Comprehensive Evaluation of a Point-of-Care Testing Platform for Decentralized Primary Healthcare: Ensuring Analytical Quality Through Central Laboratory Oversight
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Sample Collection and Handling
2.3. Point-of-Care Testing System and Procedures
2.4. Central Laboratory Reference Methods
2.5. Statistical Analysis
3. Results
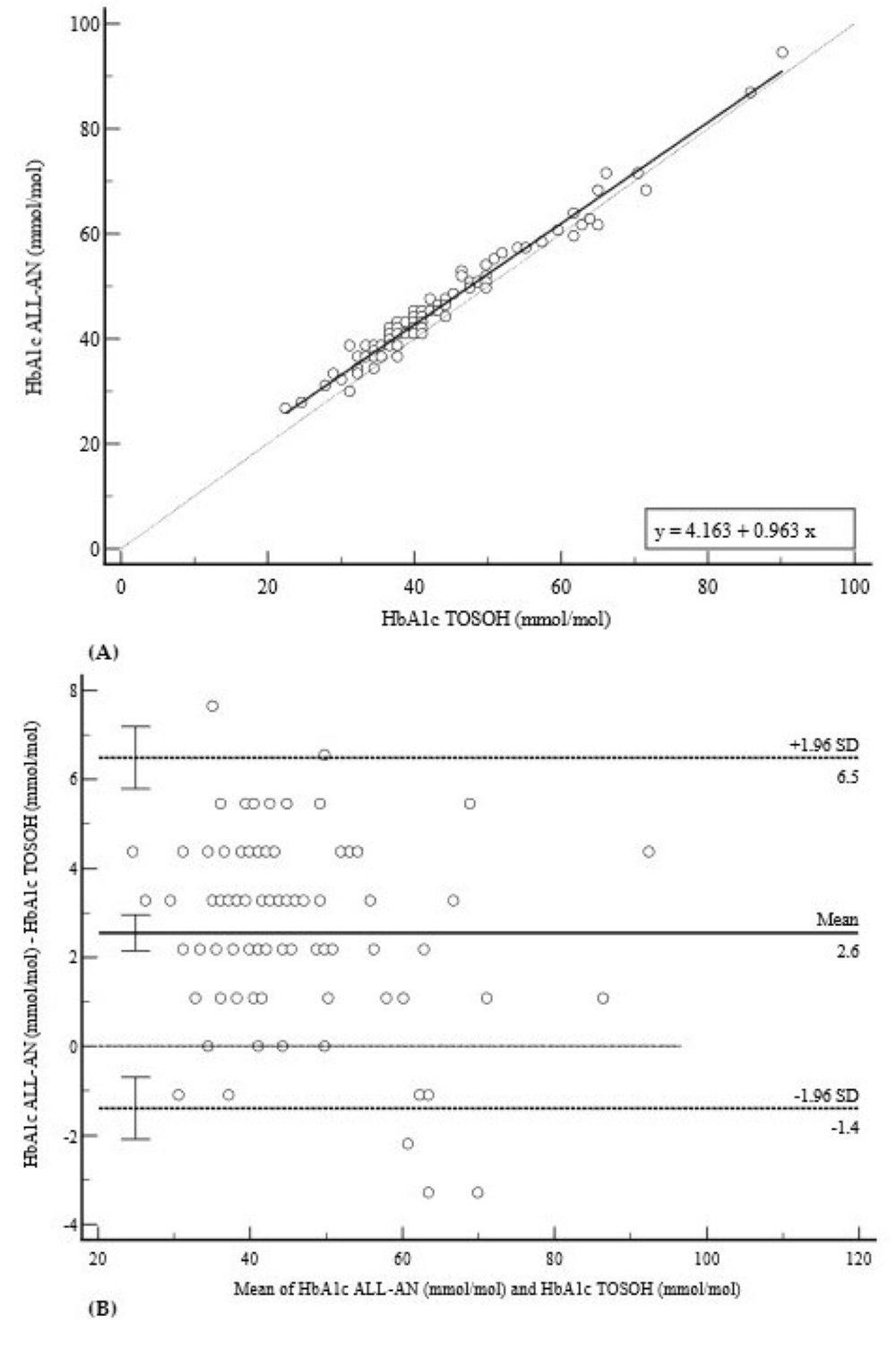
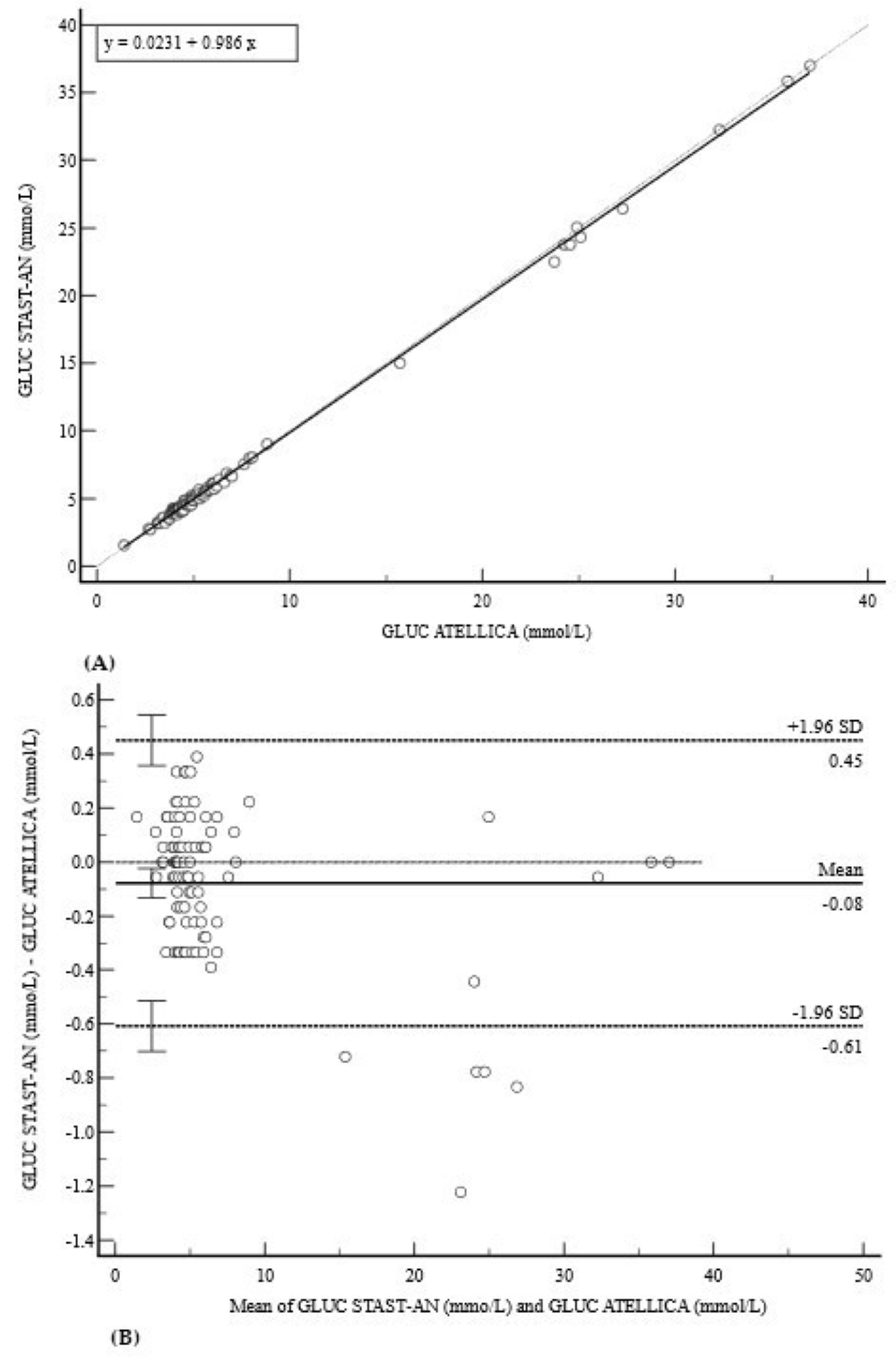
3.1. Diabetic Profile (HbA1c and GLUC)
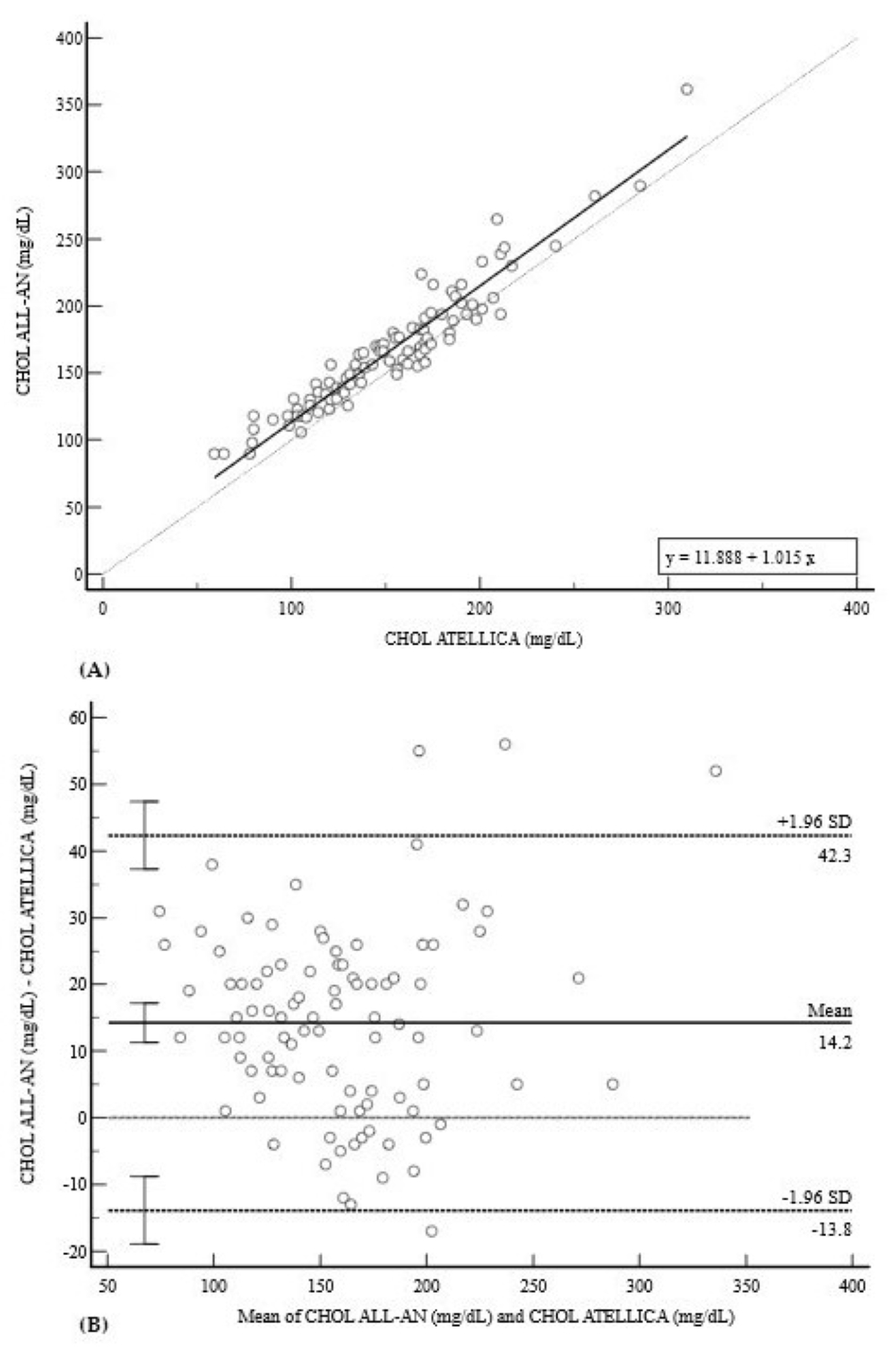
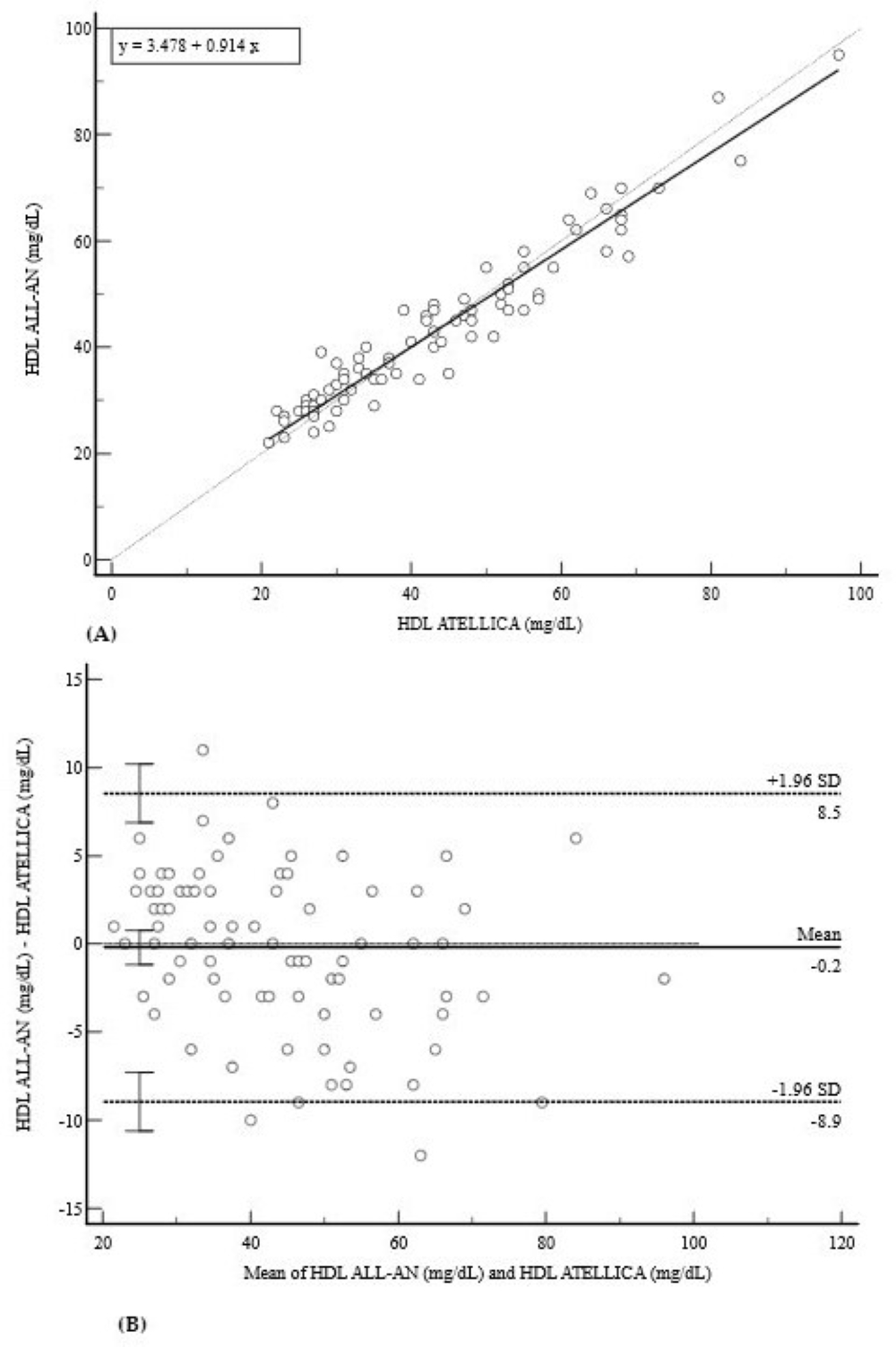
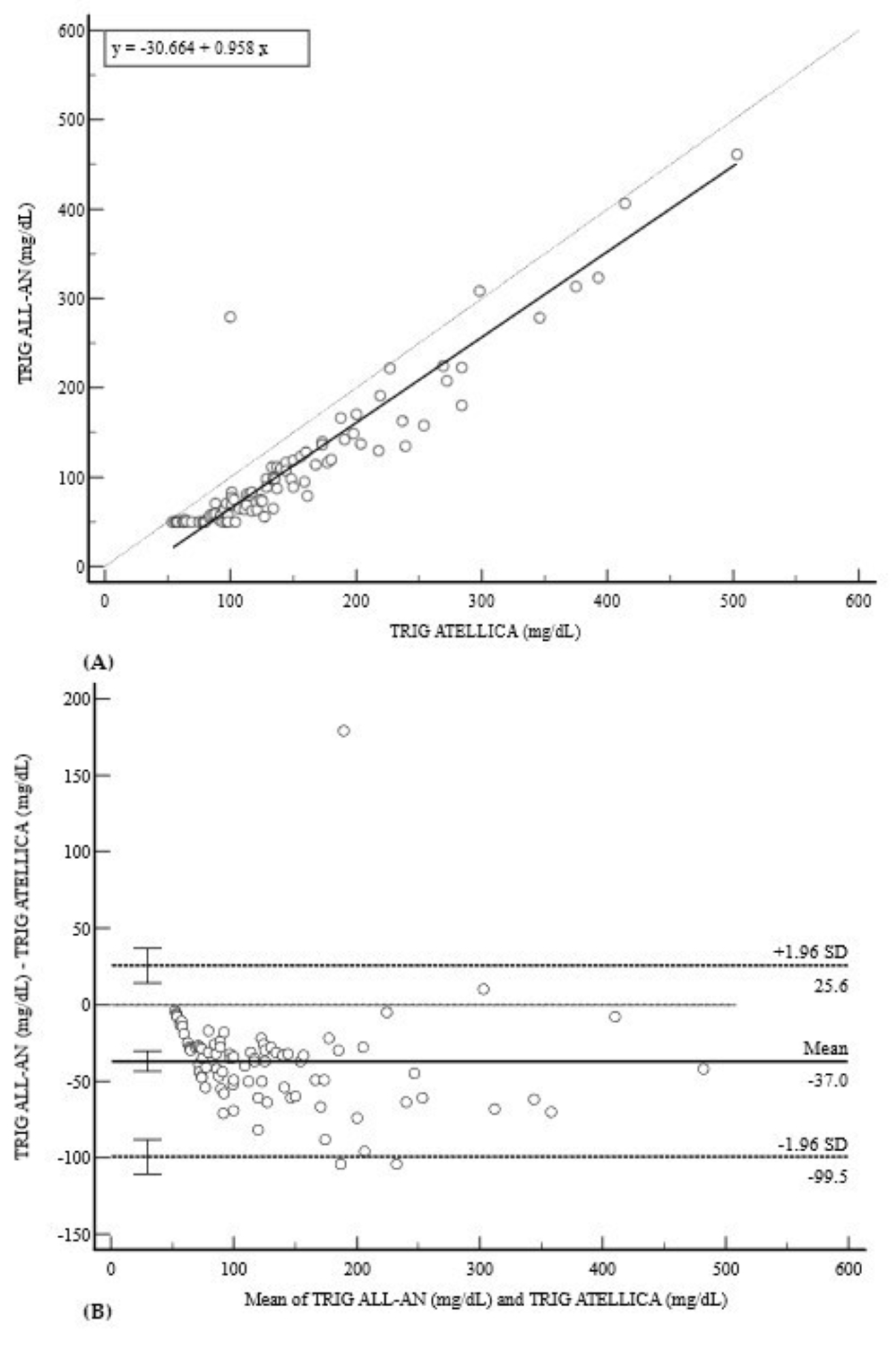
3.2. Lipid Profile (CHOL, HDL, and TRIG)
