A Challenging Diagnosis of Endometrial Stromal Sarcoma in a 50-Year-Old Patient: Case Report and Literature Review
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical Examination
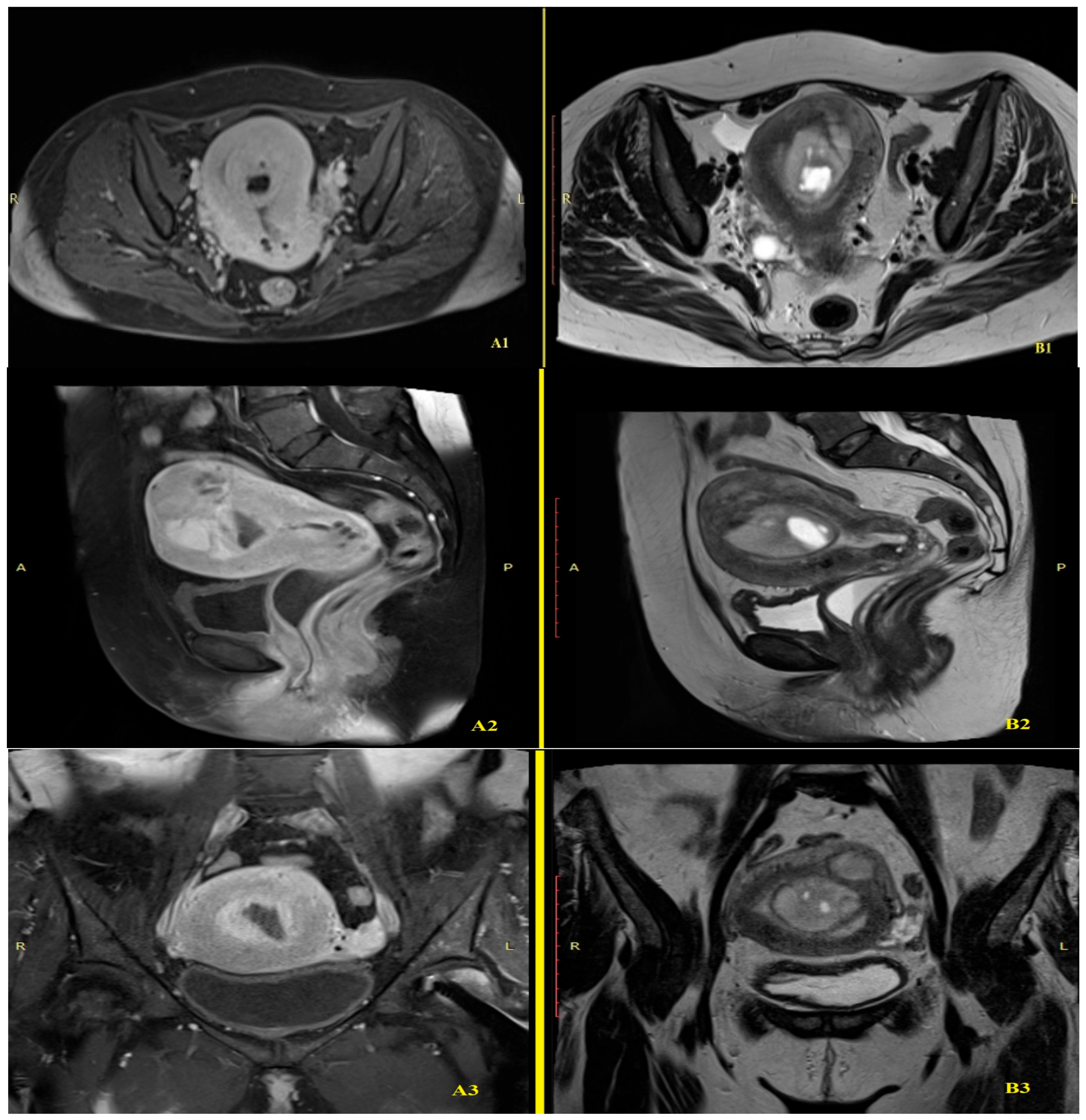
2.2. Imaging Findings
2.3. Initial Diagnosis and Management
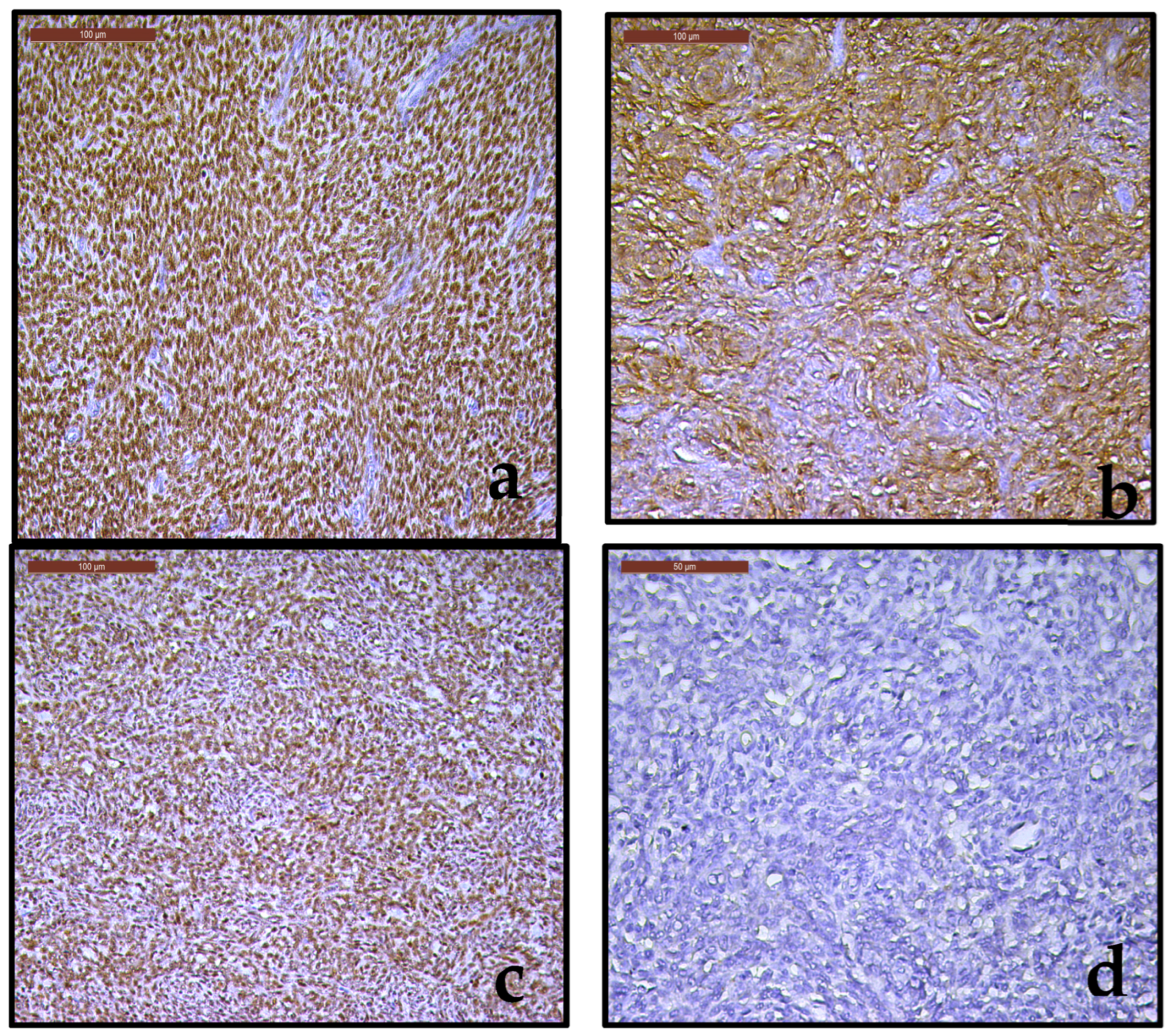
2.3.1. Pathological and Immunohistochemical Findings
2.3.2. Follow-Up
3. Discussion
3.1. Pathologic Features
- ESN: benign, non-invasive proliferation
- LG-ESS: typically associated with JAZF1, PHF1, MEAF6-PHF1, and related fusions; generally indolent
- HG-ESS: defined by YWHAE-NUTM2, BCOR, or ZC3H7B-BCOR fusions; aggressive clinical behavior
- UUS: lacks specific lineage differentiation or defining molecular markers; highly aggressive [1]
3.2. Immunohistochemical Findings
3.3. Molecular Features
| Features | LG-ESS | HG-ESS | ESN |
|---|---|---|---|
| Typical Age of Onset | 40–55 years (perimenopausal women) [3,4,5,8,45,57,58] | Any adult age, often younger than UUS [11,25,50,51,52,54,56] | Most often perimenopausal, but can occur at any age [11,12,13] |
| Growth behavior | Indolent, slow-growing [9,10,11,12,13] | Aggressive, rapid progression [9,24,49,50,51,53,56] | Benign [11,12,13] |
| Molecular hallmark | t (7;17) (p15;q21) → JAZF1-SUZ12 (~45%), JAZF1-PHF1, EPC1-PHF1, MEAF6-PHF1, BRD8-PHF1, EPC2-PHF1, rare MBTD1-CXorf67, JAZF1-BCORL1 [37,54,55] | t (10;17) (q22;p13) → YWHAE-NUTM2 fusion (NUTM2A/NUTM2B), ZC3H7B-BCOR fusion; BCOR internal tandem duplications (ITDs) [25,55,56] | t (7;17) (p15;q21) → JAZF1-SUZ12 fusion in ~75% [37,54] |
| Histology | Small, uniform spindle cells; tongue-like infiltration [9,10,11,12,13] | Large round cells in nests; high cellularity, frequent necrosis [49,50,51] | Proliferative-phase stromal cells, minimal atypia; well-circumscribed, unencapsulated; no myometrial/lymphovascular invasion [11,12,13] |
| Mitotic index | <5 mitoses/10 HPF [9,10,11,12,13] | >10–30 mitoses/10 HPF [49,50,51] | ≤5 mitoses/10 HPF [11,12,13] |
| Cyclin D1 Expression | Usually negative, Cyclin D1 <10% nuclei [9,10,11,12,13] | Cyclin D1 strong diffuse nuclear positivity (>70%) nuclei positive [9,24,49,50,51,53,56] | Negative [11,12,13] |
| Immunohistochemistry | CD10+, ER+, PR+, WT1+, vimentin+, actins+, IFITM1+; may express SMA, β-catenin, pancytokeratins; CD117, BCOR negative (non-rearranged); no BCOR protein overexpression, normal/p53 wild-type pattern (non-aberrant) [17,18,19,45,46,47,52] | ER−/PR− or only focally positive; BCOR overexpression in BCOR rearranged tumors (ZC3H7B-BCOR fusion, BCOR ITD); not all HG-ESS are BCOR-positive, CD10 variable or focal; negative for smooth-muscle markers. [9,24,49,50,51,53,56] | CD10+, ER+, PR+, and absence of infiltration, WT1 variably positive/weak/focal; AR−, SMA−, desmin−; vimentin+; β-catenin membranous; cytokeratins focal positive/weak; IFITM1+ (weak/focal positive), BCOR negative (non-rearranged pattern) [11,12] |
| Margins and invasion | Myometrial and lymphovascular invasion common [9,10,11,12,13] | Extensive myometrial and extrauterine invasion [9,24,49,50,51,53,56] | No invasion (diagnosis requires absence of myometrial and lymphovascular invasion) [11,12,13] |
| Common Sites of Recurrence | Pelvis, lungs, abdomen (late recurrence) [9,10,11,12,13] | Often extrauterine at diagnosis (e.g., lung, liver) [9,24,49,50,51,53,56] | Rare [11,12,13] |
| Recurrence risk | 10–20%, can recur decades later [9,10,11,12,13] | High, often early post-treatment [9,24,49,50,51,53,56] | Very low [11,12,13] |
| Prognosis | Favorable (5-year survival ~98% for stage I) [45,59,60] | Poor (5-year OS 25–55%) [9,24,49,50,51,53,56] | Excellent [11,12,13] |
