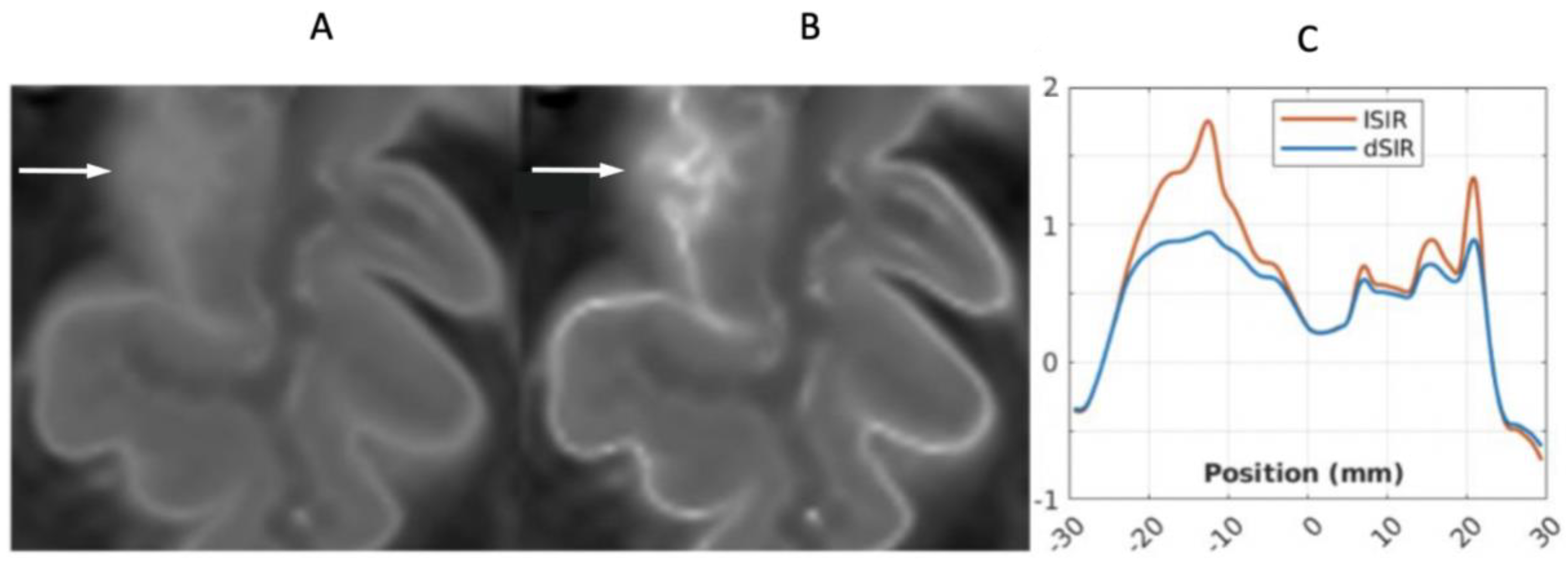
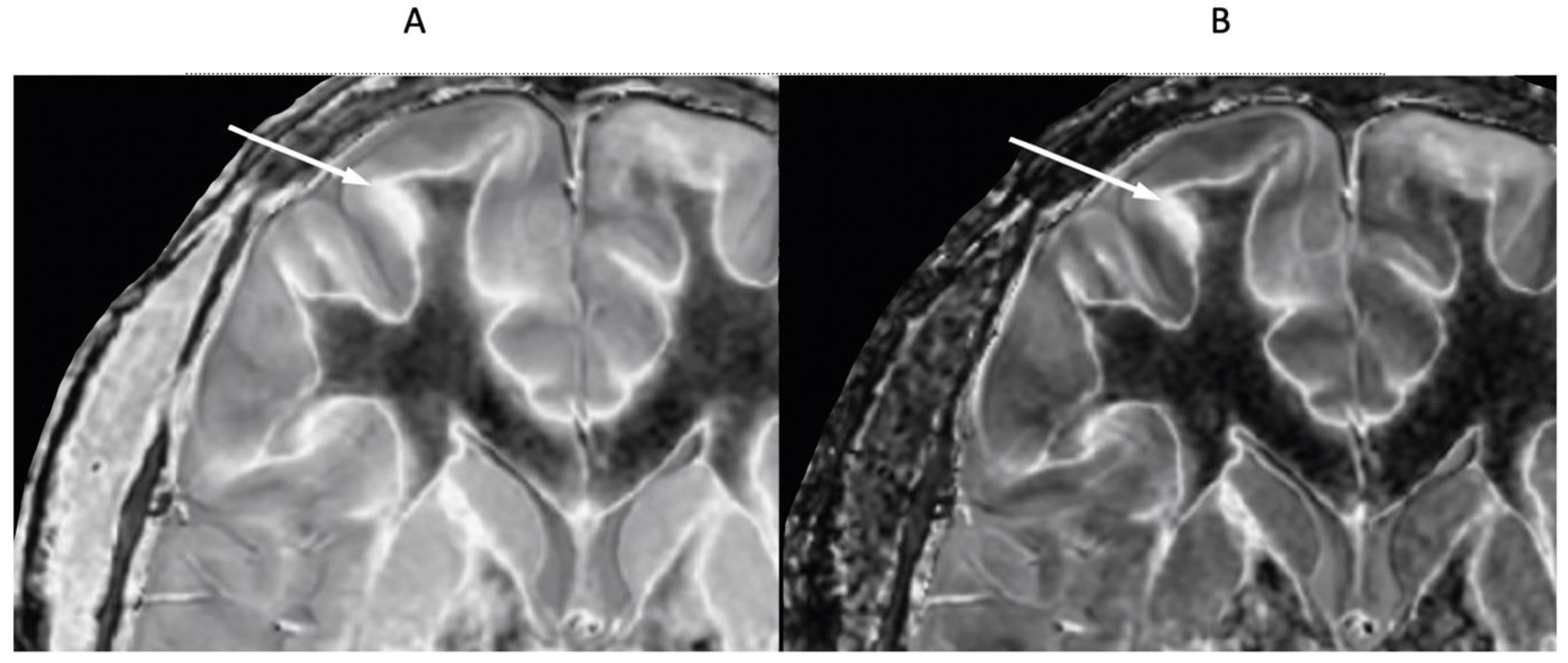
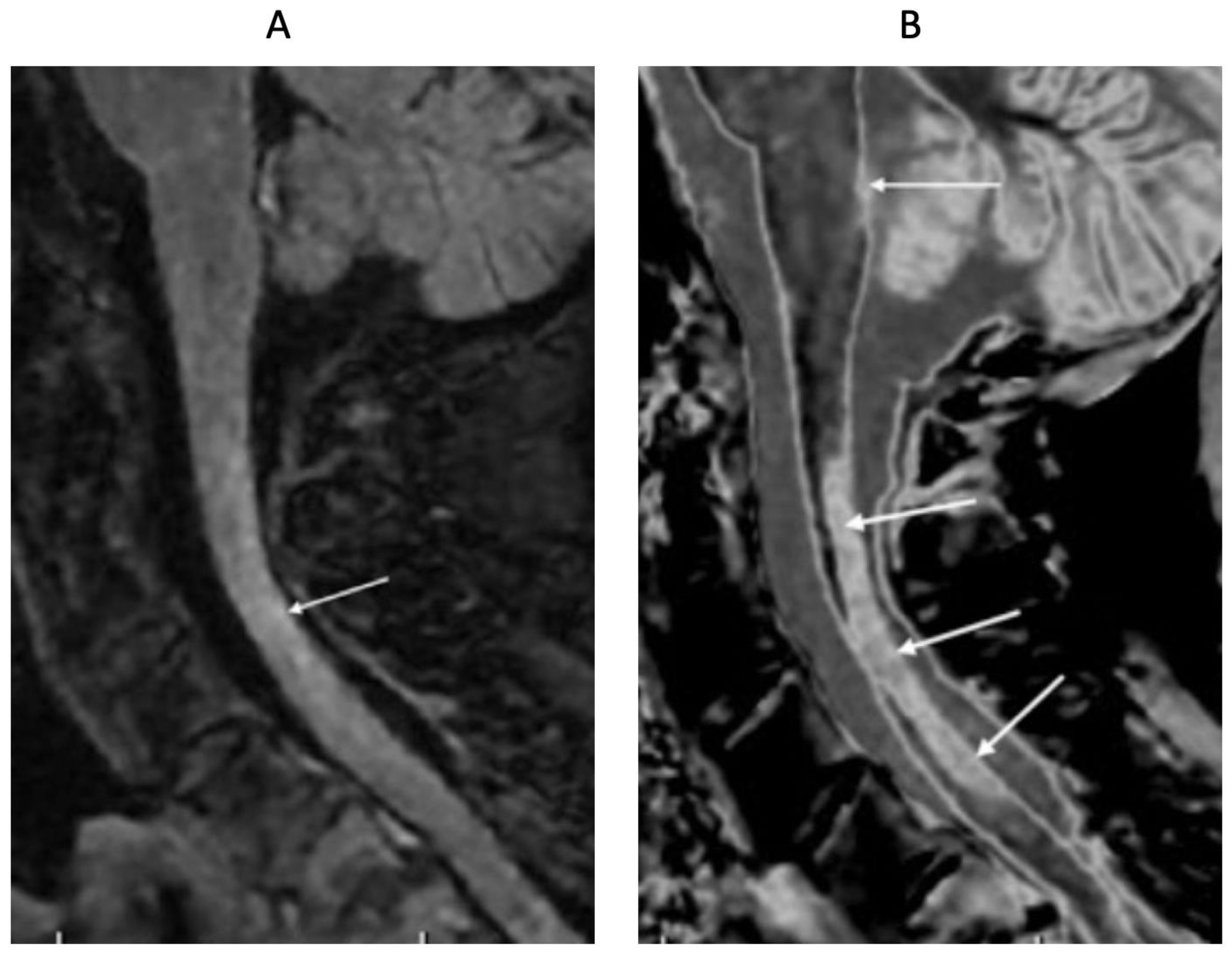
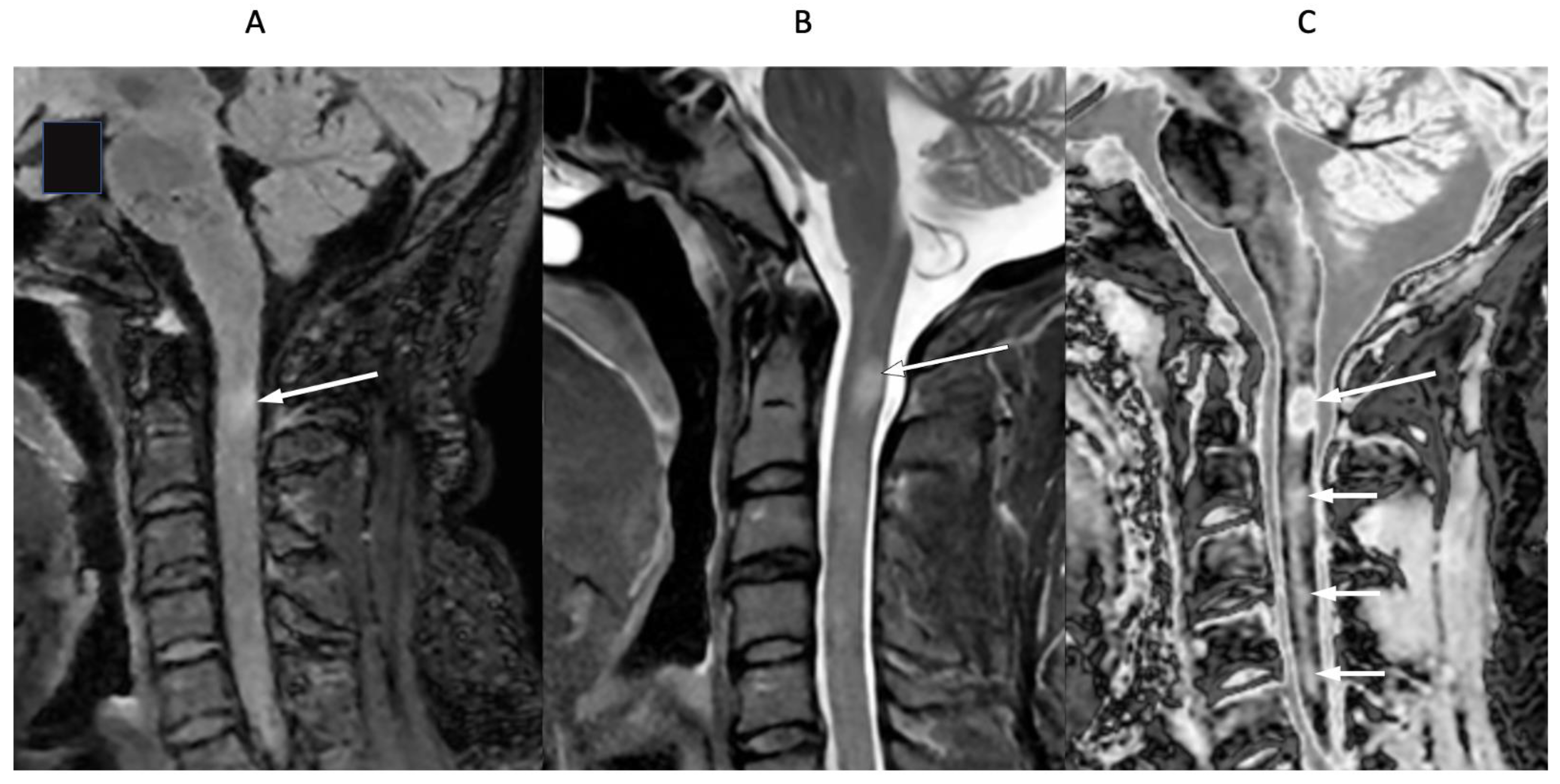
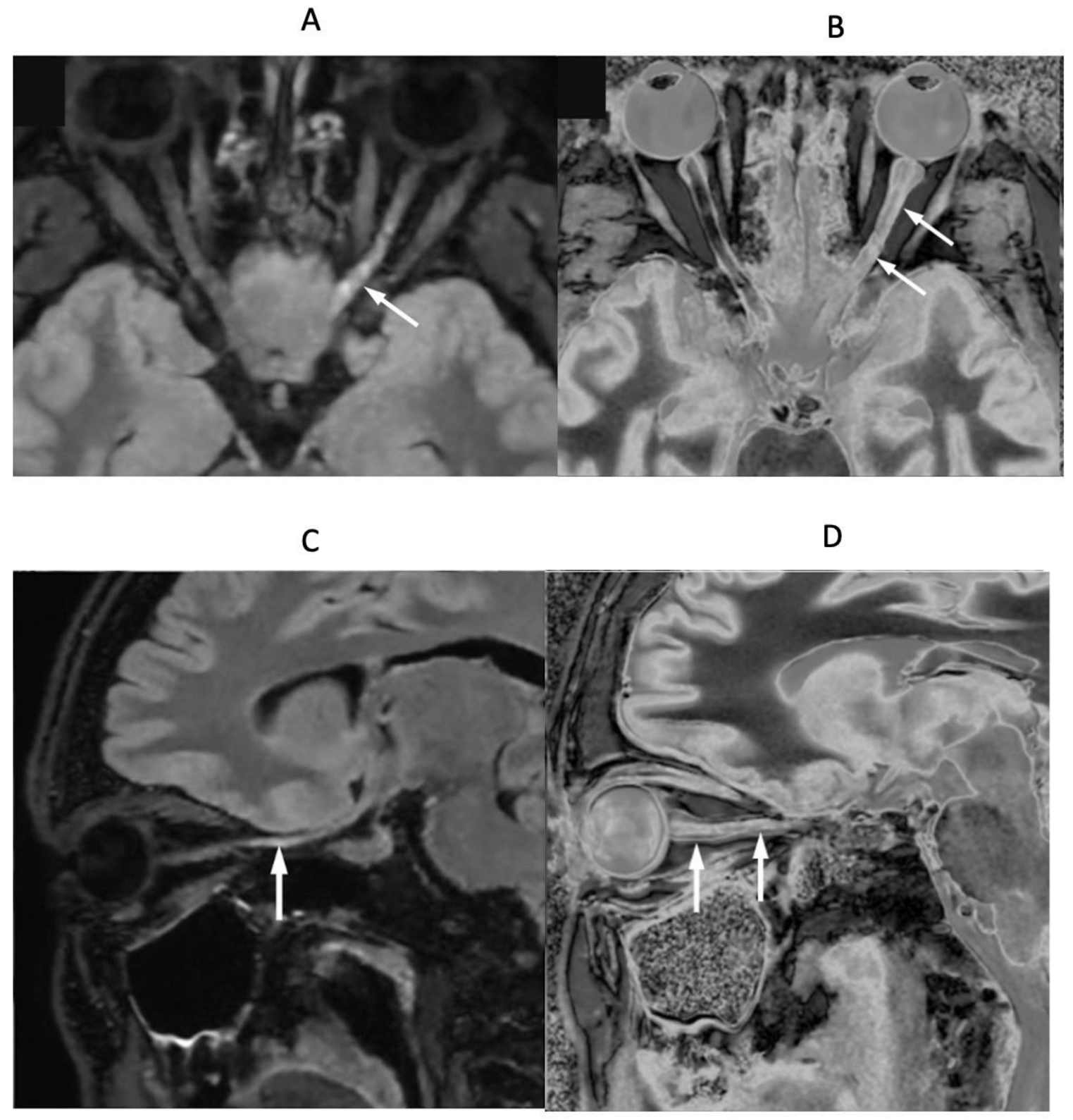
4. Illustrative Cases
5. Discussion
5.1. T1 Measurements and Magnetization Transfer (MT)
5.2. Signs
5.2.1. Whiteout Sign
5.2.2. Grayout Signs
5.3. Clinical Issues
5.3.1. Activity
5.3.2. Acute Clinical Episodes with Stable MRI (ACES)
5.3.3. Diagnostic Criteria for MS
5.3.4. Clinical Use
5.4. Other Sequences Using Two IR Sequences
5.5. Further Developments
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLAIR | BipoLAr fIlteR, bipolar filter |
| cdSIR | Composite divided Subtracted Inversion Recovery |
| clSIR | Composite logarithmic then Subtracted Inversion Recovery |
| dSIR | Divided Subtracted Inversion Recovery |
| hD | Highest Domain |
| IR | Inversion Recovery |
| lSIR | Logarithmic then Subtracted Inversion Recovery |
| mD | Middle Domain |
| MP-RAGE | Magnetization Prepared-Rapid Acquisition Gradient Echo |
| MP2RAGE | Magnetization Prepared 2 Rapid Acquisition Gradient Echo |
| MT | Magnetization Transfer |
| SIR | Subtracted Inversion Recovery |
| SOF | Signal from Other Filter |
| TP | Tissue Property |
| TP-bipolar filter | Tissue Property-bipolar filter |
| TP-filter | Tissue Property-filter |
| UHC | Ultra-High Contrast |
References
- Ma, Y.-J.; Moazamian, D.; Cornfeld, D.M.; Condron, P.; Holdsworth, S.J.; Bydder, M.; Du, J.; Bydder, G.M. Improving the understanding and performance of clinical MRI using tissue property filters and the central contrast theorem, MASDIR pulse sequences and synergistic contrast MRI. Quant. Imaging Med. Surg. 2022, 12, 4658–4690. [Google Scholar] [CrossRef] [PubMed]
