Liver Tissue Mapping in Transfusion-Dependent β-Thalassemia: Reproducibility and Clinical Insights from Multiparametric MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI Acquisition and Image Analysis
2.3. Laboratory Parameters
2.4. Diagnostic Criteria
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Reproducibility of Hepatic T2*, T1, and T2 Values
3.3. Association Among the Three Liver Relaxation Times
3.4. Demographic and Clinical Correlates of Liver Relaxation Times
3.5. Liver Relaxation Times and Complications
4. Discussion
4.1. Study Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Origa, R. beta-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef]
- Taher, A.T.; Farmakis, D.; Porter, J.B.; Cappellini, M.D.; Musallam, K.M. Guidelines for the Management of Transfusion-Dependent β-Thalassaemia, 5th ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2025. [Google Scholar]
- Andrews, P.A. Disorders of iron metabolism. N. Engl. J. Med. 2000, 342, 1293. [Google Scholar]
- Ozment, C.P.; Turi, J.L. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim. Biophys. Acta 2009, 1790, 694–701. [Google Scholar] [CrossRef]
- Shander, A.; Cappellini, M.D.; Goodnough, L.T. Iron overload and toxicity: The hidden risk of multiple blood transfusions. Vox Sang. 2009, 97, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Saliba, A.; Taher, A. Iron overload in transfusion-dependent thalassemia. Hematology 2015, 20, 311–312. [Google Scholar] [CrossRef]
- Taher, A.T.; Saliba, A.N. Iron overload in thalassemia: Different organs at different rates. Hematology 2017, 2017, 265–271. [Google Scholar] [CrossRef]
- Akiki, N.; Hodroj, M.H.; Bou-Fakhredin, R.; Matli, K.; Taher, A.T. Cardiovascular Complications in β-Thalassemia: Getting to the Heart of It. Thalass. Rep. 2023, 13, 38–50. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Maggio, A.; Sorrentino, F.; Filosa, A.; Rosso, R.; et al. National networking in rare diseases and reduction of cardiac burden in thalassemia major. Eur. Heart J. 2022, 43, 2482–2492. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [PubMed]
- Eyre, K.; Lindsay, K.; Razzaq, S.; Chetrit, M.; Friedrich, M. Simultaneous multi-parametric acquisition and reconstruction techniques in cardiac magnetic resonance imaging: Basic concepts and status of clinical development. Front. Cardiovasc. Med. 2022, 9, 953823. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Meloni, A.; Rossi, G.; Midiri, M.; Missere, M.; Valeri, G.; Sorrentino, F.; D’Ascola, D.G.; Spasiano, A.; Filosa, A.; et al. Prediction of cardiac complications for thalassemia major in the widespread cardiac magnetic resonance era: A prospective multicentre study by a multi-parametric approach. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 299–309. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- Ladis, V.; Chouliaras, G.; Berdousi, H.; Kanavakis, E.; Kattamis, C. Longitudinal study of survival and causes of death in patients with thalassemia major in Greece. Ann. N. Y. Acad. Sci. 2005, 1054, 445–450. [Google Scholar] [CrossRef]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef]
- Wood, J.C.; Otto-Duessel, M.; Aguilar, M.; Nick, H.; Nelson, M.D.; Coates, T.D.; Pollack, H.; Moats, R. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation 2005, 112, 535–543. [Google Scholar] [CrossRef]
- Torlasco, C.; Cassinerio, E.; Roghi, A.; Faini, A.; Capecchi, M.; Abdel-Gadir, A.; Giannattasio, C.; Parati, G.; Moon, J.C.; Cappellini, M.D.; et al. Role of T1 mapping as a complementary tool to T2* for non-invasive cardiac iron overload assessment. PLoS ONE 2018, 13, e0192890. [Google Scholar] [CrossRef]
