Long-Term Evolution of Skeletal Muscle Quantity and Quality After Curative-Intent Colon Cancer Surgery: A Retrospective Cohort Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CT-Based Body Composition Assessment
2.3. Statistical Analysis
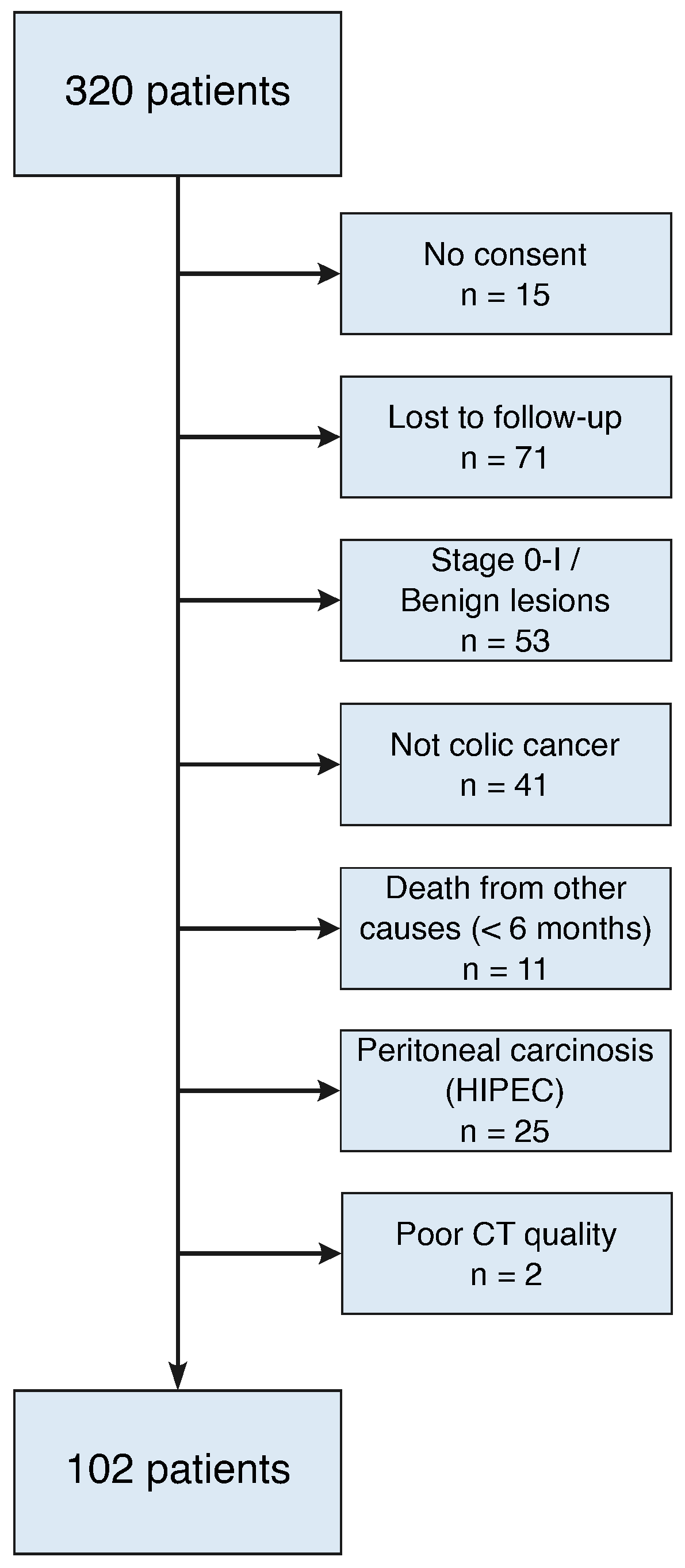
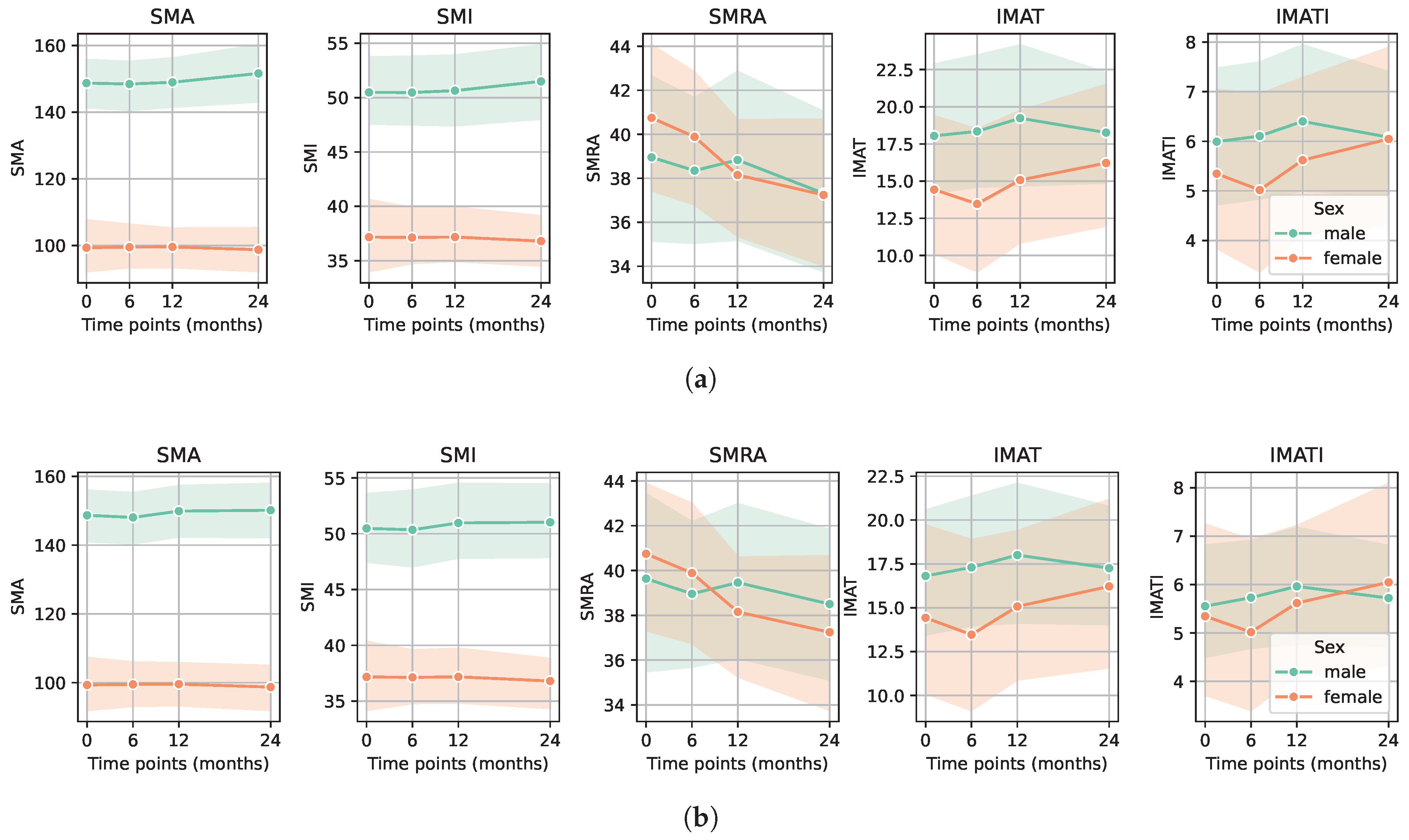
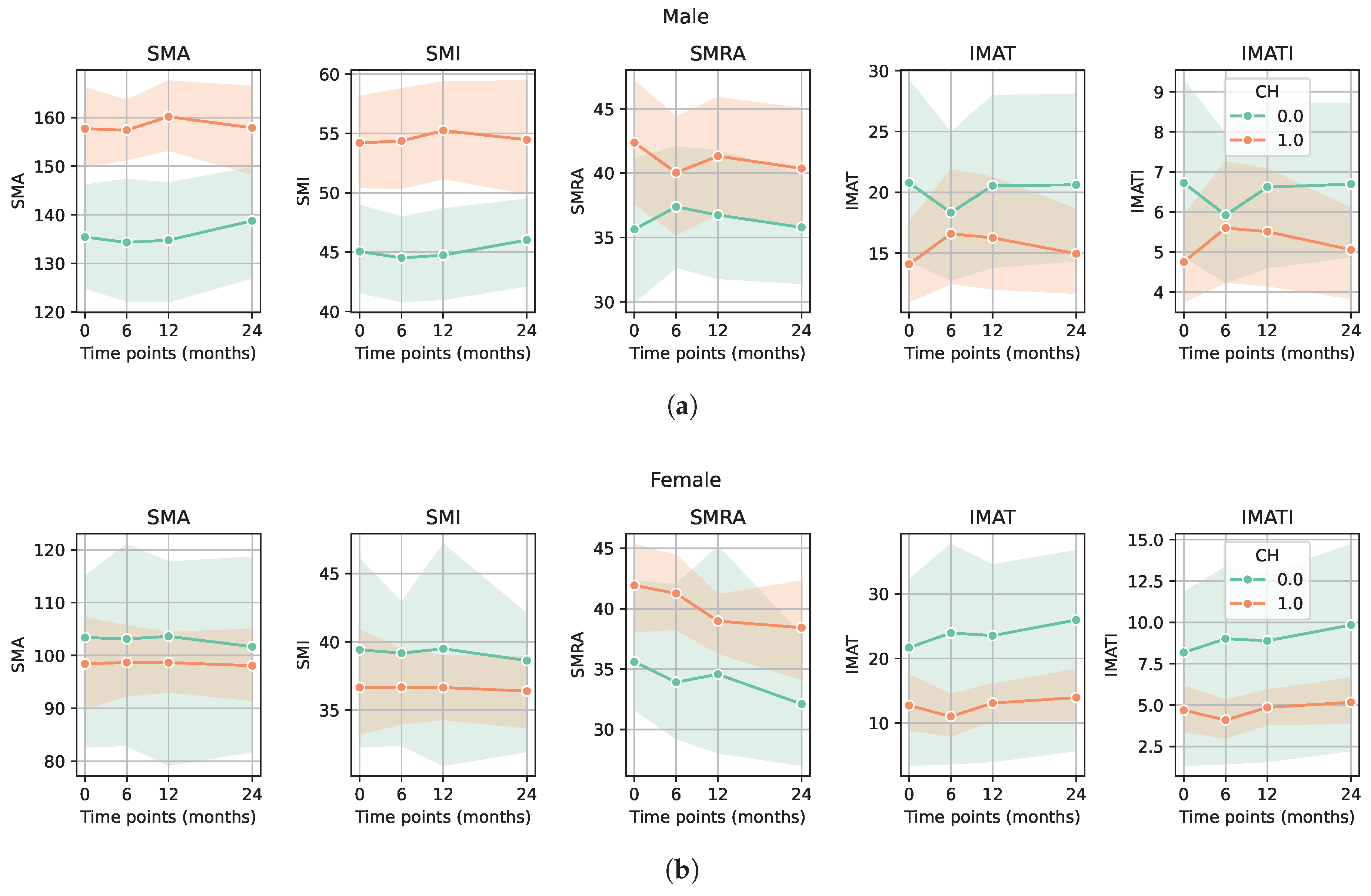
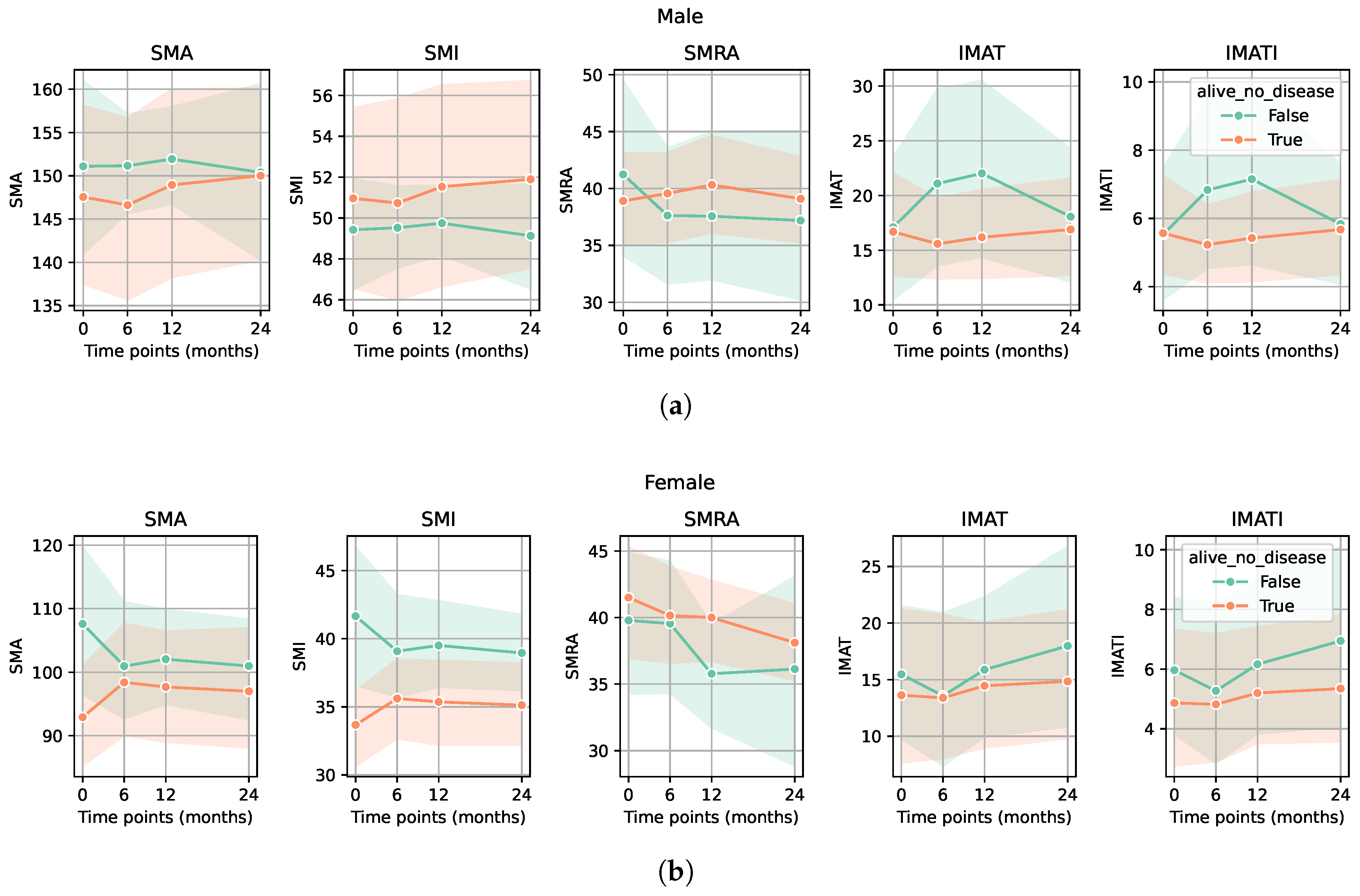
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Lee, J.S.; Visser, M.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Newman, A.B.; Health, Aging and Body Composition Study. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007, 55, 769–774. [Google Scholar] [CrossRef]
- Carvalho do Nascimento, P.R.; Bilodeau, M.; Poitras, S. How do we define and measure sarcopenia? A meta-analysis of observational studies. Age Ageing 2021, 50, 1906–1913. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Vergara-Fernandez, O.; Trejo-Avila, M.; Salgado-Nesme, N. Sarcopenia in patients with colorectal cancer: A comprehensive review. World J. Clin. Cases 2020, 8, 1188–1202. [Google Scholar] [CrossRef]
- Schneider, M.; Hübner, M.; Becce, F.; Koerfer, J.; Collinot, J.A.; Demartines, N.; Hahnloser, D.; Grass, F.; Martin, D. Sarcopenia and major complications in patients undergoing oncologic colon surgery. J. Cachexia Sarcopenia Muscle 2021, 12, 1757–1763. [Google Scholar] [CrossRef]
- Malietzis, G.; Currie, A.C.; Athanasiou, T.; Johns, N.; Anyamene, N.; Glynne-Jones, R.; Kennedy, R.H.; Fearon, K.C.H.; Jenkins, J.T. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 2016, 103, 572–580. [Google Scholar] [CrossRef]
