Analytical Validation of a Serum Biomarker Signature for Detection of Early-Stage Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Demographics
2.2. Sample Collection, Processing, and Storage
2.3. Materials and Reagents
2.4. Biomarker Measurements
2.5. Analytical Performance and Acceptance Criteria
2.5.1. Analytical Sensitivity
2.5.2. Linearity of Calibration
2.5.3. Biomarker Precision
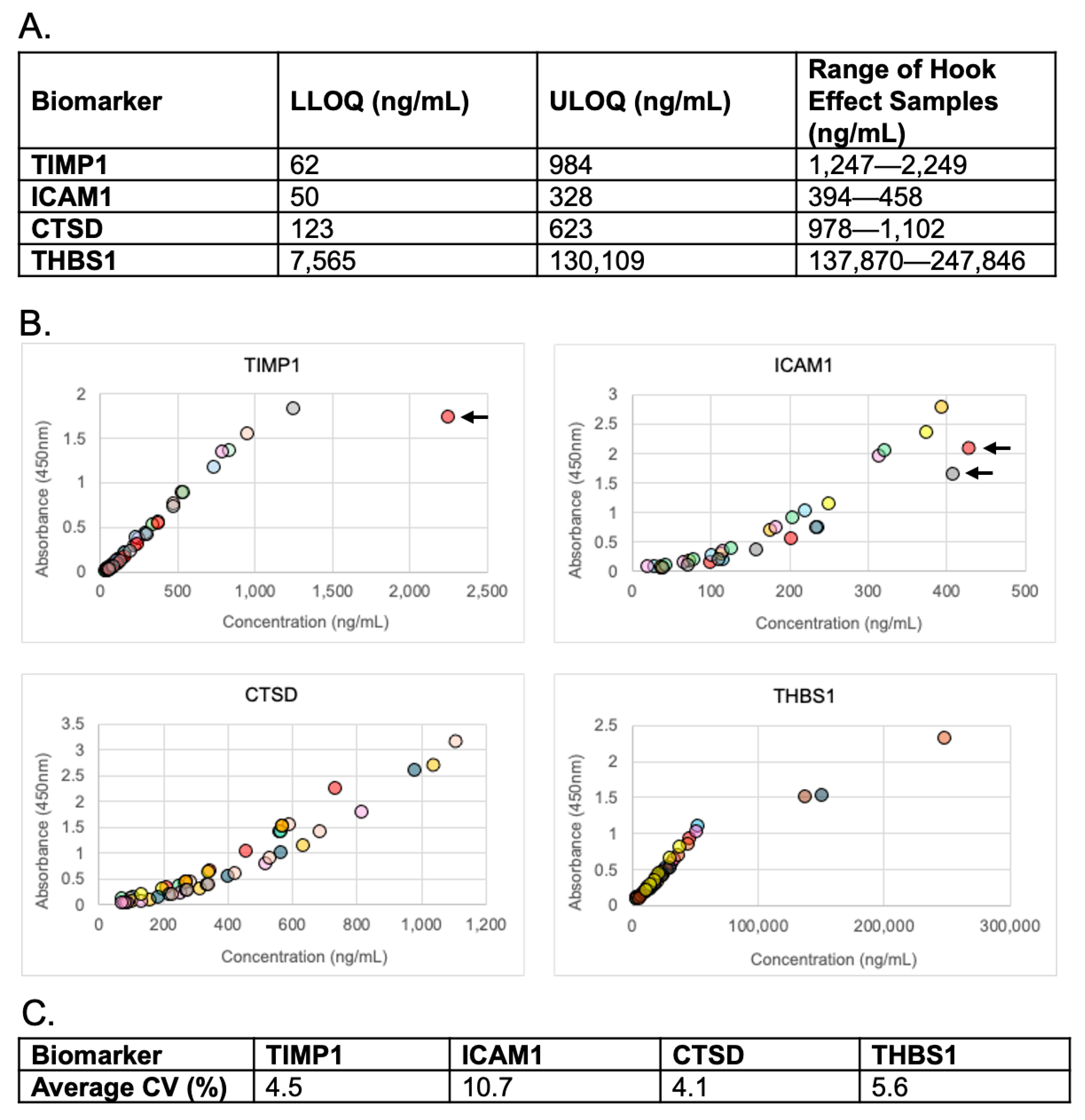
2.5.4. Reportable Range, Dilutional Linearity, and Hook Effect
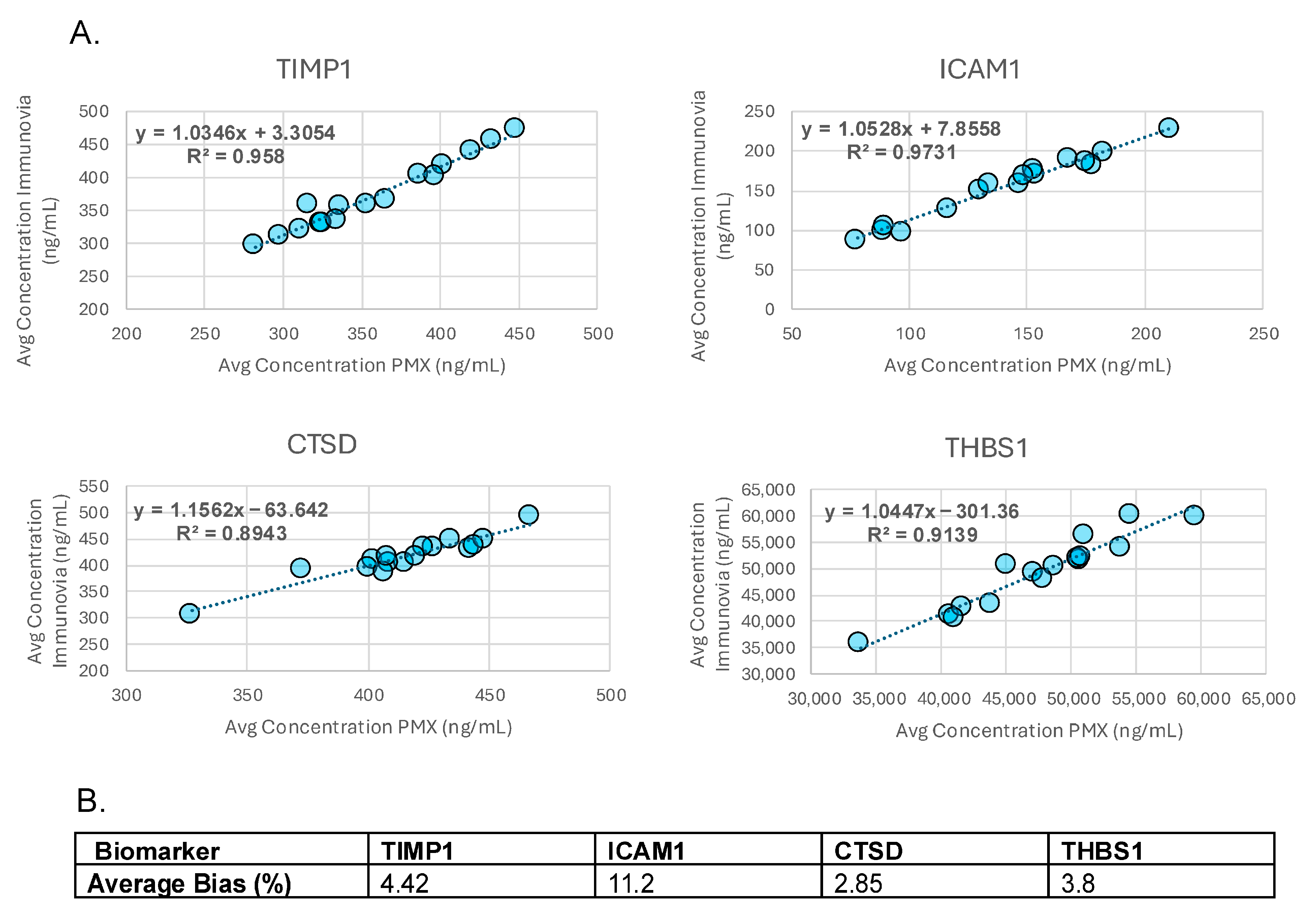
2.5.5. Accuracy
2.5.6. Stability
2.5.7. Analytical Specificity
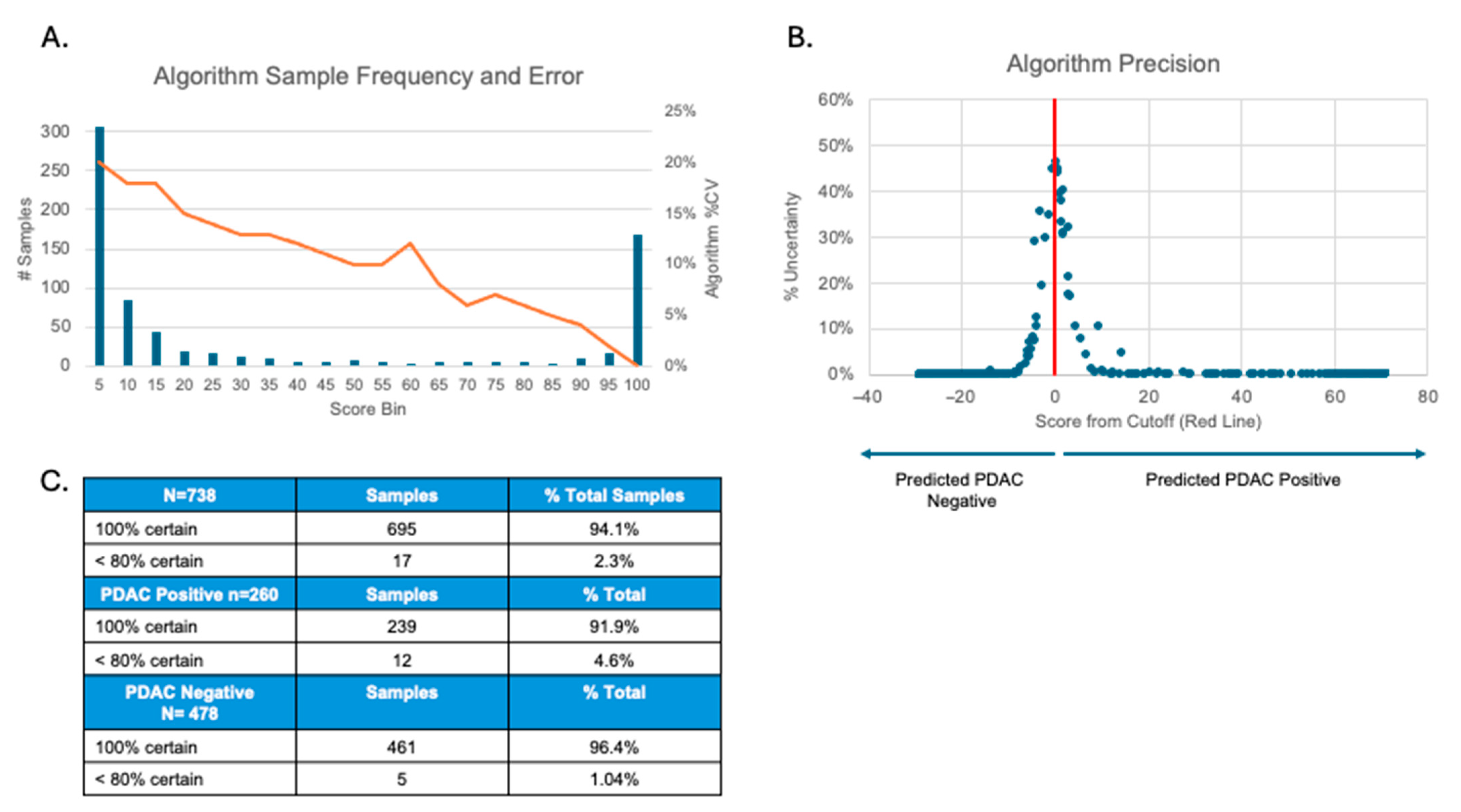