- Meloni, A.; Martini, N.; Positano, V.; De Luca, A.; Pistoia, L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Allò, M.; et al. Myocardial iron overload by cardiovascular magnetic resonance native segmental T1 mapping: A sensitive approach that correlates with cardiac complications. J. Cardiovasc. Magn. Reson. 2021, 23, 70. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Ahmed, A.T. Role of cardiac magnetic resonance T1 mapping in comparison to T2* for cardiac iron overload assessment in transfusion-dependent thalassemia major patients. Egypt. J. Radiol. Nucl. Med. 2023, 54, 197. [Google Scholar] [CrossRef]
- Selim, O.M.H.Z.; Ibrahim, A.S.A.H.; Aly, N.H.; Hegazy, S.N.A.; Ebeid, F.S.E. Early detection of myocardial iron overload in patients with β-thalassemia major using cardiac magnetic resonance T1 mapping. Magn. Reson. Imaging 2024, 114, 110250. [Google Scholar] [CrossRef] [PubMed]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Pistoia, L.; Positano, V.; Martini, N.; Borrello, R.L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Putti, M.C.; et al. Myocardial tissue characterization by segmental T2 mapping in thalassaemia major: Detecting inflammation beyond iron. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1222–1230. [Google Scholar] [CrossRef]
- Farmakis, D.; Giakoumis, A.; Angastiniotis, M.; Eleftheriou, A. The changing epidemiology of the ageing thalassaemia populations: A position statement of the Thalassaemia International Federation. Eur. J. Haematol. 2020, 105, 16–23. [Google Scholar] [CrossRef]
- Taher, A.T.; Cappellini, M.D. How I manage medical complications of beta-thalassemia in adults. Blood 2018, 132, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, K.; Bernat, A.; Wroblewska, A. Molecular pathogenesis and clinical consequences of iron overload in liver cirrhosis. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 461–479. [Google Scholar] [CrossRef]
- Wahidiyat, P.A.; Liauw, F.; Sekarsari, D.; Putriasih, S.A.; Berdoukas, V.; Pennell, D.J. Evaluation of cardiac and hepatic iron overload in thalassemia major patients with T2* magnetic resonance imaging. Hematology 2017, 22, 501–507. [Google Scholar] [CrossRef]
- Mehta, K.J.; Farnaud, S.J.; Sharp, P.A. Iron and liver fibrosis: Mechanistic and clinical aspects. World J. Gastroenterol. 2019, 25, 521–538. [Google Scholar] [CrossRef]
- Riaz, M.; Abbas, M.; Rasool, G.; Baig, I.S.; Mahmood, Z.; Munir, N.; Mahmood Tahir, I.; Ali Shah, S.M.; Akram, M. Prevalence of transfusion-transmitted infections in multiple blood transfusion-dependent thalassemic patients in Asia: A systemic review. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221096909. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Daar, S.; Alansary, N.; Kattamis, A.; Skafida, M.; Galati, M.C.; Christou, S.; Campisi, S.; Messina, G.; et al. A Concise Review on the Frequency, Major Risk Factors and Surveillance of Hepatocellular Carcinoma (HCC) in β-Thalassemias: Past, Present and Future Perspectives and the ICET-A Experience. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020006. [Google Scholar] [CrossRef]
- Fragkou, N.; Vlachaki, E.; Goulis, I.; Sinakos, E. Liver disease in patients with transfusion-dependent β-thalassemia: The emerging role of metabolism dysfunction-associated steatotic liver disease. World J. Hepatol. 2024, 16, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Hankins, J.S.; McCarville, M.B.; Loeffler, R.B.; Smeltzer, M.P.; Onciu, M.; Hoffer, F.A.; Li, C.S.; Wang, W.C.; Ware, R.E.; Hillenbrand, C.M. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 2009, 113, 4853–4855. [Google Scholar] [CrossRef]