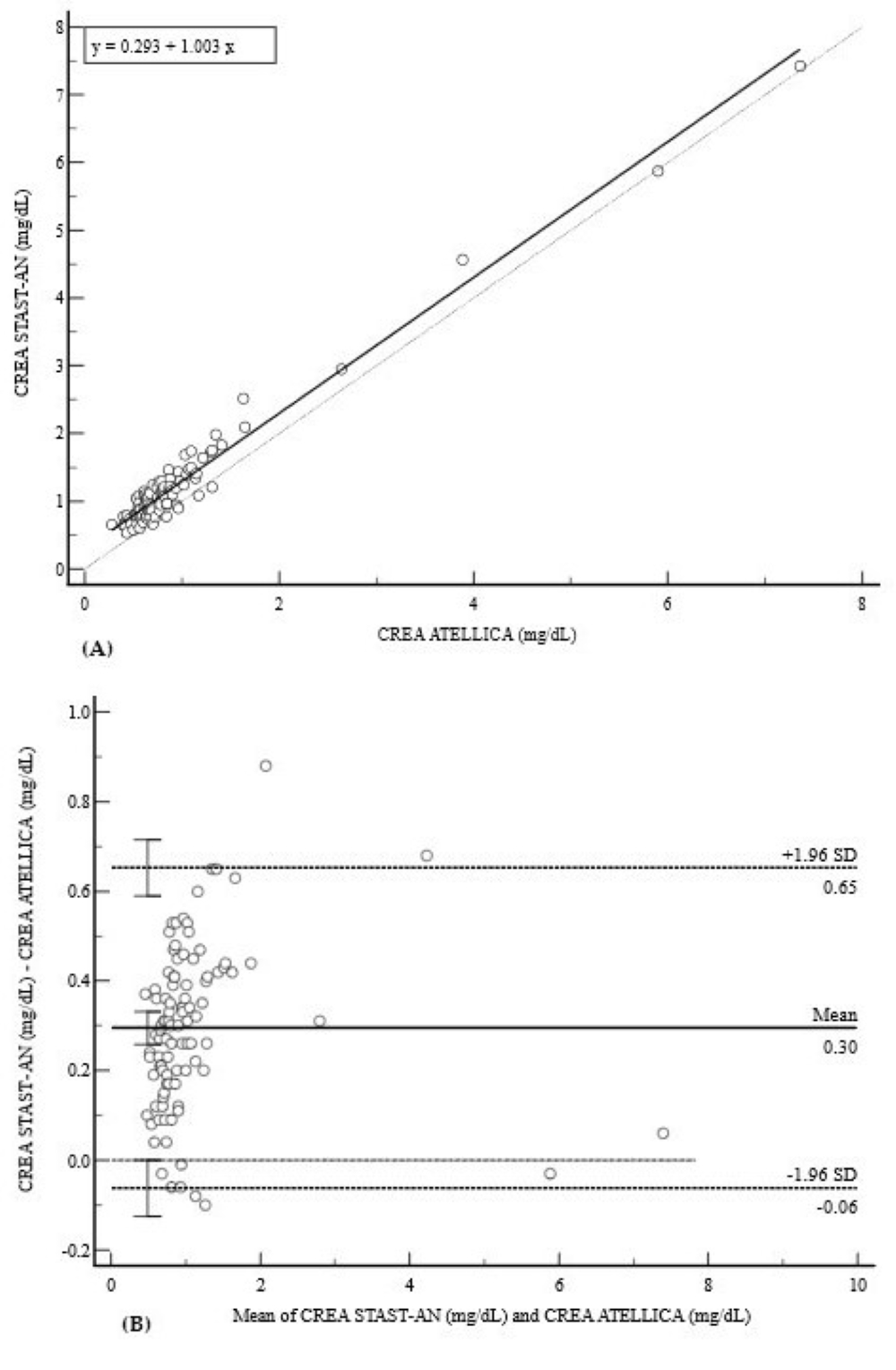
3.3. Kidney Profile (CREA)
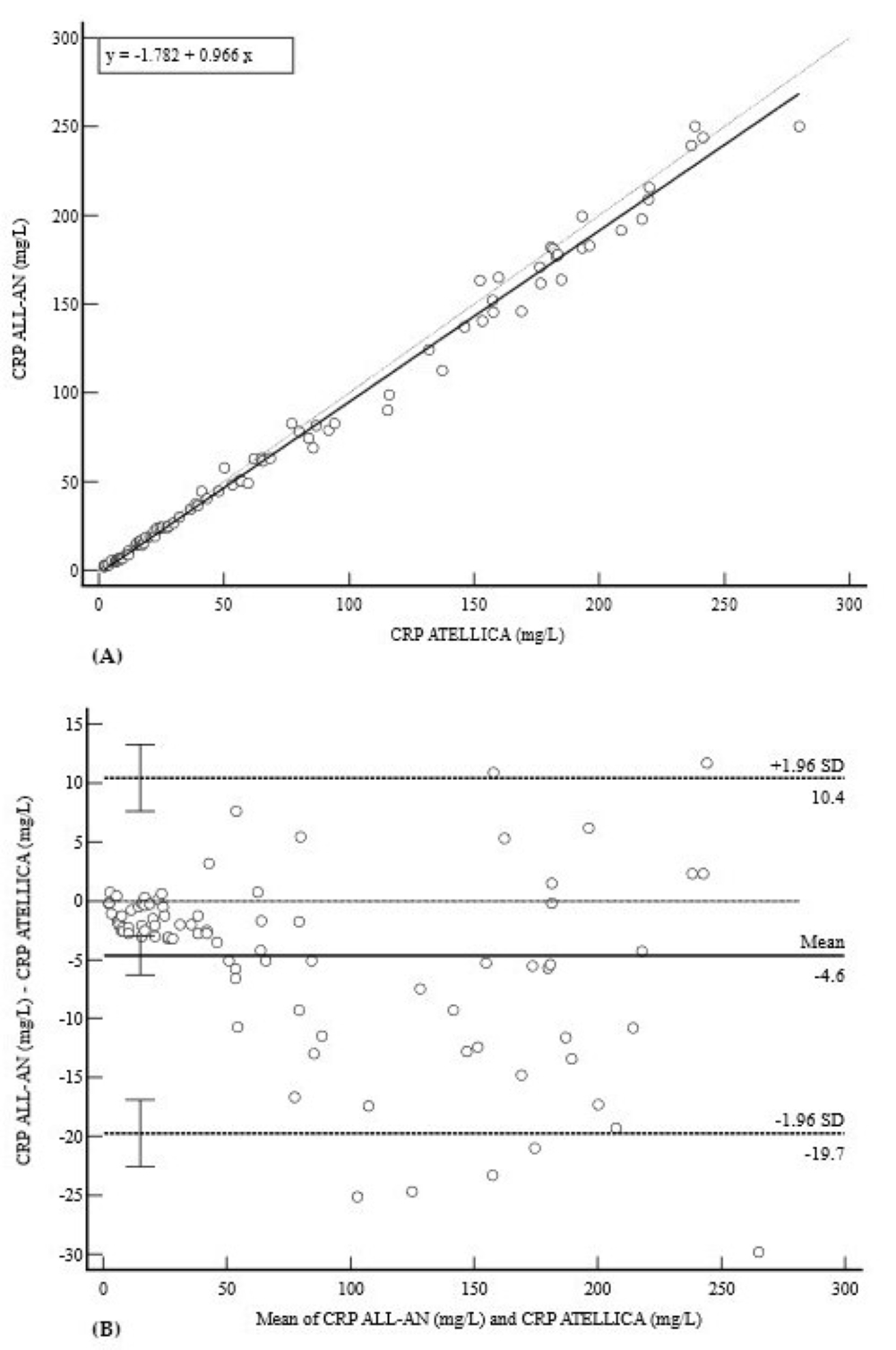
3.4. Inflammatory Profile (CRP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| POCT | Point-of-Care Testing |
| HbA1c | Glycated Hemoglobin |
| GLUC | Glucose |
| CHOL | Total Cholesterol |
| HDL | High-Density Lipoprotein Cholesterol |
| TRIG | Triglycerides |
| CREA | Creatinine |
| CRP | C-Reactive Protein |
| IQC | Internal Quality Control |
| EQA | External Quality Assessment |
| LH | Lithium Heparin |
| ALL-AN | Allegro Analyzer |
| STAST-AN | StatStrip A Meter Analyzer |
| HPLC | High-Performance Liquid Chromatography |
References