- Hopkins, J.J.; Reif, R.L.; Bigam, D.L.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.B. The Impact of Muscle and Adipose Tissue on Long-term Survival in Patients with Stage I to III Colorectal Cancer. Dis. Colon Rectum 2019, 62, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Becce, F.; Balmer, R.; Do Amaral, R.H.; Alemán-Gómez, Y.; Uldry, E.; Fraga, M.; Tsoumakidou, G.; Villard, N.; Denys, A.; et al. Prognostic value of CT-based skeletal muscle and adipose tissue mass and quality parameters in patients with liver metastases and intrahepatic cholangiocarcinoma undergoing Yttrium-90 radioembolization. Eur. Radiol. 2025, 35, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.J.; Reif, R.; Bigam, D.; Baracos, V.E.; Eurich, D.T.; Sawyer, M.M. Change in Skeletal Muscle Following Resection of Stage I-III Colorectal Cancer is Predictive of Poor Survival: A Cohort Study. World J. Surg. 2019, 43, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; MacFie, J.; Liberman, A.S.; Soop, M.; et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin. Nutr. 2012, 31, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Truninger, K.; Lugli, A.; Koeberle, D. Suivi après polypectomie coloscopique et traitement du cancer colorectal. Forum Médical Suisse–Swiss Med. Forum 2022, 22, 349–355. [Google Scholar] [CrossRef]
- Edge, S.B.; American Joint Committee on Cancer (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Amin, M.B.; American Joint Committee on Cancer. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Ibtehaz, N.; Rahman, M.S. MultiResUNet: Rethinking the U-Net architecture for multimodal biomedical image segmentation. Neural Netw. 2020, 121, 74–87. [Google Scholar] [CrossRef]