2.5.8. Algorithm Precision
2.6. Statistical Analyses
3. Results
3.1. Analytical Sensitivity
3.2. Linearity of Calibration
3.3. Biomarker Precision
3.4. Reportable Range, Dilutional Linearity, and Hook Effect
3.5. Accuracy
3.6. Stability
3.7. Analytical Specificity
3.8. Algorithm Precision
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic Ductal Adenocarcinoma |
| TIMP1 | Tissue inhibitor of metalloproteinase 1 |
| ICAM1 | Intercellular adhesion molecule 1 |
| CTSD | Cathepsin D |
| THBS1 | Thrombospondin 1 |
| CA 19-9 | Carbohydrate antigen 19-9 |
| ELISA | Enzyme-linked immunosorbent assay |
| CLSI | Clinical and Laboratory Standards Institute |
| LLOQ | Lower limit of quantitation |
| ULOQ | Upper limit of quantitation |
| CV | Coefficient of variation |
| F/T | Freeze/thaw |
References
- da Costa, W.L., Jr.; Oluyomi, A.O.; Thrift, A.P. Trends in the Incidence of Pancreatic Adenocarcinoma in All 50 United States Examined Through an Age-Period-Cohort Analysis. JNCI Cancer Spectr. 2020, 4, pkaa033. [Google Scholar] [CrossRef] [PubMed]
- Institute, N.C. Cancer Stat Facts: Common Cancer Sites. 2024. Available online: https://seer.cancer.gov/statfacts/html/common.html (accessed on 20 February 2025).