- Newburn, G.; McGeown, J.P.; Kwon, E.; Tayebi, M.; Condron, P.; Emsden, T.; Holdsworth, S.J.; Cornfeld, D.M.; Bydder, G.M. Targeted MRI (tMRI) of small increases in the T1 of normal appearing white matter in mild traumatic brain injury (mTBI) using a divided subtracted inversion recovery (dSIR) sequence. OBM Neurobiol. 2023, 7, 1–27. [Google Scholar] [CrossRef]
- Newburn, G.; Condron, P.; Kwon, E.E.; McGeown, J.P.; Melzer, T.R.; Bydder, M.; Griffin, M.; Scadeng, M.; Potter, L.; Holdsworth, S.J.; et al. Diagnosis of delayed post-hypoxic leukoencephalopathy (Grinker’s myelinopathy) with MRI using divided subtracted inversion recovery (dSIR) sequences: Time for reappraisal of the syndrome? Diagnostics 2024, 14, 418. [Google Scholar] [CrossRef]
- Cornfeld, D.; Condron, P.; Newburn, G.; McGeown, J.; Scadeng, M.; Bydder, M.; Griffin, M.; Handsfield, G.; Perera, M.R.; Melzer, T.; et al. Ultra-high contrast MRI: Using divided subtracted inversion recovery (dSIR) and divided echo subtraction (dES) sequences to study the brain and musculoskeletal system. Bioengineering 2024, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-J.; Moazamian, D.; Port, J.D.; Edjlali, M.; Pruvo, J.-P.; Hacein-Bey, L.; Hoggard, N.; Paley, M.N.J.; Menon, D.K.; Bonekamp, D.; et al. Targeted magnetic resonance imaging (tMRI) of small changes in the T1 and spatial properties of normal or near normal appearing white and gray matter in disease of the brain using divided subtracted inversion recovery (dSIR) and divided reverse subtracted inversion recovery (drSIR) sequences. Quant. Imaging Med. Surg. 2023, 13, 7304–7337. [Google Scholar]
- Condron, P.; Cornfeld, D.M.; Scadeng, M.; Melzer, T.R.; Newburn, G.; Bydder, M.; Kwon, E.E.; McGeown, J.P.; Handsfield, G.G.; Emsden, T.; et al. Ultra-high contrast MRI: The whiteout sign shown with divided subtracted inversion recovery (dSIR) sequences in post-insult leukoencephalopathy syndromes (PILS). Tomography 2024, 10, 983–1013. [Google Scholar] [CrossRef]