- Meloni, A.; Rienhoff, H.Y., Jr.; Jones, A.; Pepe, A.; Lombardi, M.; Wood, J.C. The use of appropriate calibration curves corrects for systematic differences in liver R2* values measured using different software packages. Br. J. Haematol. 2013, 161, 888–891. [Google Scholar] [CrossRef]
- Garbowski, M.W.; Carpenter, J.P.; Smith, G.; Roughton, M.; Alam, M.H.; He, T.; Pennell, D.J.; Porter, J.B. Biopsy-based calibration of T2* magnetic resonance for estimation of liver iron concentration and comparison with R2 Ferriscan. J. Cardiovasc. Magn. Reson. 2014, 16, 40. [Google Scholar] [CrossRef]
- Jhaveri, K.S.; Kannengiesser, S.A.R.; Ward, R.; Kuo, K.; Sussman, M.S. Prospective Evaluation of an R2* Method for Assessing Liver Iron Concentration (LIC) Against FerriScan: Derivation of the Calibration Curve and Characterization of the Nature and Source of Uncertainty in the Relationship. J. Magn. Reson. Imaging 2019, 49, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Zhang, P.; Rienhoff, H.; Abi-Saab, W.; Neufeld, E. R2 and R2* are equally effective in evaluating chronic response to iron chelation. Am. J. Hematol. 2014, 89, 505–508. [Google Scholar] [CrossRef]
- Henninger, B.; Kremser, C.; Rauch, S.; Eder, R.; Zoller, H.; Finkenstedt, A.; Michaely, H.J.; Schocke, M. Evaluation of MR imaging with T1 and T2* mapping for the determination of hepatic iron overload. Eur. Radiol. 2012, 22, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Pavlides, M.; Tunnicliffe, E.M.; Piechnik, S.K.; Sarania, N.; Philips, R.; Collier, J.D.; Booth, J.C.; Schneider, J.E.; Wang, L.M.; et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J. Hepatol. 2014, 60, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Erden, A.; Oz, D.K.; Peker, E.; Kul, M.; Ozalp Ates, F.S.; Erden, I.; Idilman, R. MRI quantification techniques in fatty liver: The diagnostic performance of hepatic T1, T2, and stiffness measurements in relation to the proton density fat fraction. Diagn. Interv. Radiol. 2021, 27, 7–14. [Google Scholar] [CrossRef]
- Obmann, V.C.; Berzigotti, A.; Catucci, D.; Ebner, L.; Gräni, C.; Heverhagen, J.T.; Christe, A.; Huber, A.T. T1 mapping of the liver and the spleen in patients with liver fibrosis-does normalization to the blood pool increase the predictive value? Eur. Radiol. 2021, 31, 4308–4318. [Google Scholar] [CrossRef]
- Gomes, N.B.N.; Torres, U.S.; Caiado, A.H.M.; Fucuta, P.S.; Ferraz, M.L.C.G.; D’Ippolito, G. Diagnostic accuracy of an uncorrected native T1 mapping sequence for liver fibrosis and inflammation in autoimmune hepatitis: A prospective study using histopathology as reference standard. La Radiol. Medica 2024, 129, 1431–1443. [Google Scholar] [CrossRef]
- Guimaraes, A.R.; Siqueira, L.; Uppal, R.; Alford, J.; Fuchs, B.C.; Yamada, S.; Tanabe, K.; Chung, R.T.; Lauwers, G.; Chew, M.L.; et al. T2 relaxation time is related to liver fibrosis severity. Quant. Imaging Med. Surg. 2016, 6, 103–114. [Google Scholar] [CrossRef]
- Ramazzotti, A.; Pepe, A.; Positano, V.; Rossi, G.; De Marchi, D.; Brizi, M.G.; Luciani, A.; Midiri, M.; Sallustio, G.; Valeri, G.; et al. Multicenter validation of the magnetic resonance t2* technique for segmental and global quantification of myocardial iron. J. Magn. Reson. Imaging 2009, 30, 62–68. [Google Scholar] [CrossRef]