| Symptoms | Abnormal uterine bleeding, pelvic pain [3,4,5,6,7,8] | Often presents with extrauterine disease at diagnosis (up to 40–70%), abnormal bleeding, extrauterine spread [49,50,51] | Often asymptomatic; abnormal uterine bleeding if present [11,12,13] |
| Growth pattern | Infiltrative with ‘worm-like’ extensions [9,10,11,12,13] | Polypoid, infiltrative with necrosis [49,50,51] | Intramural, submucosal, or polypoid; well-circumscribed [11,12,13] |
| Treatment | Total hysterectomy + BSO; Adjuvant endocrine therapy is generally reserved for recurrent or advanced disease, not for completely resected stage I tumors. Often presents with extrauterine disease at diagnosis (up to 40–70%) [9,10,11,12,13,45,57,58] | Surgery (hysterectomy + BSO) followed by chemotherapy (anthracycline-based regimens such as doxorubicin ± ifosfamide). Radiotherapy may be considered for local control in selected cases [9,24,49,50,51,53,56] | Hysterectomy; no adjuvant therapy needed [11,12,13] |
3.4. Imaging and Clinical Presentation
3.5. Treatment and Adjuvant Management of LG-ESS
3.5.1. Surgical Management
3.5.2. Adjuvant Hormonal Therapy
3.5.3. Adjuvant Chemotherapy
3.5.4. Radiotherapy
3.5.5. Emerging Therapies and Follow-Up
3.6. Prognostic Factors
Recurrence and Metastatic Spread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent diffusion coefficient; |
| AR | Androgen receptor; |
| BCOR | Bcl6 corepressor; |
| BSO | Bilateral salpingo-oophorectomy; |
| CA125 | Cancer antigen 125; |
| CD10 | Common acute lymphoblastic leukemia antigen; |
| CD117 | C-kit proto-oncogene; |
| CT | Computed tomography; |
| DWI | Diffusion-weighted imaging; |
| EGFR | Epidermal growth factor receptor expression; |
| ER | Estrogen receptor; |
| ESN | Endometrial stromal nodule; |
| ESS | Endometrial stromal sarcoma; |
| HG-ESS | High-grade endometrial stromal sarcoma; |
| HIPEC | Hyperthermic intraperitoneal chemotherapy; |
| HPF | High-power field; |
| IFITM1 | Interferon-induced transmembrane protein 1; |
| LG-ESS | Low-grade endometrial stromal sarcoma; |
| MRI | Magnetic resonance imaging; |
| OS | Overall survival; |
| PDGFR | Platelet-derived growth factor receptor; |
| PR | Progesterone receptor; |
| SLN | Sentinel lymph node; |
| SMA | Smooth muscle actin; |
| TH | Total hysterectomy; |
| UES | Undifferentiated endometrial sarcoma; |
| UUS | Undifferentiated uterine sarcoma; |
| WHO | World Health Organization; |
| WT1 | Wilms tumor protein 1. |
References
- Kim, K.-R.; Lax, S.F.; Lazar, A.J.; Lonagcre, T.A.; Malpica, A.; Matias-Guiu, X.; Nucci, M.R.; Oliva, E. Tumours of the uterine corpus. In Female Genital Tumours. WHO Classification of Tumours, 5th ed.; WHO Classification of Tumours Editorial Board; IARC Press: Lyon, France, 2020; pp. 283–293. [Google Scholar]
- Masand, R.P.; Euscher, E.; Deavers, M.T.; Malpica, A. Endometrial stromal sarcoma: A clinic-pathologic study of 63 cases. Am. J. Surg. Pathol. 2013, 37, 1635–1647. [Google Scholar] [CrossRef]
- Rauh-Hain, J.A.; del Carmen, M.G. Endometrial stromal sarcoma: A systematic review. Obs. Gynecol. 2013, 122, 676–683. [Google Scholar] [CrossRef]
- Hanby, A.M.; Walker, C.; Tavassoli, F.A.; Devilee, P. Pathology and Genetics: Tumours of the Breast and Female Genital Organs; WHO Classification of Tumours series; IARC Press: Lyon, France, 2004; Volume 4, p. 133. [Google Scholar]
- Denschlag, D.; Thiel, F.C.; Ackermann, S.; Harter, P.; Juhasz-Boess, I.; Mallmann, P.; Strauss, H.G.; Ulrich, U.; Horn, L.C.; Schmidt, D.; et al. Sarcoma of the Uterus. Guideline of the DGGG (S2k-Level, AWMF Registry No. 015/074, August 2015). Geburtshilfe Frauenheilkd 2015, 75, 1028–1042. [Google Scholar]