- Koitka, S.; Kroll, L.; Malamutmann, E.; Oezcelik, A.; Nensa, F. Fully automated body composition analysis in routine CT imaging using 3D semantic segmentation convolutional neural networks. Eur. Radiol. 2021, 31, 1795–1804. [Google Scholar] [CrossRef]
- Hasenauer, A.; Forster, C.; Hungerbühler, J.; Perentes, J.Y.; Abdelnour-Berchtold, E.; Koerfer, J.; Krueger, T.; Becce, F.; Gonzalez, M. CT-Derived Sarcopenia and Outcomes after Thoracoscopic Pulmonary Resection for Non-Small Cell Lung Cancer. Cancers 2023, 15, 790. [Google Scholar] [CrossRef]
- Salati, V.; Mandralis, K.; Becce, F.; Koerfer, J.; Lambercy, K.; Simon, C.; Gorostidi, F. Preoperative CT-Based Skeletal Muscle Mass Depletion and Outcomes after Total Laryngectomy. Cancers 2023, 15, 3538. [Google Scholar] [CrossRef]
- Martin, D.; Billy, M.; Becce, F.; Maier, D.; Schneider, M.; Dromain, C.; Hahnloser, D.; Hübner, M.; Grass, F. Impact of Preoperative CT-Measured Sarcopenia on Clinical, Pathological, and Oncological Outcomes After Elective Rectal Cancer Surgery. Diagnostics 2025, 15, 629. [Google Scholar] [CrossRef]
- Covello, A.; Toprover, M.; Oh, C.; Leroy, G.; Kumar, A.; LaMoreaux, B.; Mechlin, M.; Fields, T.R.; Pillinger, M.H.; Becce, F. Skeletal muscle mass and quality in gout patients versus non-gout controls: A computed tomography imaging study. Jt. Bone Spine 2024, 91, 105743. [Google Scholar] [CrossRef]