- Meloni, A.; De Marchi, D.; Pistoia, L.; Grassedonio, E.; Peritore, G.; Preziosi, P.; Restaino, G.; Righi, R.; Riva, A.; Renne, S.; et al. Multicenter validation of the magnetic resonance T2* technique for quantification of pancreatic iron. Eur. Radiol. 2019, 29, 2246–2252. [Google Scholar] [CrossRef]
- Meloni, A.; Positano, V.; Pepe, A.; Rossi, G.; Dell’Amico, M.; Salvatori, C.; Keilberg, P.; Filosa, A.; Sallustio, G.; Midiri, M.; et al. Preferential patterns of myocardial iron overload by multislice multiecho T*2 CMR in thalassemia major patients. Magn. Reson. Med. 2010, 64, 211–219. [Google Scholar] [CrossRef]
- Meloni, A.; Nicola, M.; Positano, V.; D’Angelo, G.; Barison, A.; Todiere, G.; Grigoratos, C.; Keilberg, P.; Pistoia, L.; Gargani, L.; et al. Myocardial T2 values at 1.5 T by a segmental approach with healthy aging and gender. Eur. Radiol. 2022, 32, 2962–2975. [Google Scholar] [CrossRef]
- Couinaud, C. Le Foie: Etudes Anatomiques Et Chirurgicales; Masson: Paris, France, 1957. [Google Scholar]
- Meloni, A.; Luciani, A.; Positano, V.; De Marchi, D.; Valeri, G.; Restaino, G.; Cracolici, E.; Caruso, V.; Dell’amico, M.C.; Favilli, B.; et al. Single region of interest versus multislice T2* MRI approach for the quantification of hepatic iron overload. J. Magn. Reson. Imaging 2011, 33, 348–355. [Google Scholar] [CrossRef]
- Meloni, A.; Carnevale, A.; Gaio, P.; Positano, V.; Passantino, C.; Pepe, A.; Barison, A.; Todiere, G.; Grigoratos, C.; Novani, G.; et al. Liver T1 and T2 mapping in a large cohort of healthy subjects: Normal ranges and correlation with age and sex. Magn. Reson. Mater. Phys. Biol. Med. 2024, 37, 93–100. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Yaarubi, S.A.; Skordis, N.; Khater, D.; El Kholy, M.; Stoeva, I.; Fiscina, B.; Angastiniotis, M.; et al. The ICET-A Recommendations for the Diagnosis and Management of Disturbances of Glucose Homeostasis in Thalassemia Major Patients. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016058. [Google Scholar] [CrossRef] [PubMed]
- Diedenhofen, B.; Musch, J. cocor: A Comprehensive Solution for the Statistical Comparison of Correlations. PLoS ONE 2015, 10, e0121945. [Google Scholar] [CrossRef] [PubMed]
- Zerunian, M.; Pucciarelli, F.; Masci, B.; Siciliano, F.; Polici, M.; Bracci, B.; Guido, G.; Polidori, T.; De Santis, D.; Laghi, A.; et al. Updates on Quantitative MRI of Diffuse Liver Disease: A Narrative Review. Biomed Res. Int. 2022, 2022, 1147111. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Y.; Wen, J.; Yang, W.; Liu, X.; Ruan, Y.; Feng, X.; Li, C.; Wu, X. MRI Evaluation of Liver and Cardiac Iron Overload Impact on Hematopoietic Stem Cell Transplantation with β-Thalassemia Major. Blood 2019, 134, 5738. [Google Scholar] [CrossRef]
- Seema, R.; Singh, C.K.; Anjali, V.; Singh, G.P.; Alka, Y.; Deepak, S. Role of magnetic resonance imaging (MRI) in liver iron quantification in thalassemic (thalassemia major) patients. Egypt. Liver J. 2024, 14, 26. [Google Scholar] [CrossRef]
- Fahlenkamp, U.L.; Kunkel, J.; Ziegeler, K.; Neumann, K.; Adams, L.C.; Engel, G.; Böker, S.M.; Makowski, M.R. Correlation of Native Liver Parenchyma T1 and T2 Relaxation Times and Liver Synthetic Function Tests: A Pilot Study. Diagnostics 2021, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Yu, J.S.; Park, K.S.; Kang, S.H.; Huh, J.H.; Chang, J.S.; Lee, J.H.; Kim, M.Y.; Nickel, M.D.; Kannengiesser, S.; et al. Effect of hepatic steatosis on native T1 mapping of 3T magnetic resonance imaging in the assessment of T1 values for patients with non-alcoholic fatty liver disease. Magn. Reson. Imaging 2021, 80, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Zhang, C.; Yang, S.; Wang, Y.; Chen, W.; Li, X.; Wang, D. Native T1 mapping compared to ultrasound elastography for staging and monitoring liver fibrosis: An animal study of repeatability, reproducibility, and accuracy. Eur. Radiol. 