- Lee, C.H.; Ou, W.B.; Mariño-Enriquez, A.; Zhu, M.; Mayeda, M.; Wang, Y.; Guo, X.; Brunner, A.L.; Amant, F.; French, C.A.; et al. 14-3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc. Natl. Acad. Sci. USA 2012, 109, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Félix, A.; Lennerz, J.K.; Oliva, E. Recent advances in the histological and molecular classification of endometrial stromal neoplasms. Virchows Arch. 2018, 473, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Conklin, C.M.J.; Longacre, T.A. Endometrial stromal tumors: The new WHO classification. Adv. Anat. Pathol. 2014, 21, 383–393. [Google Scholar] [CrossRef]
- Chan, J.K.; Kawar, N.M.; Shin, J.Y.; Osann, K.; Chen, L.M.; Powell, C.B.; Kapp, D.S. Endometrial stromal sarcoma: A population-based analysis. Br. J. Cancer 2008, 99, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Abeler, V.M.; Røyne, O.; Thoresen, S.; Danielsen, H.E.; Nesland, J.M.; Kristensen, G.B. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009, 54, 355–364. [Google Scholar] [CrossRef]
- Ali, R.H.; Rouzbahman, M. Endometrial stromal tumours revisited: An update based on the 2014 WHO classification. J. Clin. Pathol. 2015, 68, 325–332. [Google Scholar] [CrossRef]
- Leary, A.F.; Quinn, M.; Fujiwara, K.; Coleman, R.L.; Kohn, E.; Sugiyama, T.; Glasspool, R.; Ray-Coquard, I.; Colombo, N.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup (GCIG): Clinical trial design for rare ovarian tumours. Ann. Oncol. 2017, 28, 718–726. [Google Scholar] [CrossRef]
- Usta, T.A.; Sonmez, S.E.; Oztarhan, A.; Karacan, T. Endometrial stromal sarcoma in the abdominal wall arising from scar endometriosis. J. Obs. Gynaecol. 2014, 6, 541–542. [Google Scholar] [CrossRef]
- Laufer, J.; Scasso, S.; Kim, B.; Shahi, M.; Mariani, A. Fertility-sparing management of low-grade endometrial stromal sarcoma. Int. J. Gynecol. Cancer 2023, 33, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Puliyath, G.; Nair, M.K. Endometrial stromal sarcoma: A review of the literature. Indian J. Med. Paediatr. Oncol. 2012, 33, 1–6. [Google Scholar] [CrossRef]
- Shah, S.H.; Jagannathan, J.P.; Krajewski, K.; O’Regan, K.N.; George, S.; Ramaiya, N.H. Uterine sarcomas: Then and now. AJR Am. J. Roentgenol. 2012, 199, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Bryant, C.S.; Kumar, S.; Ali-Fehmi, R.; Malone, J.M.; Morris, R.T. Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obs. Gynecol. 2008, 112, 1102–1108. [Google Scholar] [CrossRef]
- Nucci, M.R. Practical issues related to uterine pathology: Endometrial stromal tumors. Mod. Pathol. 2016, 29, S92–S103. [Google Scholar] [CrossRef] [PubMed]
- Kontomanolis, E.N.; Sapantzoglou, I.; Nikolettos, K.; Kontogeorgi, E.; Lampraki, V.; Papageorgiou, D.; Perros, P.; Fasoulakis, Z.; Koulakmanidis, A.-M.; Daskalaki, M.-A.; et al. Clinicopathological Predictors of Recurrence in Uterine Sarcomas-A Narrative Review. J. Clin. Med. 2025, 14, 4883. [Google Scholar] [CrossRef]
- Hwang, H.; Matsuo, K.; Duncan, K.; Pakzamir, E.; Pham, H.Q.; Correa, A.; Fedenko, A.; Mhawech-Fauceglia, P. Immunohistochemical panel to differentiate endometrial stromal sarcoma, uterine leiomyosarcoma and leiomyoma: Something old and something new. J. Clin. Pathol. 2015, 68, 710–717. [Google Scholar] [CrossRef]
- Ichimura, T.; Kasai, M.; Imai, K.; Yamauchi, M.; Fukuda, T.; Yasui, T.; Sumi, T. A difficult to diagnose case of low-grade endometrial stromal sarcoma with smooth muscle differentiation treated with laparoscopic surgery: A case report. Mol. Clin. Oncol. 2022, 16, 92. [Google Scholar] [CrossRef]