- Saad, A.M.; Turk, T.; Al-Husseini, M.J.; Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018, 18, 688. [Google Scholar] [CrossRef]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Survial Rates for Pancreatic Cancer. 2025. Available online: https://www.cancer.org/cancer/types/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 20 February 2025).
- Blackford, A.L.; Canto, M.I.; Klein, A.P.; Hruban, R.H.; Goggins, M. Recent Trends in the Incidence and Survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results Analysis. J. Natl. Cancer Inst. 2020, 112, 1162–1169. [Google Scholar] [CrossRef]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018, 155, 740–751.e2. [Google Scholar] [CrossRef]
- Kang, J.D.; Clarke, S.E.; Costa, A.F. Factors associated with missed and misinterpreted cases of pancreatic ductal adenocarcinoma. Eur. Radiol. 2021, 31, 2422–2432. [Google Scholar] [CrossRef]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Schmidt, C.M.; Mao, X.; Irajizad, E.; Loftus, M.; Zhang, J.; Patel, N.; Vykoukal, J.; Dennison, J.B.; Long, J.P.; et al. Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology 2021, 160, 1373–1383.e6. [Google Scholar] [CrossRef]
- O’Brien, D.P.; Sandanayake, N.S.; Jenkinson, C.; Gentry-Maharaj, A.; Apostolidou, S.; Fourkala, E.O.; Camuzeaux, S.; Blyuss, O.; Gunu, R.; Dawnay, A.; et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: Implications for early disease detection. Clin. Cancer Res. 2015, 21, 622–631. [Google Scholar] [CrossRef]
- Boyd, L.N.C.; Ali, M.; Leeflang, M.M.G.; Treglia, G.; de Vries, R.; Le Large, T.Y.S.; Besselink, M.G.; Giovannetti, E.; van Laarhoven, H.W.M.; Kazemier, G. Diagnostic accuracy and added value of blood-based protein biomarkers for pancreatic cancer: A meta-analysis of aggregate and individual participant data. EClinicalMedicine 2023, 55, 101747. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.E.; Nolen, B.M.; Zeh, H.J.; Allen, P.J.; Eloubeidi, M.A.; Goldberg, M.; Elton, E.; Arnoletti, J.P.; Christein, J.D.; Vickers, S.M.; et al. Serum biomarker panels for the detection of pancreatic cancer. Clin. Cancer Res. 2011, 17, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Haab, B.; Qian, L.; Staal, B.; Jain, M.; Fahrmann, J.; Worthington, C.; Prosser, D.; Velokokhatnaya, L.; Lopez, C.; Tang, R.; et al. A rigorous multi-laboratory study of known PDAC biomarkers identifies increased sensitivity and specificity over CA19-9 alone. Cancer Lett. 2024, 604, 217245. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Elliott, V.L.; Evans, A.; Oldfield, L.; Jenkins, R.E.; O’Brien, D.P.; Apostolidou, S.; Gentry-Maharaj, A.; Fourkala, E.O.; Jacobs, I.J.; et al. Decreased Serum Thrombospondin-1 Levels in Pancreatic Cancer Patients Up to 24 Months Prior to Clinical Diagnosis: Association with Diabetes Mellitus. Clin. Cancer Res. 2016, 22, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Saad, Y.; Saleh, D.; Elawady, R.; Eletreby, R.; Kharalla, A.S.; Badr, E. Can Serum ICAM 1 distinguish pancreatic cancer from chronic pancreatitis? Asian Pac. J. Cancer Prev. 2016, 17, 4671–4675. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Kang, E.S.; Kim, J.W.; Lee, K.T.; Lee, K.H.; Park, Y.S.; Park, J.O.; Lee, J.; Heo, J.S.; Choi, S.H.; et al. Serum CA19-9, cathepsin D, and matrix metalloproteinase-7 as a diagnostic panel for pancreatic ductal adenocarcinoma. Proteomics 2012, 12, 3590–3597. [Google Scholar] [CrossRef]