- Bydder, M.; Ali, F.; Condron, P.; Cornfeld, D.M.; Newburn, G.; Kwon, E.E.; Tayebi, M.; Scadeng, M.; Melzer, T.R.; Holdsworth, S.J.; et al. Validation of an ultra-high contrast divided subtracted inversion recovery technique using a standard T1 phantom. NMR Biomed. 2024, 37, e5269. [Google Scholar] [CrossRef] [PubMed]
- Losa, L.; Peruzzo, D.; Galbiati, S.; Locatelli, F.; Agarwal, N. Enhancing T1 signal of normal-appearing white matter with divided subtracted inversion recovery: A pilot study in mild traumatic brain injury. NMR Biomed. 2024, 37, e5175. [Google Scholar] [CrossRef] [PubMed]
- Tillema, J.-M.; Weigand, S.; Dayan, M.; Shu, Y.; Kantarci, O.; Lucchinetti, C.; Port, J. Dark rims: Novel sequence enhances diagnostic specificity in multiple sclerosis. AJNR Am. J. Neuroradiol. 2018, 39, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Gavoille, A.; Rollot, F.; Casey, R.; Kerbrat, A.; Le Page, E.; Bigaut, K.; Mathey, G.; Michel, L.; Ciron, J.; Ruet, A.; et al. Acute clinical events identified as relapses with stable magnetic resonance imaging in multiple sclerosis. JAMA Neurol. 2024, 81, 814–823. [Google Scholar] [CrossRef]
- Dünschede, J.; Ruschil, C.; Bender, B.; Mengel, A.; Lindig, T.; Ziemann, U.; Kowarik, M.C. Clinical-radiological mismatch in multiple sclerosis patients during acute relapse: Discrepancy between clinical symptoms and active, topographically fitting MRI lesions. J. Clin. Med. 2023, 12, 739. [Google Scholar] [CrossRef]
- Nij Bijvank, J.A.; Aliaga, E.S.; Balk, L.J.; Coric, D.; Davagnanam, I.; Tan, H.S.; Uitdehaag, B.M.J.; van Rijn, L.J.; Petzold, A. A model for interrogating the clinico-radiological paradox in multiple sclerosis: Internuclear opthalmoplegia. Eur. J. Neurol. 2021, 28, 1617–1626. [Google Scholar] [CrossRef]
- Katz, D.; Taubenberger, J.K.; Cannella, B.; McFarlin, D.E.; Raine, C.S.; McFarland, H.F. Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann. Neurol. 1993, 34, 661–669. [Google Scholar] [CrossRef]