- Busca, A.; Gulavita, P.; Parra-Herran, C.; Islam, S. IFITM1 Outperforms CD10 in differentiating low-grade endometrial stromal sarcomas from smooth muscle neoplasms of the uterus. Int. J. Gynecol. Pathol. 2018, 37, 372–378. [Google Scholar] [CrossRef]
- Jung, C.K.; Jung, J.H.; Lee, A.; Lee, Y.-S.; Choi, Y.-J.; Yoon, S.-K.; Lee, K.-Y. Diagnostic use of nuclear β-catenin expression for the assessment of endometrial stromal tumors. Mod. Pathol. 2008, 21, 756–763. [Google Scholar] [CrossRef]
- Adiga, C.P.; Gyanchandani, M.; Goolahally, L.N.; Itagi, R.M.; Kalenahalli, K.V. Endometrial stromal sarcoma: An aggressive uterine malignancy. J. Radiol. Case Rep. 2016, 10, 35–43. [Google Scholar] [CrossRef]
- Rha, S.E.; Byun, J.Y.; Jung, S.E.; Lee, S.L.; Cho, S.M.; Hwang, S.S.; Lee, H.G.; Namkoong, S.E.; Lee, J.M. CT and MRI of uterine sarcomas and their mimickers. AJR Am. J. Roentgenol. 2003, 181, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Leath, C.A.; Huh, W.K.; Hyde, J.; Cohn, D.E.; Resnick, K.E.; Taylor, N.P.; Powell, M.A.; Mutch, D.G.; Bradley, W.H.; Geller, M.A.; et al. A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol. Oncol. 2007, 105, 630–634. [Google Scholar] [CrossRef]
- Gothwal, M.; Yadav, G.; Rao, M.; Singh, P.; Nalwa, A. Low-Grade Endometrial Stromal Sarcoma in a Postmenopausal Woman with Third-Degree Uterovaginal Prolapse: A Rare Case with Review of the Literature. J. Midlife Health 2018, 9, 165–167. [Google Scholar] [CrossRef]
- Cui, R.; Yuan, F.; Wang, Y.; Li, X.; Zhang, Z.; Bai, H. Clinicopathological characteristics and treatment strategies for patients with low-grade endometrial stromal sarcoma. Medicine 2017, 96, e6584. [Google Scholar] [CrossRef]
- Amant, F.; Coosemans, A.; Debiec-Rychter, M.; Timmerman, D.; Vergote, I. Clinical management of uterine sarcomas. Lancet Oncol. 2009, 10, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Toptas, T.; Oz, M.; Vardar, M.A.; Kayikcioglu, F.; Ozgul, N.; Gokcu, M.; Simsek, T.; Tunc, M.; Meydanli, M.M. Low-grade endometrial stromal sarcoma: A Turkish uterine sarcoma group study analyzing prognostic factors and disease outcomes. Gynecol. Oncol. 2021, 160, 674–680. [Google Scholar] [CrossRef]
- Singhal, S.; Jayraj, A.S.; Dhamija, E.; Khurana, S. Low-grade extrauterine endometrial stromal sarcoma arising from vaginal endometriosis: A case report and literature review. Korean J. Clin. Oncol. 2023, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Stefanko, D.P.; Eskander, R.; Aisagbonhi, O. Disseminated endometriosis and low- grade endometrioid stromal sarcoma in a patient with a history of uterine morcellation for adenomyosis. Case Rep. Obs. Gynecol. 2020, 2020, 7201930. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Clarke, B.A.; McGilvray, I.; Dickson, B.C.; Khalili, K.; Chetty, R. Metastatic low-grade endometrial stromal sarcoma of uterus presenting as a primary pancreatic tumor: Case presentation and literature review. Diagn. Pathol. 2019, 14, 30. [Google Scholar] [CrossRef]
- Smith, E.S.; Jansen, C.; Miller, K.M.; Chiang, S.; Alektiar, K.M.; Hensley, M.L.; Mueller, J.J.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Primary characteristics and outcomes of newly diagnosed low-grade endometrial stromal sarcoma. Int. J. Gynecol. Cancer 2022, 32, 882–890. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. The impact of tumor morcellation during surgery on the outcomes of patients with apparently early low- grade endometrial stromal sarcoma of the uterus. Ann. Surg. Oncol. 2011, 18, 3453–3561. [Google Scholar] [CrossRef]
- Nakabayashi, A.; Odaira, K.; Horibe, Y.; Kanno, T.; Akizawa, Y.; Tabata, T. A case of unsuspected low-grade endometrial stromal sarcoma successfully treated with two minimally invasive surgeries. Gynecol. Minim. Invasive Ther. 2020, 9, 237–240. [Google Scholar] [CrossRef]