- Poruk, K.E.; Firpo, M.A.; Scaife, C.L.; Adler, D.G.; Emerson, L.L.; Boucher, K.M.; Mulvihill, S.J. Serum osteopontin and tissue inhibitor of metalloproteinase 1 as diagnostic and prognostic biomarkers for pancreatic adenocarcinoma. Pancreas 2013, 42, 193–197. [Google Scholar] [CrossRef]
- Schoeps, B.; Eckfeld, C.; Prokopchuk, O.; Bottcher, J.; Haussler, D.; Steiger, K.; Demir, I.E.; Knolle, P.; Soehnlein, O.; Jenne, D.E.; et al. TIMP1 Triggers Neutrophil Extracellular Trap Formation in Pancreatic Cancer. Cancer Res. 2021, 81, 3568–3579. [Google Scholar] [CrossRef]
- Palma, N.A.; Lucas, A.L.; Katona, B.W.; Athanasiou, A.; Kureshi, N.M.; Ford, L.; Keller, T.; Weber, S.; Schiess, R.; King, T.; et al. A High Performing Biomarker Signature for Detecting Early-Stage Pancreatic Ductal Adenocarcinoma in High-Risk Individuals. Cancers 2025, 17, 1866. [Google Scholar] [CrossRef]
- Lucas, A.L.; Simeone, D.M.; Katona, B.W.; Paiella, S.; Zogopoulos, G.; Sears, R.C.; Grindedal, E.M.; Wadlow, R.C.; Borazanci, E.; Gordon, O.K.; et al. Validation of a Serum-Based Biomarker Signature for Detection of Early-Stage Pancreatic Ductal Adenocarcinoma. Gastroenterology, 2025; in press. [Google Scholar] [CrossRef]
- Polanco, P.M.; Gonda, T.; Borazanci, E.; Glazer, E.S.; Trevino, J.G.; DeMuth, G.; Ford, L.; King, T.; Palma, N.A.; Brand, R.E. Confirmatory Clinical Validation of a Serum-Based Biomarker Signature for Detection of Early-Stage Pancreatic Ductal Adenocarcinoma. Curr. Oncol. 2025, 32, 638. [Google Scholar] [CrossRef]
- Grunwald, B.; Harant, V.; Schaten, S.; Fruhschutz, M.; Spallek, R.; Hochst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology 2016, 151, 1011–1024.e7. [Google Scholar] [CrossRef]
- Liou, G.Y.; Doppler, H.; Necela, B.; Edenfield, B.; Zhang, L.; Dawson, D.W.; Storz, P. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov. 2015, 5, 52–63. [Google Scholar] [CrossRef]
- Dumartin, L.; Whiteman, H.J.; Weeks, M.E.; Hariharan, D.; Dmitrovic, B.; Iacobuzio-Donahue, C.A.; Brentnall, T.A.; Bronner, M.P.; Feakins, R.M.; Timms, J.F.; et al. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res. 2011, 71, 7091–7102. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Hayashi, H.; Uemura, N.; Ogata, Y.; Zhao, L.; Sato, H.; Shiraishi, Y.; Kuroki, H.; Kitamura, F.; Kaida, T.; et al. Thrombospondin-1 overexpression stimulates loss of Smad4 and accelerates malignant behavior via TGF-beta signal activation in pancreatic ductal adenocarcinoma. Transl. Oncol. 2022, 26, 101533. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Katunuma, N. Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. J. Med. Investig. 2005, 52, 1–9. [Google Scholar] [CrossRef]
- Qian, X.; Rothman, V.L.; Nicosia, R.F.; Tuszynski, G.P. Expression of thrombospondin-1 in human pancreatic adenocarcinomas: Role in matrix metalloproteinase-9 production. Pathol. Oncol. Res. 2001, 7, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. 510(k) Substantial Equivalence Determination Decision Summary. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/K050231.pdf (accessed on 17 April 2025).