2020, 30, 337–345. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Klein, S.; Träber, F.; Schmeel, F.C.; Sprinkart, A.M.; Kuetting, D.L.R.; Block, W.; Uschner, F.E.; Schierwagen, R.; Hittatiya, K.; et al. Quantification of Liver Fibrosis at T1 and T2 Mapping with Extracellular Volume Fraction MRI: Preclinical Results. Radiology 2018, 288, 748–754. [Google Scholar] [CrossRef]
- Takayama, Y.; Nishie, A.; Ishimatsu, K.; Ushijima, Y.; Fujita, N.; Kubo, Y.; Yoshizumi, T.; Kouhashi, K.I.; Maehara, J.; Akamine, Y.; et al. Diagnostic potential of T1ρ and T2 relaxations in assessing the severity of liver fibrosis and necro-inflammation. Magn. Reson. Imaging 2022, 87, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sitarcikova, D.; Poetter-Lang, S.; Bastati, N.; Ba-Ssalamah, S.; Trattnig, S.; Attenberger, U.; Ba-Ssalamah, A.; Krššák, M. Diagnostic accuracy of texture analysis applied to T(1)- and T(2)-Relaxation maps for liver fibrosis classification via machine-learning algorithms with liver histology as reference standard. Eur. J. Radiol. 2025, 183, 111887. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.; Zhang, T.; Li, J.; Liu, H.; Yao, D.; Hou, L.; Tu, B.; Wang, D. Staging Liver Fibrosis: Comparison of Native T1 Mapping, T2 Mapping, and T1ρ: An Experimental Study in Rats with Bile Duct Ligation and Carbon Tetrachloride at 11.7 T MRI. J. Magn. Reson. Imaging 2022, 55, 507–517. [Google Scholar] [CrossRef]
- Xue, S.; Zhu, Y.; Shao, M.; Zhu, K.; Rong, J.; Liu, T.; Yin, X.; Zhang, S.; Yin, L.; Wang, X. T1/T2 mapping as a non-invasive method for evaluating liver fibrosis based on correlation of biomarkers: A preclinical study. BMC Gastroenterol. 2025, 25, 122. [Google Scholar] [CrossRef]
- Wood, J.C.; Ghugre, N. Magnetic resonance imaging assessment of excess iron in thalassemia, sickle cell disease and other iron overload diseases. Hemoglobin 2008, 32, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Razmjou, S.; Akhlaghpoor, S.; Eslami, S.M.; Azarkeivan, A.; Amiri, A. T2* magnetic resonance imaging of the liver in thalassemic patients in Iran. World J. Gastroenterol. 2011, 17, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Azarkeivan, A.; Hashemieh, M.; Akhlaghpoor, S.; Shirkavand, A.; Yaseri, M.; Sheibani, K. Relation between serum ferritin and liver and heart MRI T2* in beta thalassaemia major patients. East. Mediterr. Health J. 2013, 19, 727–732. [Google Scholar] [CrossRef]
- Khadivi Heris, H.; Nejati, B.; Rezazadeh, K.; Sate, H.; Dolatkhah, R.; Ghoreishi, Z.; Esfahani, A. Evaluation of iron overload by cardiac and liver T2* in β-thalassemia: Correlation with serum ferritin, heart function and liver enzymes. J. Cardiovasc. Thorac. Res. 2021, 13, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Luo, T.; Huang, L.; Lu, Q.; Yan, D.; Meng, Y.; Xie, J.; Chen, W.; Chen, J.; Long, L. Magnetic Resonance Imaging Quantification of the Liver Iron Burden and Volume Changes Following Treatment with Thalidomide in Patients with Transfusion-Dependent ß-Thalassemia. Front. Pharmacol. 2022, 13, 810668. [Google Scholar] [CrossRef]