- Scalfari, A.; Traboulsee, A.; Oh, J.; Airas, L.; Bittner, S.; Calabrese, M.; Dominguez, J.M.G.; Granziera, C.; Greenberg, B.; Hellwig, K.; et al. Smouldering-associated worsening in multiple sclerosis: An international consensus statement on definition, biology, clinical implications and future directions. Ann. Neurol. 2024, 96, 826–845. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, F.; Sati, P.; Hemond, C.C.; Elliott, C.; A Gauthier, S.; Harrison, D.M.; Mainero, C.; Oh, J.; Pitt, D.; Shinohara, R.T.; et al. Imaging chronic active lesions in multiple sclerosis: A consensus statement. Brain 2024, 147, 2913–2933. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Popescu, V.; Wuerfel, J.; Hellwig, K.; Iacobaeus, E.; Jensen, M.B.; Scalfari, A. Smouldering multiple sclerosis: The ‘real MS’. Ther. Adv. Neurol. Disord. 2022, 15, 1–18. [Google Scholar] [CrossRef]
- Singh, V.; Zheng, Y.; Ontaneda, D.; Mahajan, K.R.; Holloman, J.; Fox, R.J.; Nakamura, K.; Trapp, B.D. Disability independent of cerebral white matter demyelination in progressive multiple sclerosis. Acta Neuropathol. 2024, 148, 34. [Google Scholar] [CrossRef]
- AlTokhis, A.I.; AlOtaibi, A.M.; Felmban, G.A.; Constantinescu, C.S.; Evangelou, N. Iron rims as an imaging biomarker in MS: A systematic mapping review. Diagnostics 2020, 10, 968. [Google Scholar] [CrossRef]
- Dal-Bianco, A.; Grabner, G.; Kronnerwetter, C.; Weber, M.; Kornek, B.; Kasprian, G.; Berger, T.; Leutmezer, F.; Rommer, P.S.; Trattnig, S.; et al. Long-term evolution of multiple sclerosis iron rim lesions in 7T MRI. Brain 2021, 144, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Hemond, C.C.; Dundamadappa, S.K.; Deshpande, M.; Baek, J.; Brown, R.H., Jr.; Ionete, C.; Reich, D.S. Paramagnetic rim lesions are highly specific for multiple sclerosis in real-world data. medRxiv 2024. 2024.08.14.24312000 [Version 1]. [Google Scholar]