- Westenberg, L.B.; Zorgdrager, M.; Swaab, T.D.A.; van Londen, M.; Bakker, S.J.L.; Leuvenink, H.G.D.; Viddeleer, A.R.; Pol, R.A. Reference values for low muscle mass and myosteatosis using tomographic muscle measurements in living kidney donors. Sci. Rep. 2023, 13, 5835. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Avila, M.; Bozada-Gutiérrez, K.; Valenzuela-Salazar, C.; Herrera-Esquivel, J.; Moreno-Portillo, M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 1077–1096. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Kong, M.; Geng, N.; Zhou, Y.; Lin, N.; Song, W.; Xu, M.; Li, S.; Piao, Y.; Han, Z.; Guo, R.; et al. Defining reference values for low skeletal muscle index at the L3 vertebra level based on computed tomography in healthy adults: A multicentre study. Clin. Nutr. 2022, 41, 396–404. [Google Scholar] [CrossRef]
- van der Werf, A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Nurmohamed, S.A.; van der Pant, K.A.M.I.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef]
- Choi, C.S.; Kin, K.; Cao, K.; Hutcheon, E.; Lee, M.; Chan, S.T.F.; Arafat, Y.; Baird, P.N.; Yeung, J.M.C. The association of body composition on chemotherapy toxicities in non-metastatic colorectal cancer patients: A systematic review. ANZ J. Surg. 2024, 94, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Kimura, T.; Miyoshi, Y.; Muto, S.; Horie, S. 236P The effects of chemotherapy on body composition in patients with advanced urothelial carcinoma. Ann. Oncol. 2023, 34, S1565–S1566. [Google Scholar] [CrossRef]