- Makise, N.; Sekimizu, M.; Kobayashi, E.; Yoshida, H.; Fukayama, M.; Kato, T.; Kawai, A.; Ichikawa, H.; Yoshida, A. Low-grade endometrial stromal sarcoma with a novel MEAF6-SUZ12 fusion. Virchows Arch. 2019, 475, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Choi, Y.S.; Song, I.C.; Yun, H.J.; Jo, D.Y.; Kim, S.; Lee, H.J. Long-term treatment of residual or recurrent low-grade endometrial stromal sarcoma with aromatase inhibitors: A report of two cases and a review of the literature. Oncol. Lett. 2015, 10, 3310–3314. [Google Scholar] [CrossRef] [PubMed]
- Dahhan, T.; Fons, G.; Buist, M.R.; Ten Kate, F.J.W.; van der Velden, J. The efficacy of hormonal treatment for residual or recurrent low-grade endometrial stromal sarcoma. A retrospective study. Eur. J. Obs. Gynecol. Reprod. Biol. 2009, 144, 80–84. [Google Scholar] [CrossRef]
- Bai, H.; Yang, J.; Cao, D.; Huang, H.; Xiang, Y.; Wu, M.; Cui, Q.; Chen, J.; Lang, J.; Shen, K. Ovary and uterus-sparing procedures for low-grade endometrial stromal sarcoma: A retrospective study of 153 cases. Gynecol. Oncol. 2014, 132, 654–660. [Google Scholar] [CrossRef]
- Comert, G.K.; Turkmen, O.; Kar, I.; Yucel, O.; Kilic, C.; Boran, N.; Basaran, D.; Karalok, A.; Turan, T. Hormone therapy following surgery in low-grade endometrial stromal sarcoma: Is it related to a decrease in recurrence rate? J. Chin. Med. Assoc. 2019, 82, 385–389. [Google Scholar] [CrossRef]
- Nasioudis, D.; Ko, E.M.; Kolovos, G.; Vagios, S.; Kalliouris, D.; Giuntoli, R.L. Ovarian preservation for low-grade endometrial stromal sarcoma: A systematic review of the literature and meta-analysis. Int. J. Gynecol. Cancer 2019, 29, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Clair, K.; Wolford, J.; Veran-Taguibao, S.; Kim, G.; Eskander, R.N. Primary low-grade endometrial stromal sarcoma of the omentum. Gynecol. Oncol. Rep. 2017, 21, 119–121. [Google Scholar] [CrossRef]
- Niu, S. Diagnostic challenges and considerations of low-grade endometrial stromal sarcoma (LGESS) outside the female genital tract. Transl. Cancer Res. 2022, 11, 3445–3447. [Google Scholar] [CrossRef]
- Chang, K.L.; Crabtree, G.S.; Lim-Tan, S.K.; Kempson, R.L.; Hendrickson, M.R. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am. J. Surg. Pathol. 1990, 14, 415–438. [Google Scholar] [CrossRef]
- Günter, K.; Matthias, E. Uterine Sarkome und Mischtumoren: Handbuch und Bildatlas zur Diagnostik und Therapie; De Gruyter: Berlin, Germany; New York, NY, USA, 2009. [Google Scholar]
- Kurihara, S.; Oda, Y.; Ohishi, Y.; Iwasa, A.; Takahira, T.; Kaneki, E.; Kobayashi, H.; Wake, N.; Tsuneyoshi, M. Endometrial stromal sarcomas and related high-grade sarcomas: Immunohistochemical and molecular genetic study of 31 cases. Am. J. Surg. Pathol. 2008, 32, 1228–1238. [Google Scholar] [CrossRef]
- Rabban, J.T.; Gilks, C.B.; Malpica, A.; Matias-Guiu, X.; Mittal, K.; Mutter, G.L.; Oliva, E.; Parkash, V.; Ronnett, B.M.; Staats, P.; et al. Issues in the Differential Diagnosis of Uterine Low-grade Endometrioid Carcinoma, Including Mixed Endometrial Carcinomas: Recommendations from the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2019, 38, S25–S39. [Google Scholar] [CrossRef]
- Mariño-Enriquez, A.; Lauria, A.; Przybyl, J.; Ng, T.L.; Kowalewska, M.; Debiec-Rychter, M.; Ganesan, R.; Sumathi, V.; George, S.; McCluggage, W.G.; et al. BCOR Internal Tandem Duplication in High-grade Uterine Sarcomas. Am. J. Surg. Pathol. 2018, 42, 335–341. [Google Scholar] [CrossRef]