- Rocca, M.A.; Preziosa, P.; Barkhof, F.; Brownlee, W.; Calabrese, M.; De Stefano, N.; Granziera, C.; Ropele, S.; Toosy, A.T.; Vidal-Jordana, À.; et al. Current and future role of MRI in the diagnosis and prognosis of multiple sclerosis. Lancet Reg. Health Eur. 2024, 44, 100978. [Google Scholar] [CrossRef] [PubMed]
- Hemond, C.C.; Gaitán, M.I.; Absinta, M.; Reich, D.S. New imaging markers in multiple sclerosis and related disorders, smoldering inflammation and the central vein sign. Neuroimag. Clin. N. Am. 2024, 34, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Sati, P.; Oh, J.; Constable, R.T.; Evangelou, N.; Guttmann, C.R.G.; Henry, R.G.; Klawiter, E.C.; Mainero, C.; Massacesi, L.; McFarland, H.; et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: A consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat. Rev. Neurol. 2016, 12, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Castellaro, M.; Tamanti, A.; Pisani, A.I.; Pizzini, F.B.; Crescenzo, F.; Calabrese, M. The use of the central vein sign in the diagnosis of multiple sclerosis: A systematic review and meta-analysis. Diagnostics 2020, 10, 1025. [Google Scholar] [CrossRef]
- Solomon, A.J.; Watts, R.; Ontaneda, D.; Absinta, M.; Sati, P.; Reich, D.S. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult. Scler. J. 2018, 24, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Nistri, R.; Ianniello, A.; Pozzilli, V.; Giannì, C.; Pozzilli, C. Advanced MRI techniques: Diagnosis and follow-up of multiple sclerosis. Diagnostics 2024, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, M.P.; Ciccarelli, O.; Reich, D.S.; Banwell, B.; de Stefano, N.; Enzinger, C.; Fazekas, F.; Filippi, M.; Frederiksen, J.; Gasperini, C.; et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021, 20, 653–670. [Google Scholar] [CrossRef]