- Godinho-Mota, J.C.M.; Vaz-Gonçalves, L.; Dias Custódio, I.D.; Schroeder de Souza, J.; Mota, J.F.; Gonzalez, M.C.; Rodrigues Vilella, P.; Anusca Martins, K.; de Paiva Maia, Y.C.; Verde, S.M.M.L.; et al. Impact of Chemotherapy Regimens on Body Composition of Breast Cancer Women: A Multicenter Study across Four Brazilian Regions. Nutrients 2023, 15, 1689. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.M.G.A.; Kok, D.E.; Visser, M.; de Vries, J.H.M.; de Kruif, J.T.C.M.; de Vries, Y.; Posthuma, L.; Sommeijer, D.W.; Timmer-Bonte, A.; Los, M.; et al. Changes in body composition during and after adjuvant or neo-adjuvant chemotherapy in women with breast cancer stage I-IIIB compared with changes over a similar timeframe in women without cancer. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 1685–1693. [Google Scholar] [CrossRef]
- Zanforlini, B.M.; Alessi, A.; Pontarin, A.; De Rui, M.; Zoccarato, F.; Seccia, D.M.; Trevisan, C.; Brunello, A.; Basso, U.; Manzato, E.; et al. Association of body surface area with fat mass, free fat mass and total weight in healthy individuals, and implications for the dosage of cytotoxic drugs. Clin. Nutr. ESPEN 2021, 43, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, M.; Jouinot, A.; Burns, R.; Pigneur, F.; Mounier, R.; Gondin, J.; Neuzillet, C.; Goldwasser, F. Body composition and sarcopenia: The next-generation of personalized oncology and pharmacology? Pharmacol. Ther. 2019, 196, 135–159. [Google Scholar] [CrossRef]