- Lee, C.H.; Mariño-Enriquez, A.; Ou, W.; Zhu, M.; Ali, R.H.; Chiang, S.; Amant, F.; Gilks, C.B.; van de Rijn, M.; Oliva, E.; et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: A histologically high-grade and clinically aggressive tumor. Am. J. Surg. Pathol. 2012, 36, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Garg, G.; Shah, J.P.; Toy, E.P.; Bryant, C.S.; Kumar, S.; Morris, R.T. Stage IA vs. IB endometrial stromal sarcoma: Does the new staging system predict survival? Gynecol. Oncol. 2010, 118, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, Y.; Qin, M.; Cai, Y.; Jin, Y.; Pan, L.Y. High-grade endometrial stromal sarcoma: A retrospective study of factors influencing prognosis. Cancer Manag. Res. 2019, 11, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Baek, M.H.; Park, Y.; Kim, Y.T.; Nam, J.H. Investigation of hormone receptor expression and its prognostic value in endometrial stromal sarcoma. Virchows Arch. 2018, 473, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tsuyoshi, H.; Yoshida, Y. Molecular biomarkers for uterine leiomyosarcoma and endometrial stromal sarcoma. Cancer Sci. 2018, 109, 1743–1752. [Google Scholar] [CrossRef]
- Micci, F.; Heim, S.; Panagopoulos, I. Molecular pathogenesis and prognostication of “low-grade’’ and “high-grade” endometrial stromal sarcoma. Genes. Chromosomes Cancer 2021, 60, 160–167. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Sung, W.J.; Hong, J. High-Grade Endometrial Stromal Sarcoma: Molecular Alterations and Potential Immunotherapeutic Strategies. Front. Immunol. 2022, 13, 837004. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.; Clough, E.; Goldsmith, P.; Burke, J.R. Peritoneal inclusion cyst presenting as an umbilical hernia: Case report and systematic review of the literature. J. Surg. Case Rep. 2024, 2024, rjae258. [Google Scholar] [CrossRef] [PubMed]
- Reich, O.; Singer, C.; Hudelist, G.; Regauer, S. Estrogen sulfotransferase expression in endometrial stromal sarcomas: An immunohistochemical study. Pathol. Res. Pract. 2007, 203, 85–87. [Google Scholar] [CrossRef]
- Gadducci, A. Prognostic factors in uterine sarcoma. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Cosio, S.; Romanini, A.; Genazzani, A.R. The management of patients with uterine sarcoma: A debated clinical challenge. Crit. Rev. Oncol. Hematol. 2008, 65, 129–142. [Google Scholar] [CrossRef]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in gynecological disease (15): Clinical and ultrasound characteristics of uterine sarcoma. Ultrasound Obs. Gynecol. 2019, 54, 676–687. [Google Scholar]
- Santos, P.; Cunha, T.M. Uterine sarcomas: Clinical presentation and MRI features. Diagn. Interv. Radiol. 2015, 21, 4–9. [Google Scholar] [CrossRef]
- Sala, E.; Rockall, A.G.; Freeman, S.J.; Mitchell, D.G.; Reinhold, C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: What the radiologist needs to know. Radiology 2013, 266, 717–740. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ueng, S.H.; Chen, K.; Huang, Y.T.; Lu, H.Y.; Ng, K.K.; Chang, T.C.; Lai, C.H.; Lin, G. Utility of diffusion-weighted and contrast-enhanced magnetic resonance imaging in diagnosing and differentiating between high- and low-grade uterine endometrial stromal sarcoma. Cancer Imaging 2019, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, V.; Bizzarri, N.; Guida, F.; Vascone, C.; Costantini, B.; Scambia, G.; Fagotti, A. Role of surgery in gynaecological sarcomas. Oncotarget 2019, 10, 2561–2575. [Google Scholar] [CrossRef]
- Amant, F.; De Knijf, A.; Van Calster, B.; Leunen, K.; Neven, P.; Berteloot, P.; Vergote, I.; Van Huffel, S.; Moerman, P. Clinical study investigating the role of lymphadenectomy, surgical castration and adjuvant hormonal treatment in endometrial stromal sarcoma. Br. J. Cancer 2007, 97, 1194–1199. [Google Scholar] [CrossRef]