- Diagnostics, R. Elecsys CA 19-9 Package Insert. 2025. Available online: https://elabdoc-prod.roche.com/eLD/api/downloads/97be00ff-6a2d-ef11-2491-005056a772fd?countryIsoCode=XG (accessed on 15 March 2025).
- Athanasiou, A.; Kureshi, N.; Wittig, A.; Sterner, M.; Huber, R.; Palma, N.A.; King, T.; Schiess, R. Biomarker Discovery for Early Detection of Pancreatic Ductal Adenocarcinoma (PDAC) Using Multiplex Proteomics Technology. J. Proteome Res. 2025, 24, 315–322. [Google Scholar] [CrossRef]
- McEnroe, R.J.; Dimeski, G.; Miller, W.G.; Sonntag, O. EP37 Supplemental Tables for Interference Testing in Clinical Chemistry, 1st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Klee, G.G. Human anti-mouse antibodies. Arch. Pathol. Lab. Med. 2000, 124, 921–923. [Google Scholar] [CrossRef]
| Marker | Sample | Average Concentration (ng/mL) ± SD | Inter-Day CV (%) | Average Intra-Day CV (%) |
|---|---|---|---|---|
| TIMP1 | High | 833.0 ± 41.5 | 5.0 | 3.5 |
| Medium | 330.0 ± 18.3 | 5.6 | 3.2 | |
| Low | 156.0 ± 13.0 | 8.3 | 4.5 | |
| LLOQ | 70.6 ± 11.0 | 15.6 | 3.7 | |
| ICAM1 | High | 279.0 ± 19.7 | 7.1 | 5.6 |
| Medium | 152.0 ± 21.2 | 14.0 | 6.3 | |
| Low | 120.0 ± 13.1 | 10.9 | 7.5 | |
| LLOQ | 30.2 ± 11.1 | 36.7 | 17.5 | |
| CTSD | High | 455.0 ± 20.6 | 4.5 | 2.8 |
| Medium | 451.0 ± 29.2 | 6.5 | 5.5 | |
| Low | 265.0 ± 26.6 | 10.0 | 6.7 | |
| LLOQ | 180.0 ± 19.7 | 11.0 | 5.7 | |
| THBS1 | High | 53,811.0 ± 3033.0 | 5.6 | 2.7 |
| Medium | 42,014.0 ± 2728.0 | 6.5 | 3.2 | |
| Low | 27,165.0 ± 2219.0 | 8.2 | 4.2 | |
| LLOQ | 11,059.0 ± 856.0 | 7.7 | 3.0 | |
| Room Temperature (Average % Bias) | |||||||||
| 24 h | 48 h | 72 h | |||||||
| High | Medium | Low | High | Medium | Low | High | Medium | Low | |
| TIMP1 | −11.4 | −7.5 | −5.8 | −18.9 | −9.1 | −10.0 | −18.9 | −11.3 | −12.5 |
| ICAM1 | 1.6 | −4.7 | −5.2 | −3.0 | −6.3 | −1.0 | −8.0 | −1.1 | −3.9 |
| CTSD | −14.6 | −22.3 | −20.7 | −23.4 | −39.5 | −24.4 | −28.5 | −50.1 | −30.9 |
| THBS1 | −5.3 | −9.4 | −0.4 | −9.6 | −9.9 | −2.4 | −12.7 | −4.9 | −11.6 |
| 8.5 h | |||||||||
| High | Medium | Low | |||||||
| CTSD | −11.7 | −13.0 | −12.0 | ||||||
| 4 °C (average % bias) | |||||||||
| 24 h | 48 h | 72 h | |||||||
| High | Medium | Low | High | Medium | Low | High | Medium | Low | |
| TIMP1 | −8.3 | −3.4 | −7.8 | −6.9 | −4.7 | −10.3 | −12.9 | −10.3 | −10.1 |
| ICAM1 | −1.2 | −2.3 | −4.3 | −2.5 | −3.7 | −3.1 | −6.5 | −3.4 | −1.1 |
| CTSD | −0.7 | −7.5 | −8.3 | −6.1 | −6.3 | −6.5 | −3.9 | −13.1 | −17.1 |