- Mitra, P.; Sharma, P. POCT in Developing Countries. eJIFCC 2021, 32, 195–199. [Google Scholar] [PubMed]
- Wilson, S.; Bohn, M.K.; Adeli, K. POCT: An Inherently Ideal Tool in Pediatric Laboratory Medicine. eJIFCC 2021, 32, 145–157. [Google Scholar] [PubMed]
- Gout-Zwart, J.J.; Olde Hengel, E.H.J.; Hoogland, P.; Postma, M.J. Budget Impact Analysis of a Renal Point-of-Care Test in Dutch Community Pharmacies to Prevent Antibiotic-Related Hospitalizations. Appl. Health Econ. Health Policy 2019, 17, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Griggs, J.E.; Lyon, R.M.; Sherriff, M.; Barrett, J.W.; Wareham, G.; Ter Avest, E. Air Ambulance Charity Kent Surrey Sussex. Predictive clinical utility of pre-hospital point of care lactate for transfusion of blood product in patients with suspected traumatic haemorrhage: Derivation of a decision-support tool. Scand. J. Trauma. Resusc. Emerg. Med. 2022, 30, 72. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, E.L.; Lewandrowski, K. Implementing point-of-care testing to improve outcomes. J. Hosp. Adm. 2013, 2, 2. [Google Scholar] [CrossRef]
- Goyder, C.; Tan, P.S.; Verbakel, J.; Ananthakumar, T.; Lee, J.J.; Hayward, G.; Turner, P.J.; Van Den Bruel, A. Impact of point-of-care panel tests in ambulatory care: A systematic review and meta-analysis. BMJ Open 2020, 10, e032132. [Google Scholar]