- Gusella, M.; Toso, S.; Ferrazzi, E.; Ferrari, M.; Padrini, R. Relationships between body composition parameters and fluorouracil pharmacokinetics. Br. J. Clin. Pharmacol. 2002, 54, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, A.; Schneider, M.; Grass, F.; Hahnloser, D.; Becce, F.; Hübner, M. Long-Term Evolution of Skeletal Muscle Quantity and Quality After Curative-Intent Colorectal Surgery: A Retrospective Cohort Study. BJS 2025, 112, znaf092.062. [Google Scholar] [CrossRef]
| Overall Population (n = 102) | Male (n = 67) | Female (n = 35) | p-Value |
|---|---|---|---|
| Alive | 53 (79.1) | 32 (91.4) | |
| without disease | 39 (58.2) | 23 (65.7) | |
| with disease | 14 (20.9) | 9 (25.7) | |
| Death | 14 (20.9) | 3 (8.6) | |
| due to the disease | 11 (16.4) | 3 (8.6) | |
| due to other cause | 3 (4.5) | ||
| Age | 66.2 (13.3) | 65.9 (13.5) | 0.912 |
| BMI | 26.2 (5.4) | 24.8 (6.0) | 0.239 |
| Preoperative weight | 76.6 (15.0) | 66.1 (14.4) | 0.001 |
| Height | 171.2 (10.2) | 163.6 (5.7) | <0.001 |
| ASA | |||
| 1 | 3 (4.5) | 4 (11.4) | |
| 2 | 34 (50.7) | 21 (60.0) | |
| 3 | 27 (40.3) | 9 (25.7) | |
| 4 | 3 (4.5) | 1 (2.9) | |
| Chemotherapy | |||
| Neoadjuvant | 12 (18.2) | 4 (11.4) | |
| Adjuvant | 37 (56.9) | 23 (65.7) | |
| Pathologic tumor stage | |||
| II | 21 (31.4) | 13 (37.2) | |
| III | 25 (37.3) | 12 (34.3) | |
| IV | 21 (31.3) | 10 (28.6) | |
| Charlson Comorbidity Index | 1.2 (1.8) | 0.4 (0.7) | 0.006 |
| CCI | 9.0 (14.7) | 7.7 (15.7) | 0.664 |
| Preoperative sarcopenia markers | |||
| IMAT area (SD) | 18.2 (13.1) | 16.7 (10.2) | 0.541 |
| IMATI (SD) | 6.4 (5.5) | 6.3 (3.9) | 0.863 |
| SMA (SD) | 141.9 (29.0) | 103.6 (17.4) | <0.001 |
| SMI (SD) | 48.4 (9.4) | 38.9 (7.7) | <0.001 |