- Deshmukh, U.; Black, J.; Perez-Irizarry, J.; Passarelli, R.; Levy, K.; Rostkowski, A.; Hui, P.; Rutherford, T.J.; Santin, A.D.; Azodi, M.; et al. Adjuvant Hormonal Therapy for Low-Grade Endometrial Stromal Sarcoma. Reprod. Sci. 2019, 26, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.; Schultheiss, T.E.; Ryu, J.K.; Wong, J.Y.C. The role of adjuvant radiation in uterine sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 728–734. [Google Scholar] [CrossRef]
- Yamazaki, H.; Todo, Y.; Mitsube, K.; Hareyama, H.; Shimada, C.; Kato, H.; Yamashiro, K. Long-term survival of patients with recurrent endometrial stromal sarcoma: A multicenter, observational study. J. Gynecol. Oncol. 2015, 26, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Seagle, B.L.L.; Sobecki-Rausch, J.; Strohl, A.E.; Shilpi, A.; Grace, A.; Shahabi, S. Prognosis and treatment of uterine leiomyosarcoma: A National Cancer Database study. Gynecol. Oncol. 2017, 145, 61–70. [Google Scholar] [CrossRef]
- Reed, N.S.; Mangioni, C.; Malmström, H.; Scarfone, G.; Poveda, A.; Pecorelli, S.; Tateo, S.; Franchi, M.; Jobsen, J.J.; Coens, C.; et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur. J. Cancer 2008, 44, 808–818. [Google Scholar] [CrossRef]
- Hoang, L.; Chiang, S.; Lee, C.H. Endometrial stromal sarcomas and related neoplasms: New developments and diagnostic considerations. Pathology 2018, 50, 162–177. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, G.; Schmeler, K.M.; Coleman, R.L.; Tu, X.; Liu, J.; Kavanagh, J.J. Recurrence patterns and prognosis of endometrial stromal sarcoma and the potential of tyrosine kinase-inhibiting therapy. Gynecol. Oncol. 2011, 121, 323–327. [Google Scholar] [CrossRef]
- Akahira, J.; Tokunaga, H.; Toyoshima, M.; Takano, T.; Nagase, S.; Yoshinaga, K.; Tase, T.; Wada, Y.; Ito, K.; Niikura, H.; et al. Prognoses and prognostic factors of carcinosarcoma, endometrial stromal sarcoma and uterine leiomyosarcoma: A comparison with uterine endometrial adenocarcinoma. Oncology 2006, 71, 333–430. [Google Scholar] [CrossRef]
- Borella, F.; Bertero, L.; Cassoni, P.; Piovano, E.; Gallio, N.; Preti, M.; Cosma, S.; Ferraioli, D.; Pace, L.; Mariani, L.; et al. Low-Grade Uterine Endometrial Stromal Sarcoma: Prognostic Analysis of Clinico-Pathological Characteristics, Surgical Management, and Adjuvant Treatments. Experience From Two Referral Centers. Front. Oncol. 2022, 12, 883344. [Google Scholar] [CrossRef]
- Scher, D.; Nghiem, W.; Aziz, S.; Rahbar, R.; Banks, W.; Venbrux, A.; Sarin, S. Endometrial Stromal Sarcoma Metastatic from the Uterus to the Inferior Vena Cava and Right Atrium. Tex Heart Inst. J. 2015, 42, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H. Surgical treatment of uterine sarcoma. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Reuss, A.; Baumann, K.; Hanker, L.; Kimmig, R.; Schröder, W.; Burges, A.; Gropp-Meier, M.; Kurzeder, C.; et al. Phase II Study Evaluating PegLiposomal Doxorubicin and Carboplatin Combination Chemotherapy in Gynecologic Sarcomas and Mixed Epithelial-Mesenchymal Tumors A Phase II Protocol of the Arbeitsgemeinschaft Gynaekologische Onkologie Study Group (AGO-GYN 7). Int. J. Gynecol. Cancer 2016, 26, 1636–1641. [Google Scholar] [CrossRef]
- Kalender, M.E.; Sevinc, A.; Yilmaz, M.; Ozsarac, C.; Camci, C. Detection of complete response to imatinib mesylate (Glivec/Gleevec) with 18F-FDG PET/CT for low-grade endometrial stromal sarcoma. Cancer Chemother. Pharmacol. 2009, 63, 555–559. [Google Scholar] [PubMed]



| ESN |