- Crocker, J.B.; Lee-Lewandrowski, E.; Lewandrowski, N.; Baron, J.; Gregory, K.; Lewandrowski, K. Implementation of point-of-care testing in an ambulatory practice of an academic medical center. Am. J. Clin. Pathol. 2014, 142, 640–646. [Google Scholar]
- Schols, A.M.R.; Stakenborg, J.P.G.; Dinant, G.-J.; Willemsen, R.T.A.; Cals, J.W.L. Point-of-care testing in primary care patients with acute cardiopulmonary symptoms: A systematic review. Fam. Pract. 2018, 35, 4–12. [Google Scholar]
- Gubbins, P.O.; Klepser, M.E.; Dering-Anderson, A.M.; Bauer, K.A.; Darin, K.M.; Klepser, S.A.; Matthias, K.R.; Scarsi, K. Point-of-care testing for infectious diseases: Opportunities, barriers, and considerations in community pharmacy. J. Am. Pharm. Assoc. 2014, 54, 163–171. [Google Scholar]
- Kosack, C.S.; de Kieviet, W.; Bayrak, K.; Milovic, A.; Page, A.L. Evaluation of the Nova StatSensor® Xpress™ Creatinine Point-Of-Care Handheld Analyzer. PLoS ONE 2015, 10, e0122433. [Google Scholar]
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar]
- Zaninotto, M.; Miolo, G.; Guiotto, A.; Marton, S.; Plebani, M. Quality performance of laboratory testing in pharmacies: A collaborative evaluation. Clin. Chem. Lab. Med. 2016, 54, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Walther, L.H.; Zegers, F.; Nybo, M.; Mogensen, C.B.; Christensen, E.F.; Lassen, A.T.; Mikkelsen, S. Accuracy of a point-of-care blood lactate measurement device in a prehospital setting. J. Clin. Monit. Comput. 2022, 36, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- van Lint, C.L.; van der Boog, P.J.; Wang, W.; Brinkman, W.P.; Rövekamp, T.J.; Neerincx, M.A.; Rabelink, T.J.; van Dijk, S. Patient experiences with self-monitoring renal function after renal transplantation: Results from a single-center prospective pilot study. Patient Prefer. Adherence 2015, 9, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- ISO 15189; Medical Laboratories—Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 22870; Point-of-Care Testing (POCT)—Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2016.