- Fernandez, M.; Lokan, J.; Leung, C.; Grigg, A. A critical evaluation of the role of iron overload in fatty liver disease. J. Gastroenterol. Hepatol. 2022, 37, 1873–1883. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Tantiworawit, A.; Leerapun, A.; Soontornpun, A.; Thongsawat, S. Secondary Iron Overload and the Liver: A Comprehensive Review. J. Clin. Transl. Hepatol. 2023, 11, 932–941. [Google Scholar] [CrossRef]
- Liu, D.; Luo, Y.; Zheng, Y.; Ji, R.; Zhou, Y. Effect of elevated serum ferritin on the risk of death in patients with decompensated cirrhosis: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 795–802. [Google Scholar] [CrossRef]
- Spasiano, A.; Meloni, A.; Costantini, S.; Quaia, E.; Cademartiri, F.; Cinque, P.; Pepe, A.; Ricchi, P. Setting for “Normal” Serum Ferritin Levels in Patients with Transfusion-Dependent Thalassemia: Our Current Strategy. J. Clin. Med. 2021, 10, 5985. [Google Scholar] [CrossRef]
- Ali, M.S.; Borhany, M.; Butt, A.J.; Munawar Ali, R.; Kashif, S.; Wahaj, M.; Shamsi, T. Correlation Between Serum Ferritin and Degree of Hepatic Fibrosis on Fibroscan in Thalassemic Patients. Cureus 2023, 15, e42069. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, P.; Meloni, A.; Spasiano, A.; Costantini, S.; Pepe, A.; Cinque, P.; Filosa, A. The impact of liver steatosis on the ability of serum ferritin levels to be predictive of liver iron concentration in non-transfusion-dependent thalassaemia patients. Br. J. Haematol. 2018, 180, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, P.; Pistoia, L.; Positano, V.; Spasiano, A.; Casini, T.; Putti, M.C.; Borsellino, Z.; Cossu, A.; Messina, G.; Keilberg, P.; et al. Liver steatosis in patients with transfusion-dependent thalassaemia. Br. J. Haematol. 2024, 204, 2458–2467. [Google Scholar] [CrossRef]
- Breit, H.C.; Block, K.T.; Winkel, D.J.; Gehweiler, J.E.; Henkel, M.J.; Weikert, T.; Stieltjes, B.; Boll, D.T.; Heye, T.J. Evaluation of liver fibrosis and cirrhosis on the basis of quantitative T1 mapping: Are acute inflammation, age and liver volume confounding factors? Eur. J. Radiol. 2021, 141, 109789. [Google Scholar] [CrossRef] [PubMed]
- Kupczyk, P.A.; Mesropyan, N.; Isaak, A.; Endler, C.; Faron, A.; Kuetting, D.; Sprinkart, A.M.; Mädler, B.; Thomas, D.; Attenberger, U.I.; et al. Quantitative MRI of the liver: Evaluation of extracellular volume fraction and other quantitative parameters in comparison to MR elastography for the assessment of hepatopathy. Magn. Reson. Imaging 2021, 77, 7–13. [Google Scholar] [CrossRef] [PubMed]
- von Ulmenstein, S.; Bogdanovic, S.; Honcharova-Biletska, H.; Blümel, S.; Deibel, A.R.; Segna, D.; Jüngst, C.; Weber, A.; Kuntzen, T.; Gubler, C.; et al. Assessment of hepatic fibrosis and inflammation with look-locker T1 mapping and magnetic resonance elastography with histopathology as reference standard. Abdom. Radiol. 2022, 47, 3746–3757. [Google Scholar] [CrossRef]
- Özyurt, G.M.; Esen, K.; Üçbilek, E.; Apaydın, F.D. Liver/spleen magnetic resonance elastography and T1/T2 mapping in chronic liver disease: A prospective study. Rev. Assoc. Médica Bras. 2024, 70, e20241008. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Merkel, P.A.; Simonson, D.C.; Amiel, S.A.; Plewe, G.; Sherwin, R.S.; Pearson, H.A.; Tamborlane, W.V. Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. N. Engl. J. Med. 1988, 318, 809–814. [Google Scholar] [CrossRef]
- Venou, T.M.; Kyriakidis, F.; Barmpageorgopoulou, F.; Theodoridou, S.; Vyzantiadis, A.; Klonizakis, P.; Gavriilaki, E.; Vlachaki, E. Risk Factors for Impaired Glucose Metabolism in Transfusion-Dependent Patients with β-Thalassemia: A Single-Center Retrospective Observational Study. Hematol. Rep. 2025, 17, 6. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Peluso, A.; Messina, G.; Spasiano, A.; Allo, M.; Bisconte, M.G.; Putti, M.C.; et al. The Close Link of Pancreatic Iron with Glucose Metabolism and with Cardiac Complications in Thalassemia Major: A Large, Multicenter Observational Study. Diabetes Care 2020, 43, 2830–2839. [Google Scholar] [CrossRef]