| THBS1 | −6.3 | −5.8 | −4.6 | −5.5 | −7.9 | 1.0 | −13.6 | −6.6 | −3.0 |
| 3 F/T Cycles (Average % Bias) | 5 F/T Cycles (Average % Bias) | |||||
|---|---|---|---|---|---|---|
| High | Medium | Low | High | Medium | Low | |
| TIMP1 | −3.0 | −5.8 | −0.5 | −7.0 | −4.8 | −4.3 |
| ICAM1 | −1.1 | −7.1 | −4.3 | −1.4 | −12.2 | −4.1 |
| CTSD | −5.7 | −5.6 | −10.4 | −5.5 | −13.8 | −16.5 |
| THBS1 | −4.8 | −3.7 | −3.2 | −4.6 | −11.2 | −1.3 |
| Bilirubin | Closely Related Proteins | HAMA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin | Conjugated | Unconjugated | Triglycerides | Biotin | TIMP 2 | Sample 1 | 3.2 | ||
| TIMP 1 | High Conc. | −1.60 | −0.90 | 5.70 | 1.90 | −5.30 | 2.2 | Sample 2 | 3.5 |
| Low Conc. | −8.90 | 7.60 | 1.20 | 8.00 | 0.80 | 6.9 | Sample 3 | 1.8 | |
| ICAM 4 | Sample 1 | 8.13 | |||||||
| ICAM 1 | High Conc. | −7.10 | −6.60 | 9.60 | 15.10 | −1.90 | 2.1 | Sample 2 | 15.3 |
| Low Conc. | 1.50 | −1.50 | 8.90 | −9.90 | −6.70 | 11.1 | Sample 3 | 20.7 | |
| CTSB | Sample 1 | −3.10 | |||||||
| CTSD | High Conc. | −10.30 | −3.70 | −5.10 | −4.50 | 0.80 | −1.55 | Sample 2 | 28.8 |
| Low Conc. | −7.6 | −3.10 | 3.60 | 19.70 | 15.60 | 4.5 | Sample 3 | 22.1 | |
| THBS 2 | Sample 1 | −0.2 | |||||||
| THBS1 | High Conc. | 1.70 | 4.60 | −4.80 | 3.60 | −0.70 | 6.0 | Sample 2 | 7.3 |
| Low Conc. | −6.40 | −3.10 | 7.40 | 5.50 | 1.30 | 2.5 | Sample 3 | 1.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pescatore, R.; Milliken, N.; King, T.; Josey, D.; Palma, N.A.; Ford, L. Analytical Validation of a Serum Biomarker Signature for Detection of Early-Stage Pancreatic Ductal Adenocarcinoma. Diagnostics 2025, 15, 3177. https://doi.org/10.3390/diagnostics15243177
Pescatore R, Milliken N, King T, Josey D, Palma NA, Ford L. Analytical Validation of a Serum Biomarker Signature for Detection of Early-Stage Pancreatic Ductal Adenocarcinoma. Diagnostics. 2025; 15(24):3177. https://doi.org/10.3390/diagnostics15243177
Chicago/Turabian StylePescatore, Robyn, Naphtali Milliken, Thomas King, Dillon Josey, Norma A. Palma, and Lisa Ford. 2025. "Analytical Validation of a Serum Biomarker Signature for Detection of Early-Stage Pancreatic Ductal Adenocarcinoma" Diagnostics 15, no. 24: 3177. https://doi.org/10.3390/diagnostics15243177
APA StylePescatore, R., Milliken, N., King, T., Josey, D., Palma, N. A., & Ford, L. (2025). Analytical Validation of a Serum Biomarker Signature for Detection of Early-Stage Pancreatic Ductal Adenocarcinoma. Diagnostics, 15(24), 3177. https://doi.org/10.3390/diagnostics15243177