| SMRA (SD) | 37.7 (11.5) | 37.6 (8.9) | 0.981 |
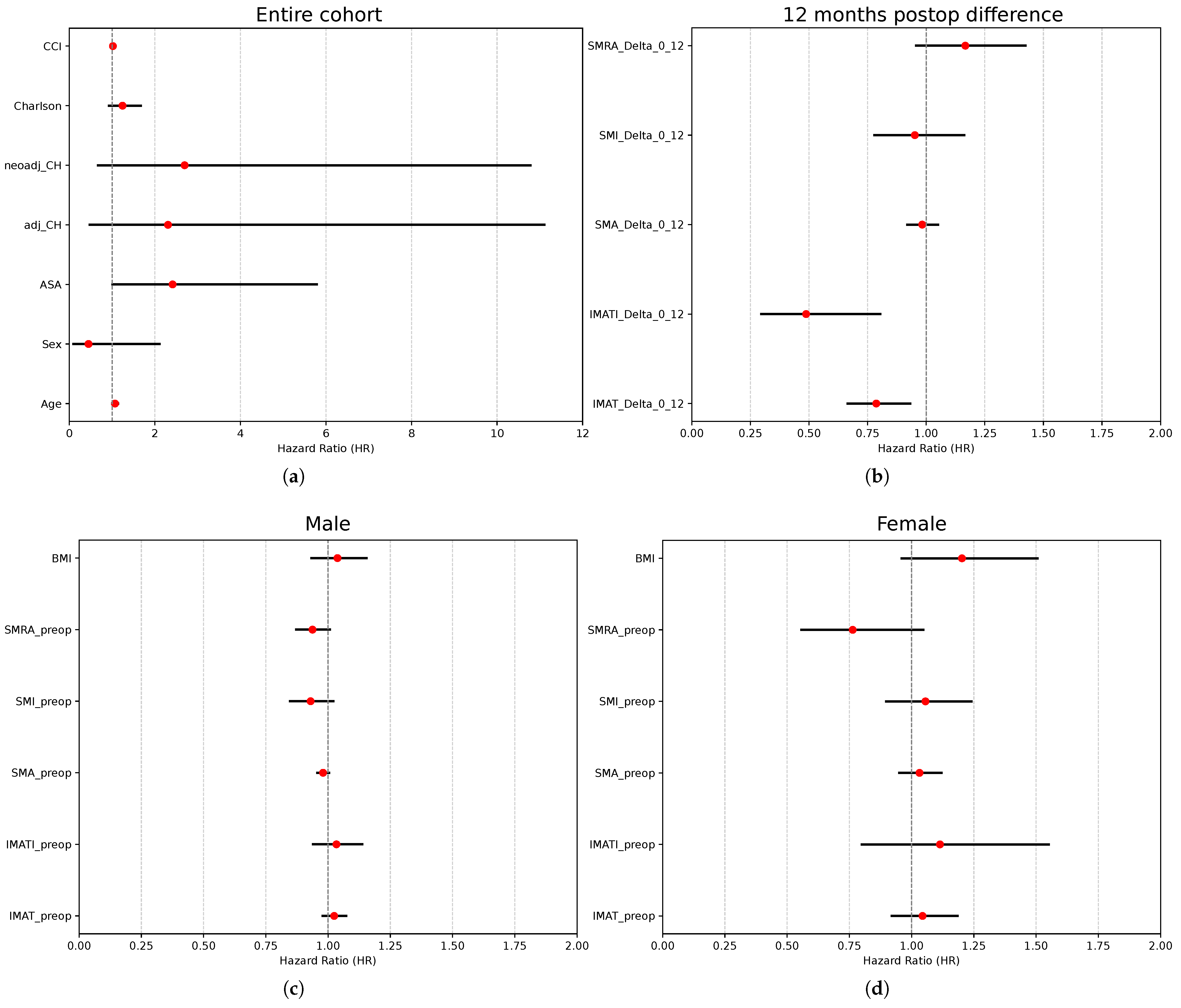
| Variable | Coefficient (SE) | HR (95% CI) | p-Value |
|---|---|---|---|
| Age | 0.07 (0.03) | 1.07 (1.00–1.14) | 0.045 |
| Sex | −0.80 (0.79) | 0.45 (0.10–2.11) | 0.309 |
| ASA | 0.88 (0.45) | 2.41 (1.01–5.80) | 0.048 |
| Neoadjuvant CH | 0.84 (0.80) | 2.31 (0.48–11.10) | 0.297 |
| Adjuvant CH | 0.99 (0.71) | 2.70 (0.67–10.78) | 0.161 |
| Charlson Comorbidity Index | 0.22 (0.15) | 1.24 (0.93–1.67) | 0.146 |
| CCI | 0.02 (0.02) | 1.02 (0.10–1.06) | 0.312 |
| IMAT (0–12) | −0.24 (0.09) | 0.79 (0.67–0.93) | 0.005 |
| IMATI (0–12) | −0.72 (0.26) | 0.49 (0.30–0.80) | 0.005 |
| SMA (0–12) | −0.02 (0.03) | 0.98 (0.92–1.05) | 0.618 |
| SMI (0–12) | −0.05 (0.10) | 0.95 (0.78–1.16) | 0.626 |
| SMRA (0–12) | 0.15 (0.10) | 1.17 (0.96–1.42) | 0.127 |
| Variable | Coefficient (SE) | HR (95% CI) | p-Value |
|---|---|---|---|
| Preoperative IMAT area | 0.02 (0.02) | 1.02 (0.98–1.07) | 0.307 |
| Preoperative IMATI | 0.03 (0.05) | 1.03 (0.94–1.14) | 0.498 |
| Preoperative SMA | −0.02 (0.01) | 0.98 (0.96–1.00) | 0.097 |
| Preoperative SMI | −0.07 (0.05) | 0.93 (0.85–1.02) | 0.128 |
| Preoperative SMRA | −0.06 (0.04) | 0.94 (0.87–1.01) | 0.078 |
| BMI | 0.04 (0.05) | 1.04 (0.93–1.15) | 0.496 |
| Variable | Coefficient (SE) | HR (95% CI) | p-Value |
|---|---|---|---|
| Preoperative IMAT area | 0.04 (0.06) | 1.04 (0.92–1.18) | 0.504 |
| Preoperative IMATI | 0.11 (0.17) | 1.11 (0.80–1.55) | 0.522 |
| Preoperative SMA | 0.03 (0.04) | 1.03 (0.95–1.12) | 0.454 |
| Preoperative SMI | 0.05 (0.08) | 1.06 (0.90–1.24) | 0.511 |