- Klaus, J.B.; Goerke, U.; Klarhöfer, M.; Keerthivasan, M.B.; Jung, B.; Berzigotti, A.; Ebner, L.; Roos, J.; Christe, A.; Obmann, V.C.; et al. MRI Dixon Fat-Corrected Look-Locker T1 Mapping for Quantification of Liver Fibrosis and Inflammation—A Comparison with the Non-Fat-Corrected Shortened Modified Look-Locker Inversion Recovery Technique. Investig. Radiol. 2024, 59, 754–760. [Google Scholar] [CrossRef]
- Bradley, C.R.; Cox, E.F.; Palaniyappan, N.; Aithal, G.P.; Francis, S.T.; Guha, I.N. Variability of noninvasive MRI and biological markers in compensated cirrhosis: Insights for assessing disease progression. Eur. Radiol. Exp. 2022, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, M.; Banerjee, R.; Sellwood, J.; Kelly, C.J.; Robson, M.D.; Booth, J.C.; Collier, J.; Neubauer, S.; Barnes, E. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J. Hepatol. 2016, 64, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.R.; Serai, S.D.; Miethke, A.G.; Singh, R.; Tkach, J.A.; Trout, A.T. Comparison of liver T1 relaxation times without and with iron correction in pediatric autoimmune liver disease. Pediatr. Radiol. 2020, 50, 935–942. [Google Scholar] [CrossRef] [PubMed]


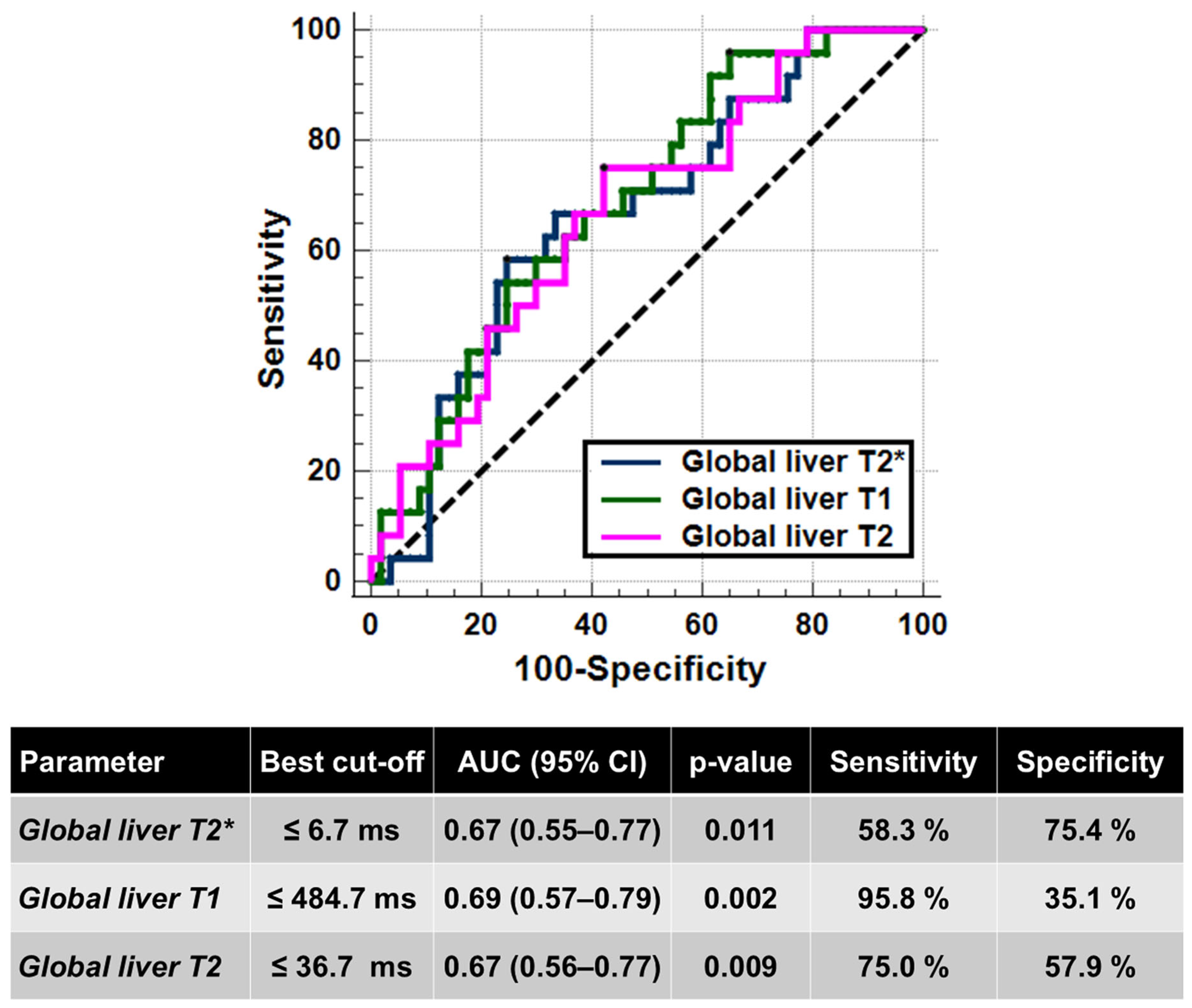
| Global Liver T2* Values | Global Liver T1 Values | Global Liver T2 Values | ||
|---|---|---|---|---|
| Categorical Variable | ||||
| Frequency, N (%) | Difference in relaxation times between two groups (absent vs. present) | |||
| Female sex | 52 (64.2) | 14.19 ± 9.52 ms vs. 12.86 ± 9.63 ms (p = 0.497) | 458.65 ± 80.21 ms vs. 431.61 ± 70.90 ms (p = 0.129) | 39.11 ± 7.46 ms vs. 37.13 ± 8.24 ms (p = 0.279) |
| Splenectomy | 32 (39.5) | 13.24 ± 9.18 ms vs. 13.49 ± 10.25 ms (p = 0.988) | 434.78 ± 68.07 ms vs. 451.27 ± 84.72 ms (p = 0.417) | 37.22 ± 7.24 ms vs. 38.79 ± 9.03 ms (p = 0.364) |
| Past/active HCV infection | 50 (61.7) | 12.46 ± 9.59 ms vs. 12.88 ± 9.59 ms (p = 0.553) | 430.72 ± 75.59 ms vs. 447.97 ± 74.62 ms (p = 0.252) | 37.13 ± 8.96 ms vs. 38.28 ±7.37 ms (p = 0.627) |
| Continuous variables | ||||
| Mean value | Correlation (R, p-value) with relaxation times | |||
| Age | 38.13 ± 10.79 years | R = 0.161, p = 0.150 | R = 0.264, p = 0.017 | R = 0.178 p = 0.112 |
| Age at start of regular transfusions | 10.74 ± 8.67 months | R = 0.149, p = 0.401 | R = 0.262, p = 0.134 | R = 0.098, p = 0.582 |
| Pre-transfusion hemoglobin | 9.71 ± 0.42 g/dL | R = 0128, p = 0.319 | R = −0.027, p = 0.832 | R = −0.089, p = 0.486 |
| Mean serum ferritin | 845.23 ± 746.01 ng/mL | R = −0.545, p < 0.0001 | R = −0.423, p = 0.001 | R = −0.662, p < 0.0001 |
| Mean alanine aminotransferase | 21.87 ± 10.22 U/L | R = −0.309, p = 0.017 | R = −0.157, p = 0.236 | R = −0.320, p = 0.013 |
| Mean aspartate aminotransferase | 21.68 ± 7.27 U/L | R = −0.186, p = 0.163 | R = −0.040, p = 0.767 | R = −0.179, p = 0.179 |
| Mean gamma-glutamyl transferase | 17.80 ± 12.01 U/L | R = −0.220, p = 0.161 | R = 0.192, p = 0.224 | R = 0.022, p = 0.892 |
| Global liver T2* values | R = 0.643 p < 0.0001 | R = 0.764, p < 0.0001 | ||
| Global liver T1 values | R = 0.643, p < 0.0001 | R = 0.761, p < 0.0001 | ||
| Global liver T2 values | R = 0.764, p < 0.0001 | R = 0.761, p < 0.0001 | ||
| Region | Intra-Operator | ||||