- Traboulsee, A.; Li, D.K. Routine MR imaging protocol and standardization in central nervous system demyelinating diseases. Neuroimag. Clin. N. Am. 2024, 34, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.; Kober, T.; Krueger, G.; van der Zwaag, W.; Van de Moortele, P.-F.; Gruetter, R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010, 49, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, J.; Saint-Jalmes, H.; Acosta, O.; Kober, T.; Tanner, M.; Ferré, J.; Salvado, O.; Fripp, J.; Gambarota, G. Multi T1-weighted contrast MRI with fluid and white matter suppression at 1.5T. Magn. Reson. Imaging 2019, 63, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, J.; Gambarota, G.; Saint-Jalmes, H.; Acosta, O.; Ferré, J.C.; Raniga, P.; Fripp, J. High-resolution multi-T1-weighted contrast and T1 mapping with low B1 sensitivity using the fluid and white matter suppression (FLAWS) sequence at 7T. Magn. Reson. Med. 2021, 85, 1364–1378. [Google Scholar] [CrossRef]
- Müller, J.; La Rosa, F.; Beaumont, J.; Tsagkas, C.; Rahmanzadeh, R.; Weigel, M.; Bach Cuadra, M.; Gambarota, G.; Granziera, C. Fluid and white matter suppression. New sensitive 3T magnetic resonance imaging contrasts for cortical lesion detection in multiple sclerosis. Investig. Radiol. 2022, 57, 592–600. [Google Scholar] [CrossRef]
- Durozard, P.; Maarouf, A.; Zaaraoui, W.; Stellmann, J.-P.; Boutière, C.; Rico, A.; Demortière, S.; Guye, M.; Le Troter, A.; Dary, H.; et al. Cortical lesions as an early hallmark of Multiple Sclerosis: Visualization by 7T MRI. Investig. Radiol. 2024, 59, 747–753. [Google Scholar] [CrossRef] [PubMed]

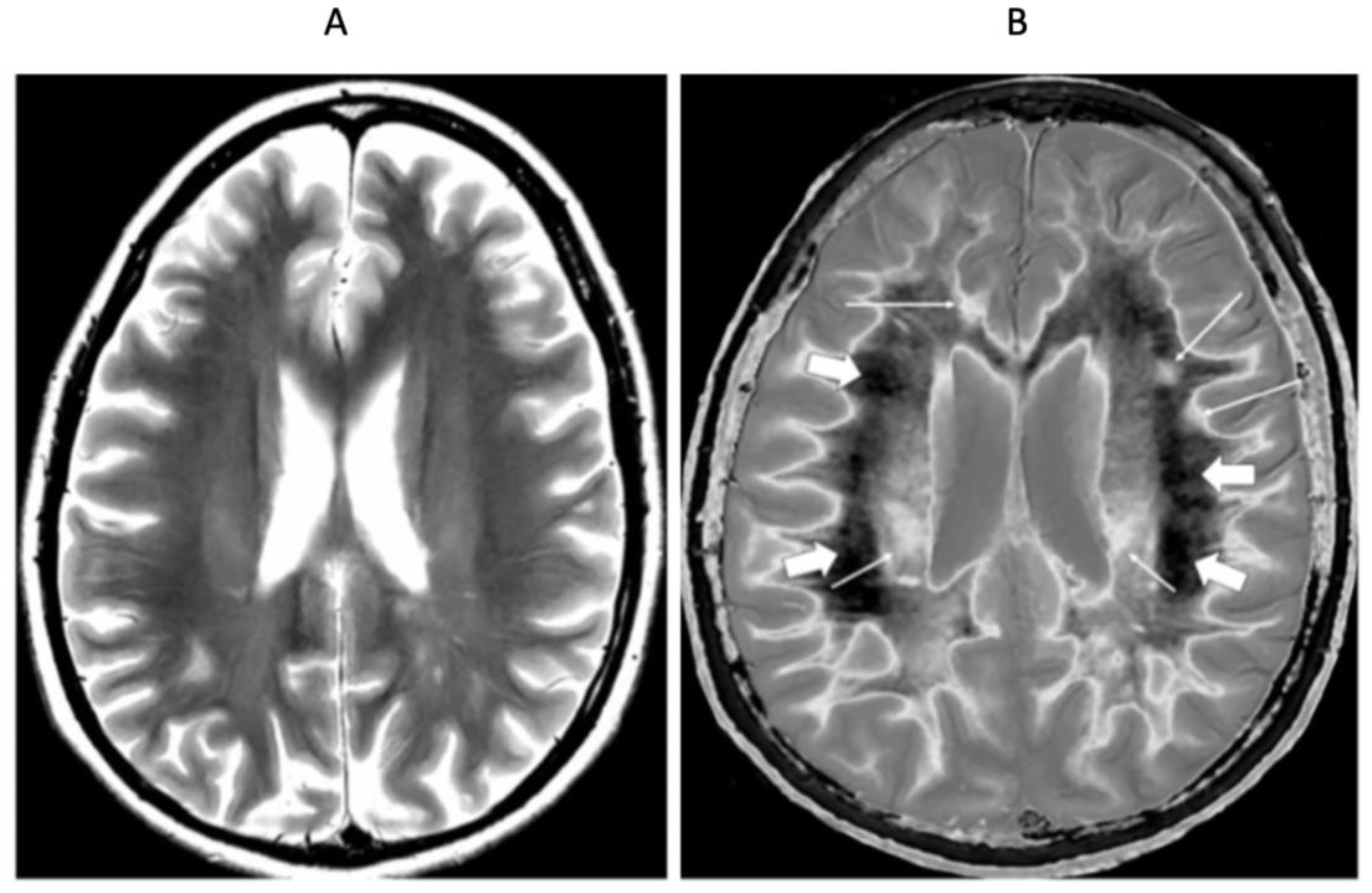
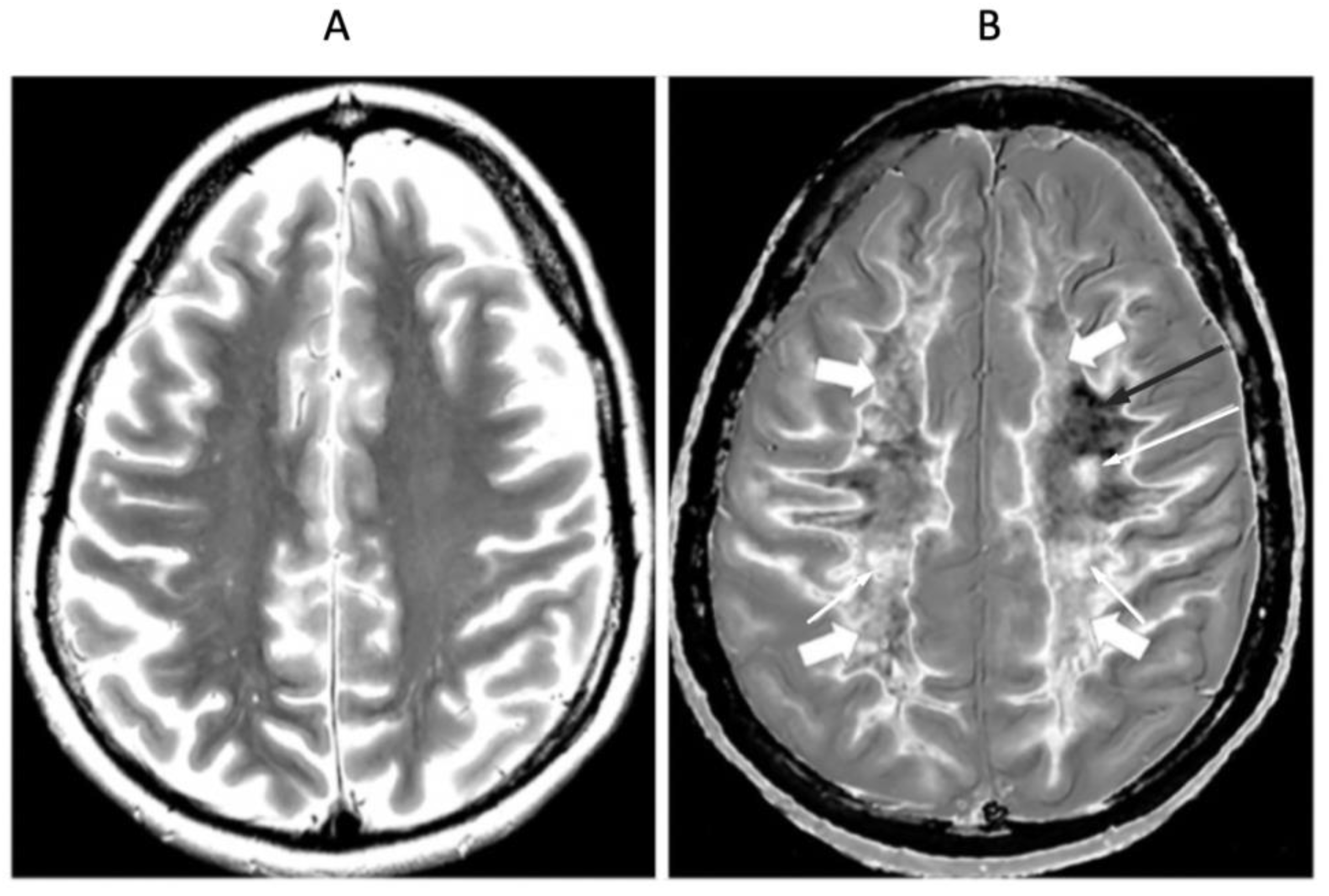
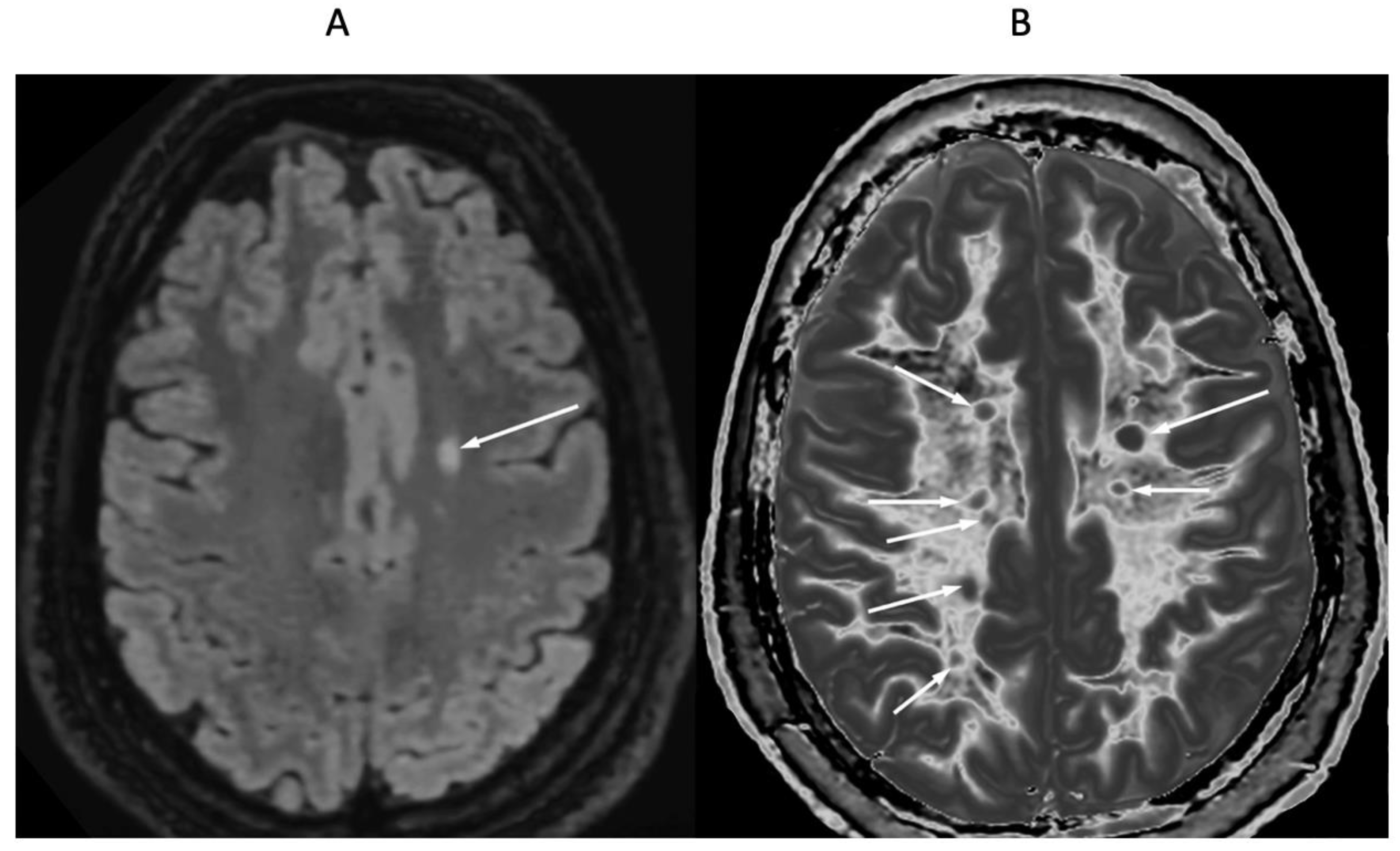

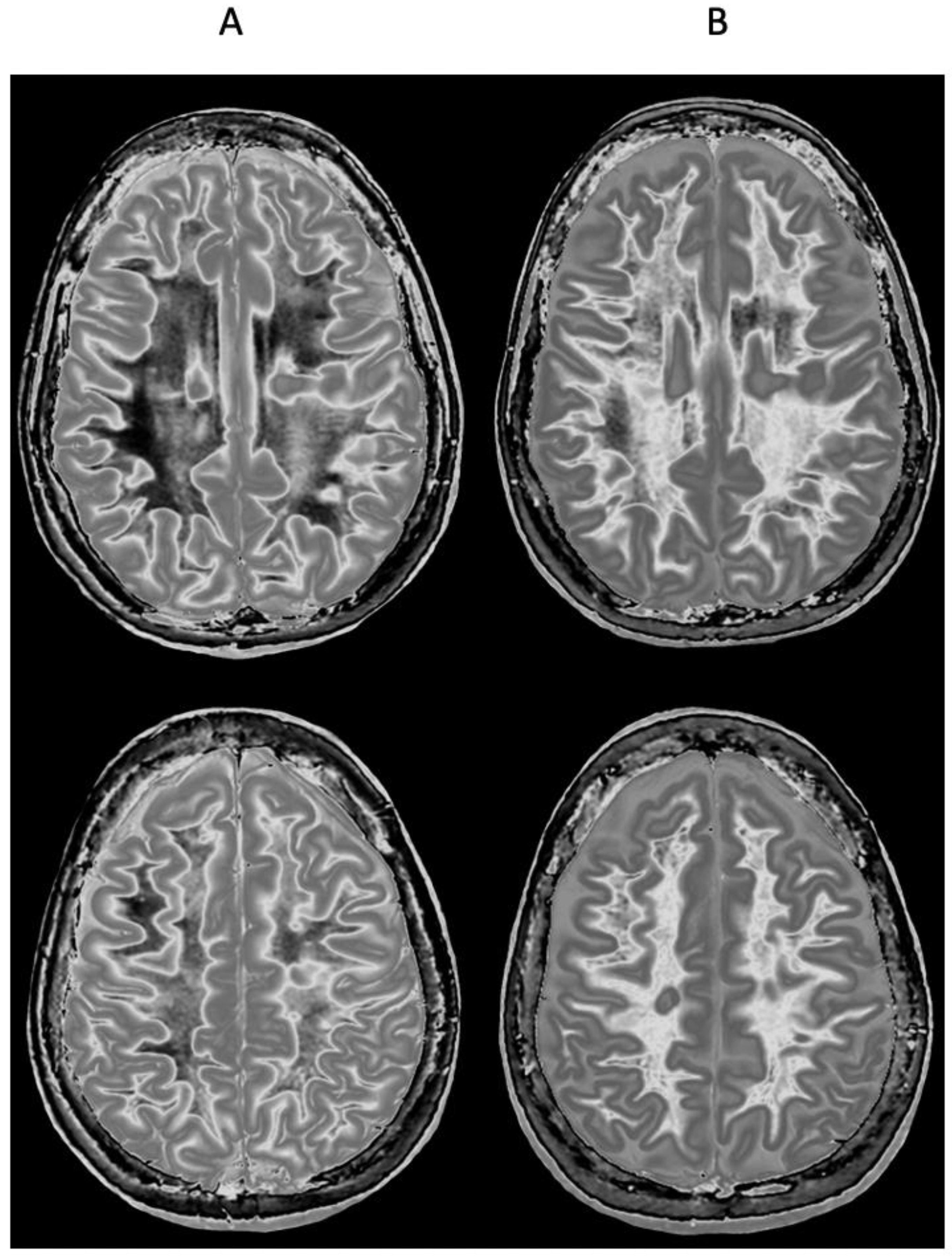

| # | Sequence | TR (ms) | TI (ms) | TE (ms) | Matrix Size Voxel Sizes (mm) | Number of Slices | Slice Thickness (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 2D FSE IR (for white matter nulling) | 9192 | 350 | 7 | 256 × 224 | 26 | 4 |