| Absence of myometrial invasion, or no more than 3 tongues of invasion, each <3 mm No lymphovascular invasion Histology shows well-circumscribed, non-infiltrative nodules resembling proliferative endometrial stroma. IHC profile: CD10+, ER+, PR+, vimentin+ (variable intensity); WT1: usually focal or negative (important distinction from LG-ESS); Cytokeratin, SMA: focal/weak; Desmin: negative; β-catenin membranous, not nuclear (nuclear → favors LG-ESS with CTNNB1 mutation) |
| HG-ESS |
| Significant cytologic atypia High mitotic activity (>10–30 mitoses/10 HPF) Frequent necrosis Immunophenotype: Cyclin D1: strong, diffuse nuclear positivity (>70% of tumor nuclei); BCOR: strong, diffuse nuclear positivity in BCOR-rearranged cases; ER/PR: typically negative; CD10: usually negative or only focal Molecular alterations: YWHAE–NUTM2A/B fusion (hallmark); ZC3H7B–BCOR fusion; BCOR internal tandem duplications (ITDs); HG-ESS categories correspond to these genetic events. |
| CELLULAR LEIOMYOMA |
| Uniform smooth muscle cells with fascicular architecture Thick-walled blood vessels Cleft-like spaces No infiltrative margins Molecular profile: Lacks endometrial stromal fusions (JAZF1, PHF1) IHC: SMA+, desmin+, h-caldesmon+; CD10: may be focal but not diffuse; ER/PR: can be positive |
| LEIOMYOSARCOMA |
| Marked cytologic atypia High mitotic index Coagulative tumor cell necrosis Thick-walled, hyalinized vessels Molecular profile: Does NOT harbor JAZF1 or PHF1 fusions IHC: SMA+, desmin+, h-caldesmon+ (strong, diffuse); CD10: usually negative; ER/PR: variable |
| UTROSCT |
| Lacks conventional endometrial stromal differentiation Architectural patterns mimicking ovarian sex cord tumors Molecular alterations: Gene fusions involving ESR1 or GREB1 (e.g., ESR1-NCOA2, GREB1-NCOA2) IHC: Positive for sex cord markers: inhibin, calretinin, SF-1, CD99; CD10: usually negative; ER/PR variably positive |
| ENDOMETRIAL POLYP |
| Does not show expansile stromal growth Does not displace surrounding endometrium No infiltrative pattern Often contains thick-walled vessels and fibrous stroma |
| GLAND-POOR ADENOMYOSIS |
| No confluent stromal proliferation Does not displace or infiltrate myometrium Endometrial stroma present only around ectopic glands |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haliciu, A.-M.; Furnică, C.; Stan, C.I.; Gemanariu, R.-M.; Pavaleanu, I.; Buțureanu, T.A.; Pruteanu, A.; Balan, T.A.; Anghel, B.G.; Balan, R.A. A Challenging Diagnosis of Endometrial Stromal Sarcoma in a 50-Year-Old Patient: Case Report and Literature Review. Diagnostics 2025, 15, 3215. https://doi.org/10.3390/diagnostics15243215
Haliciu A-M, Furnică C, Stan CI, Gemanariu R-M, Pavaleanu I, Buțureanu TA, Pruteanu A, Balan TA, Anghel BG, Balan RA. A Challenging Diagnosis of Endometrial Stromal Sarcoma in a 50-Year-Old Patient: Case Report and Literature Review. Diagnostics. 2025; 15(24):3215. https://doi.org/10.3390/diagnostics15243215
Chicago/Turabian StyleHaliciu, Ana-Maria, Cristina Furnică, Cristinel Ionel Stan, Raluca-Mihaela Gemanariu, Ioana Pavaleanu, Tudor Andrei Buțureanu, Andreea Pruteanu, Teodora Ana Balan, Bogdan Gabriel Anghel, and Raluca Anca Balan. 2025. "A Challenging Diagnosis of Endometrial Stromal Sarcoma in a 50-Year-Old Patient: Case Report and Literature Review" Diagnostics 15, no. 24: 3215. https://doi.org/10.3390/diagnostics15243215
APA StyleHaliciu, A.-M., Furnică, C., Stan, C. I., Gemanariu, R.-M., Pavaleanu, I., Buțureanu, T. A., Pruteanu, A., Balan, T. A., Anghel, B. G., & Balan, R. A. (2025). A Challenging Diagnosis of Endometrial Stromal Sarcoma in a 50-Year-Old Patient: Case Report and Literature Review. Diagnostics, 15(24), 3215. https://doi.org/10.3390/diagnostics15243215