| Preoperative SMRA | −0.27 (0.16) | 0.76 (0.56–1.05) | 0.094 |
| BMI | 0.18 (0.11) | 1.20 (0.96–1.51) | 0.109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadimitriou, A.; Schneider, M.; Zenkhri, S.; Hahnloser, D.; Martin, D.; Xu, H.A.; Maier, D.; Becce, F.; Grass, F.; Hübner, M. Long-Term Evolution of Skeletal Muscle Quantity and Quality After Curative-Intent Colon Cancer Surgery: A Retrospective Cohort Study. Diagnostics 2025, 15, 3092. https://doi.org/10.3390/diagnostics15233092
Papadimitriou A, Schneider M, Zenkhri S, Hahnloser D, Martin D, Xu HA, Maier D, Becce F, Grass F, Hübner M. Long-Term Evolution of Skeletal Muscle Quantity and Quality After Curative-Intent Colon Cancer Surgery: A Retrospective Cohort Study. Diagnostics. 2025; 15(23):3092. https://doi.org/10.3390/diagnostics15233092
Chicago/Turabian StylePapadimitriou, Argyri, Michael Schneider, Salim Zenkhri, Dieter Hahnloser, David Martin, He Ayu Xu, Damien Maier, Fabio Becce, Fabian Grass, and Martin Hübner. 2025. "Long-Term Evolution of Skeletal Muscle Quantity and Quality After Curative-Intent Colon Cancer Surgery: A Retrospective Cohort Study" Diagnostics 15, no. 23: 3092. https://doi.org/10.3390/diagnostics15233092
APA StylePapadimitriou, A., Schneider, M., Zenkhri, S., Hahnloser, D., Martin, D., Xu, H. A., Maier, D., Becce, F., Grass, F., & Hübner, M. (2025). Long-Term Evolution of Skeletal Muscle Quantity and Quality After Curative-Intent Colon Cancer Surgery: A Retrospective Cohort Study. Diagnostics, 15(23), 3092. https://doi.org/10.3390/diagnostics15233092