| 0.9 × 0.1 | |||||||
| Z512 | |||||||
| 0.4 × 0.4 | |||||||
| 2 | 2D FSE IR (used with #1 for narrow mD dSIR) | 5796 | 500 | 7 | 256 × 224 | 26 | 4 |
| 0.9 × 0.1 | |||||||
| Z512 | |||||||
| 0.4 × 0.4 | |||||||
| 3 | 2D FSE IR (used with T1 for wide mD dSIR) | 5796 | 800 | 7 | 256 × 224 | 26 | 4 |
| 0.9 × 0.1 | |||||||
| Z512 | |||||||
| 0.4 × 0.4 | |||||||
| 4 | 3D BRAVO (for white matter nulling) | 2000 | 400 | 256 × 256 | 220 | 0.8 | |
| 0.8 × 0.8 | |||||||
| Z512 | |||||||
| 5 | 3D BRAVO (used with #4 for wide mD dSIR) | 2000 | 800 | 256 × 256 | 220 | 0.8 | |
| 0.8 × 0.8 | |||||||
| Z512 | |||||||
| 6 | 2D T2-FLAIR | 6300 | 1851 | 102 | 320 × 240 | 26 | 4 |
| 0.7 × 0.7 | |||||||
| Z512 | |||||||
| 0.4 × 0.4 | |||||||
| 7 | 3D T2-FLAIR without/with fat saturation | 6300 | 1850 | 102 | 256 × 256 | 252 | 0.8 |
| 0.8 × 0.8 | |||||||
| Z512 | |||||||
| 0.6 × 0.6 | |||||||
| 8 | 3D susceptibility weighted | 40 | - | 32 | 300 × 300 | 110 | 2 |
| 0.8 × 0.8 | |||||||
| Z512 |
| Bipolar Filter | Reverse Bipolar Filter | Tissue Property |
|---|---|---|
| SIR | rSIR | T1 |
| dSIR | drSIR | T1 |
| cdSIR | cdrSIR | T1, T2, T2*, D* |
| lSIR | lrSIR | T1 |
| clSIR | clrSIR | T1, T2, T2*, D* |
| Synthetic SIR, dSIR, lSIR | rSIR, drSIR, lrSIR | T1 |
| Synthetic cdSIR, clSIR | cdrSIR, clrSIR | T1, T2, T2*, D* |
| # | Filter, Other Functions | Signal Equation | Figure # |
|---|---|---|---|
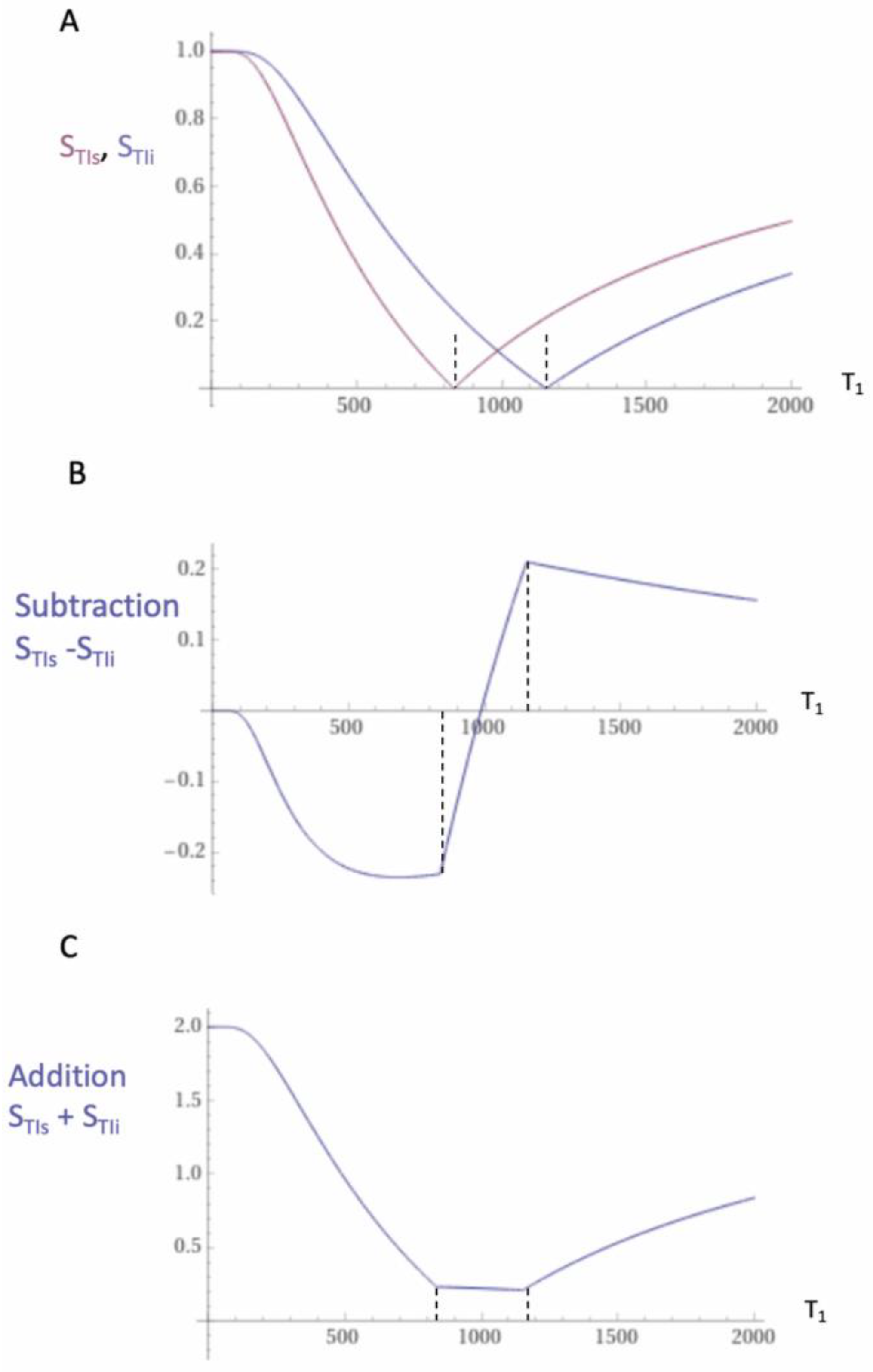
| 1 | IR, TIs | STIs = 1 − 2e−TIs/T1 | Figure 1, Figure 2 and Figure 3 |
| 2 | IR, TIi | STIi = 1 − 2e−TIi/T1 | Figure 1, Figure 2 and Figure 3 |
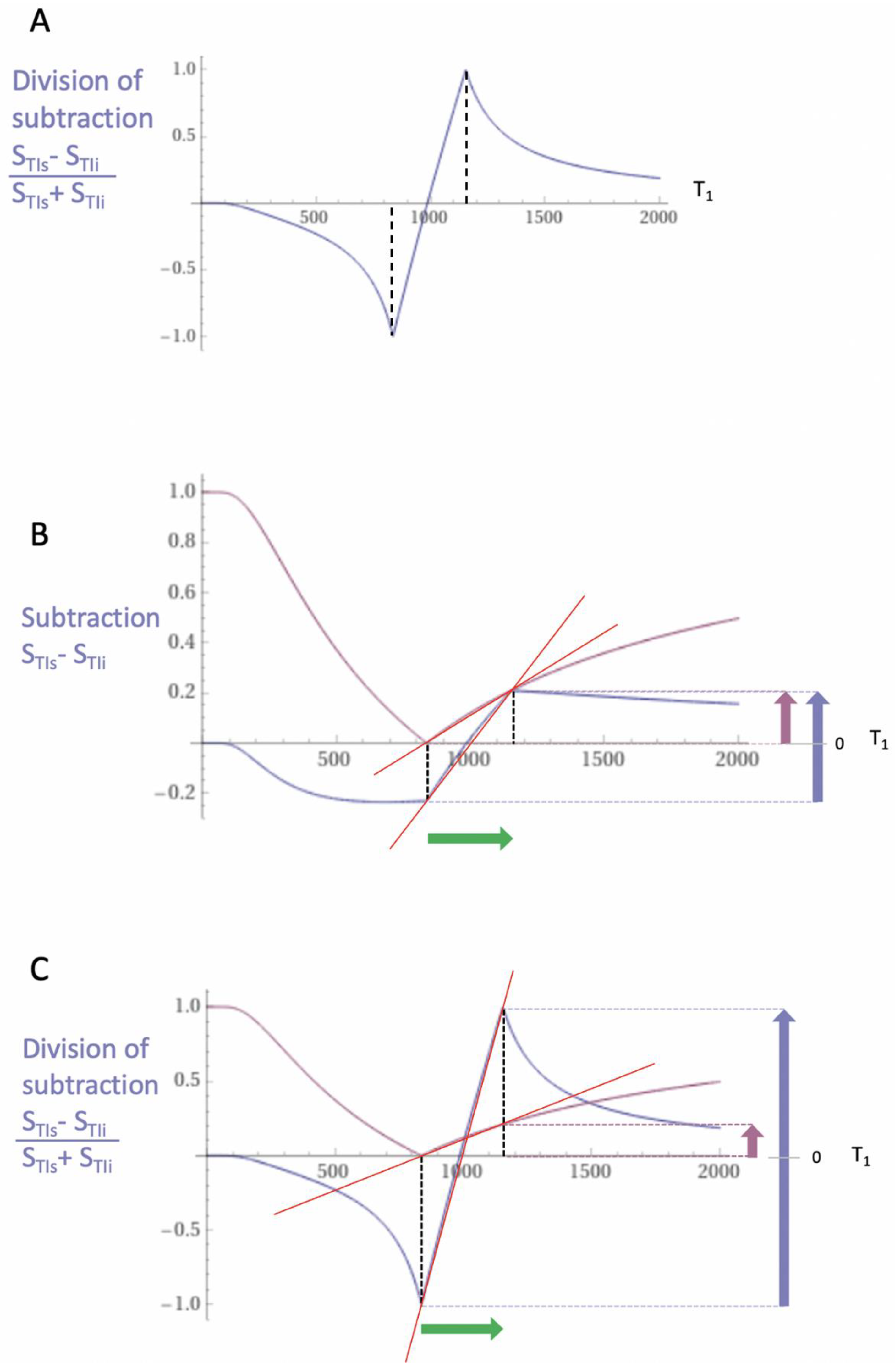
| 3 | SIR | SSIR = STIs − STIi | Figure 1 |
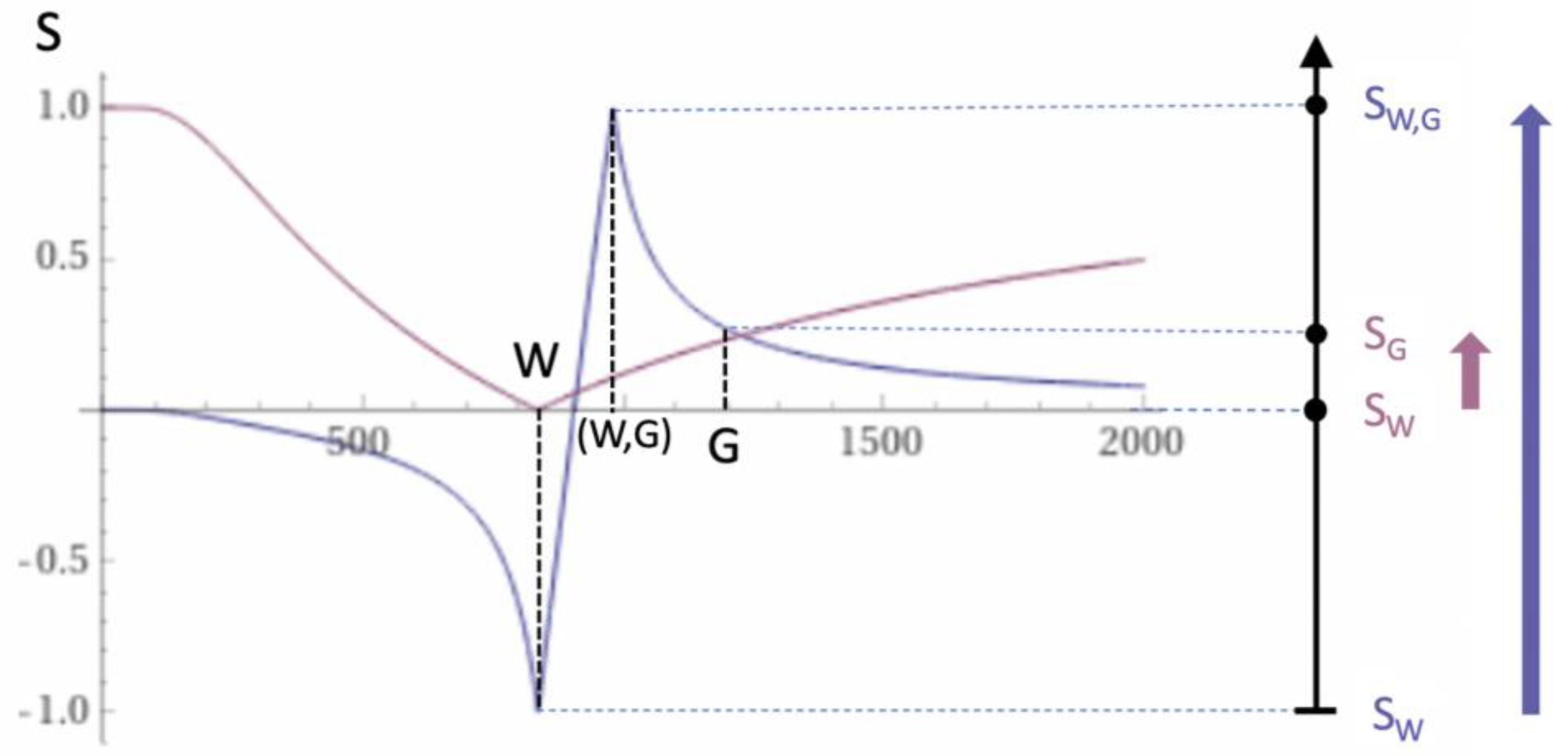
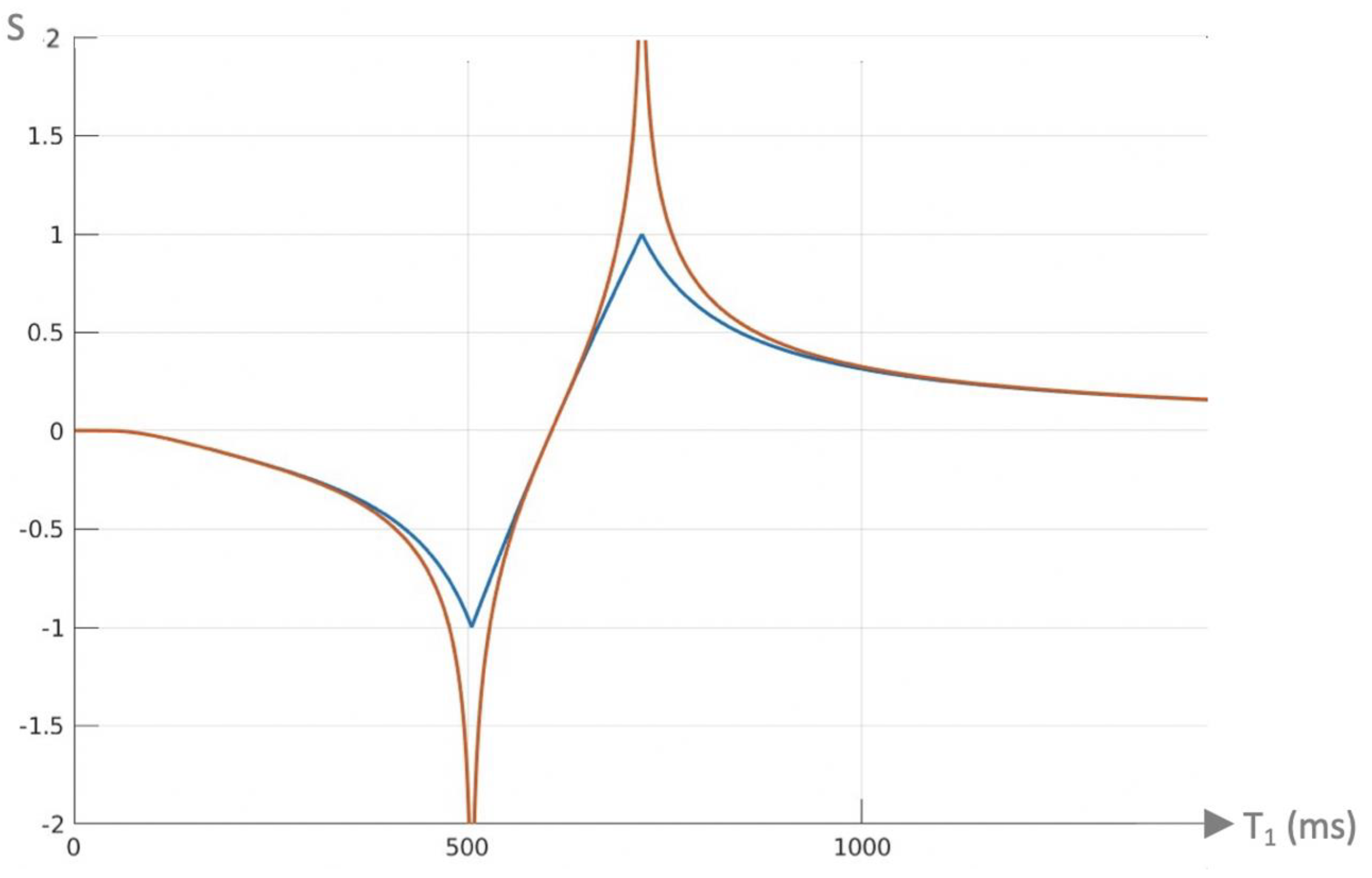
| 4 | dSIR | Figure 2 and Figure 3 | |
| 5 | cdSIR | Figure 5 | |
| 6 | cdSIR, SOF | SOF = ±e−ΔTE/T2, ±e−ΔTE/T2*, ±e−ΔbD*, etc. | Figure 5 |