|---|---|---|---|---|---|
| Paired Test | Bland–Altman Limits (ms) | CoV (%) | ICC | ||
| Mean Difference (ms) | p-Value | ||||
| T2* values | |||||
| Segment 2 | 0.14 ± 0.63 | 0.223 | −1.11 to 1.38 | 3.76 | 0.999 |
| Segment 3 | 0.17 ± 0.67 | 0.587 | −1.13 to 1.47 | 4.49 | 0.998 |
| Segment 4 | −0.45 ± 1.07 | 0.241 | −2.55 to 1.65 | 5.27 | 0.994 |
| Global | 0.06 ± 0.61 | 0.543 | −1.13 to 1.25 | 3.53 | 0.999 |
| T1 values | |||||
| Segment 2 | 6.19 ± 28.66 | 0.367 | −49.98 to 62.37 | 4.59 | 0.974 |
| Segment 3 | 2.31 ± 33.36 | 0.231 | −63.09 to 67.70 | 5.29 | 0.967 |
| Segment 4 | 0.02 ± 21.33 | 0.721 | −41.79 to 41.83 | 3.25 | 0.992 |
| Global | 4.33 ± 18.31 | 0.158 | −31.55 to 40.22 | 2.98 | 0.989 |
| T2 values | |||||
| Segment 2 | −0.62 ± 2.46 | 0.142 | −5.45 to 4.21 | 4.72 | 0.983 |
| Segment 3 | 0.18 ± 2.55 | 0.616 | −4.83 to 5.18 | 4.87 | 0.983 |
| Segment 4 | 0.78 ± 2.29 | 0.314 | −3.71 to 5.26 | 4.23 | 0.979 |
| Global | −0.11 ± 1.18 | 0.983 | −2.41 to 2.20 | 2.23 | 0.996 |
| Region | Inter-Operator | ||||
|---|---|---|---|---|---|
| Paired Test | Bland–Altman Limits (ms) | CoV (%) | ICC | ||
| Mean Difference (ms) | p-Value | ||||
| T2* values | |||||
| Segment 2 | 0.02 ± 0.86 | 0.808 | −1.68 to 1.69 | 4.95 | 0.997 |
| Segment 3 | 0.19 ± 0.83 | 0.407 | −1.44 to 1.82 | 4.59 | 0.996 |
| Segment 4 | −0.29 ± 1.10 | 0.721 | −2.45 to 1.87 | 5.17 | 0.994 |
| Global | 0.03 ± 0.69 | 0.661 | −1.33 to 1.39 | 3.99 | 0.998 |
| T1 values | |||||
| Segment 2 | −5.41 ± 29.40 | 0.443 | −63.04 to 52.22 | 4.62 | 0.974 |
| Segment 3 | 5.42 ± 32.21 | 0.216 | −57.72 to 68.56 | 5.18 | 0.967 |
| Segment 4 | −8.83 ± 19.99 | 0.386 | −48.01 to 30.35 | 3.33 | 0.991 |
| Global | −0.95 ± 20.21 | 0.882 | −40.57 to 38.66 | 3.18 | 0.988 |
| T2 values | |||||
| Segment 2 | −0.34 ± 2.89 | 0.647 | −6.01 to 5.33 | 5.42 | 0.978 |
| Segment 3 | 0.98 ± 2.42 | 0.184 | −3.76 to 5.72 | 5.05 | 0.981 |
| Segment 4 | 0.82 ± 2.60 | 0.477 | −4.28 to 5.92 | 4.77 | 0.972 |
| Global | 0.32 ± 1.75 | 0.632 | −3.10 to 3.74 | 3.37 | 0.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Bisi, R.; Positano, V.; Carnevale, A.; Pegoraro, N.; Pistoia, L.; Spasiano, A.; Corigliano, E.; Cossu, A.; De Marco, E.; et al. Liver Tissue Mapping in Transfusion-Dependent β-Thalassemia: Reproducibility and Clinical Insights from Multiparametric MRI. Diagnostics 2025, 15, 3085. https://doi.org/10.3390/diagnostics15233085
Meloni A, Bisi R, Positano V, Carnevale A, Pegoraro N, Pistoia L, Spasiano A, Corigliano E, Cossu A, De Marco E, et al. Liver Tissue Mapping in Transfusion-Dependent β-Thalassemia: Reproducibility and Clinical Insights from Multiparametric MRI. Diagnostics. 2025; 15(23):3085. https://doi.org/10.3390/diagnostics15233085
Chicago/Turabian StyleMeloni, Antonella, Riccardo Bisi, Vincenzo Positano, Aldo Carnevale, Nicola Pegoraro, Laura Pistoia, Anna Spasiano, Elisabetta Corigliano, Antonella Cossu, Emanuela De Marco, and et al. 2025. "Liver Tissue Mapping in Transfusion-Dependent β-Thalassemia: Reproducibility and Clinical Insights from Multiparametric MRI" Diagnostics 15, no. 23: 3085. https://doi.org/10.3390/diagnostics15233085
APA StyleMeloni, A., Bisi, R., Positano, V., Carnevale, A., Pegoraro, N., Pistoia, L., Spasiano, A., Corigliano, E., Cossu, A., De Marco, E., Fotzi, I., Keilberg, P., Clemente, A., & Cossu, A. (2025). Liver Tissue Mapping in Transfusion-Dependent β-Thalassemia: Reproducibility and Clinical Insights from Multiparametric MRI. Diagnostics, 15(23), 3085. https://doi.org/10.3390/diagnostics15233085