| 7 | dSIR, SdSIR | (in mD) | Figure 2 |
| 8 | dSIR, T1 | (in mD) | Figure 2 |
| 9 | lSIR | SlSIR = ½(ln STIs − ln STIi) | Figure 4 |
| 10 | clSIR | Figure 4 | |
| 11 | clSIR, lSIR | , ±ΔbD*, etc | Figure 4 |
| 12 | lSIR, dSIR † | SlSIR = atanh SdSIR | Figure 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condron, P.; Cornfeld, D.M.; Bydder, M.; Kwon, E.E.; Whitehead, K.; Pravatà, E.; Danesh-Meyer, H.; Shi, C.; Emsden, T.C.; Newburn, G.; et al. Ultra-High Contrast (UHC) MRI of the Brain, Spinal Cord and Optic Nerves in Multiple Sclerosis Using Directly Acquired and Synthetic Bipolar Filter (BLAIR) Images. Diagnostics 2025, 15, 329. https://doi.org/10.3390/diagnostics15030329
Condron P, Cornfeld DM, Bydder M, Kwon EE, Whitehead K, Pravatà E, Danesh-Meyer H, Shi C, Emsden TC, Newburn G, et al. Ultra-High Contrast (UHC) MRI of the Brain, Spinal Cord and Optic Nerves in Multiple Sclerosis Using Directly Acquired and Synthetic Bipolar Filter (BLAIR) Images. Diagnostics. 2025; 15(3):329. https://doi.org/10.3390/diagnostics15030329
Chicago/Turabian StyleCondron, Paul, Daniel M. Cornfeld, Mark Bydder, Eryn E. Kwon, Karen Whitehead, Emanuele Pravatà, Helen Danesh-Meyer, Catherine Shi, Taylor C. Emsden, Gil Newburn, and et al. 2025. "Ultra-High Contrast (UHC) MRI of the Brain, Spinal Cord and Optic Nerves in Multiple Sclerosis Using Directly Acquired and Synthetic Bipolar Filter (BLAIR) Images" Diagnostics 15, no. 3: 329. https://doi.org/10.3390/diagnostics15030329
APA StyleCondron, P., Cornfeld, D. M., Bydder, M., Kwon, E. E., Whitehead, K., Pravatà, E., Danesh-Meyer, H., Shi, C., Emsden, T. C., Newburn, G., Scadeng, M., Holdsworth, S. J., & Bydder, G. M. (2025). Ultra-High Contrast (UHC) MRI of the Brain, Spinal Cord and Optic Nerves in Multiple Sclerosis Using Directly Acquired and Synthetic Bipolar Filter (BLAIR) Images. Diagnostics, 15(3), 329. https://doi.org/10.3390/diagnostics15030329