- Shephard, M.; Peake, M.; Corso, O.; Shephard, A.; Mazzachi, B.; Spaeth, B.; Barbara, J.; Mathew, T. Assessment of the Nova StatSensor whole blood point-of-care creatinine analyzer for the measurement of kidney function in screening for chronic kidney disease. Clin. Chem. Lab. Med.Clin. Chem. 2010, 48, 1113–1119. [Google Scholar]
- Lenters-Westra, E.; Slingerland, R.J. Three of four point-of-care Hba1c instruments do not meet generally accepted analytical performance criteria. Clin. Chem. 2014, 60, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Lippi, G. Point of care testing: Evolving scenarios and innovative perspectives. Clin. Chem. Lab Med. 2014, 52, 309–311. [Google Scholar]
- Price, C.P.; St John, A.; Nichols, J.H. Point-of-Care Testing. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th ed.; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: St. Louis, MO, USA, 2018; pp. 280–308. [Google Scholar]
| Analyte | System | Method/Principle | Required Volume (µL) | Time to Result | Linearity Range | Reference Range | Matrix |
|---|---|---|---|---|---|---|---|
| HbA1c | Allegro (Nova Biomedical Italia S.r.l.®, Lainate, Milan, Italy, ALL-AN) | Latex Agglutination Immunoassay | 1.5 | 6 min | 10–130 mmol/mol | <42 mmol/mol | Whole Blood (K3EDTA) |
| Central Lab (Tosoh Bioscience® S.r.l., Turin, Italy, TOSOH) | HPLC | 4 | 1.6 min | N/A (calibrated range) | <42 mmol/mol | Whole Blood (K3EDTA) | |
| Glucose | Allegro (STAST-AN) | Amperometric Biosensor | 1.2 | 6 s | 10–600 mg/dL | 65–100 mg/dL | Whole Blood (LH) |
| Central Lab (Siemens Healthcare® s.r.l., Milan, Italy, Atellica) | Enzymatic (Hexokinase) | 5 | 10 min | 4–700 mg/dL | 65–100 mg/dL | Plasma (LH) | |
| Cholesterol | Allegro (ALL-AN) | Enzymatic-Colorimetric | 80 | 10 min | 25–620 mg/dL | <200 mg/dL | Whole Blood (LH) |
| Central Lab (Atellica) | Enzymatic-Colorimetric | 5 | 10 min | 90–500 mg/dL | <200 mg/dL | Plasma (LH) | |
| HDL | Allegro (ALL-AN) | Immunometric | 80 | 10 min | 5–129 mg/dL | >45 mg/dL | Whole Blood (LH) |
| Central Lab (Atellica) | Enzymatic-Colorimetric (Direct) | 5 | 10 min | 20–100 mg/dL | >45 mg/dL | Plasma (LH) | |
| Triglycerides | Allegro (ALL-AN) | Enzymatic-Colorimetric | 80 | 10 min | 10–600 mg/dL | <150 mg/dL | Whole Blood (LH) |
| Central Lab (Atellica) | Enzymatic-Colorimetric | 5 | 10 min | 50–550 mg/dL | <150 mg/dL | Plasma (LH) | |
| Creatinine | Allegro (STAST-AN) | Amperometric Biosensor | 1.2 | 30 s | 0.3–12 mg/dL | 0.67–1.17 mg/dL | Whole Blood (LH) |
| Central Lab (Atellica) | Enzymatic (Creatinase) | 5 | 10 min | 0.1–30 mg/dL | 0.67–1.17 mg/dL | Plasma (LH) | |
| CRP | Allegro (ALL-AN) | Latex-Enhanced Immunoturbidimetry | 5 | 7 min | 2.0–250 mg/L | <5.0 mg/L | Whole Blood (LH) |
| Central Lab (Atellica) | Immunoturbidimetry | 40 | 10 min | 0.5–156 mg/L | <5.0 mg/L | Plasma (LH) |
| Analyte (Units) | Pearson’s r (95% CI) | Deming Intercept (95% CI) | Deming Slope (95% CI) | Mean Bias (95% LoA) |
|---|---|---|---|---|
| HbA1c (mmol/mol) | 0.9863 (0.98–0.99) | 4.16 (2.50 to 5.82) * | 0.96 (0.93 to 0.99) * | +2.6 (−1.4 to 6.5) |
| GLUC (mmol/L) | 0.9994 (0.99–1.00) | 0.02 (−0.05 to 0.10) | 0.98 (0.97 to 1.00) | −0.08 (−0.61 to 0.45) |
| CHOL (mg/dL) | 0.9510 (0.93–0.97) | 11.89 (4.50 to 19.28) * | 1.01 (0.98 to 1.05) | +14.2 (−13.8 to 42.3) |
| HDL (mg/dL) | 0.9640 (0.95–0.97) | 3.48 (−0.50 to 7.46) | 0.91 (0.86 to 0.97) | −0.2 (−8.9 to 8.5) |
| TRIG (mg/dL) | 0.9287 (0.90–0.95) | −30.66 (−45.1 to −16.22) * | 0.96 (0.91 to 1.01) | −37.0 (−99.5 to 25.6) |
| CREA (mg/dL) | 0.9816 (0.97–0.99) | 0.29 (0.24 to 0.35) * | 1.00 (0.95 to 1.05) | +0.3 (−0.06 to 0.65) |
| CRP (mg/L) | 0.9955 (0.99–1.00) | −1.78 (−3.5 to 0.0) | 0.97 (0.95 to 0.99) | −4.6 (−19.7 to 10.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, G.; Alcaro, F.D.; Colacicco, L.; Urbani, A. Comprehensive Evaluation of a Point-of-Care Testing Platform for Decentralized Primary Healthcare: Ensuring Analytical Quality Through Central Laboratory Oversight. Diagnostics 2025, 15, 2977. https://doi.org/10.3390/diagnostics15232977
Moretti G, Alcaro FD, Colacicco L, Urbani A. Comprehensive Evaluation of a Point-of-Care Testing Platform for Decentralized Primary Healthcare: Ensuring Analytical Quality Through Central Laboratory Oversight. Diagnostics. 2025; 15(23):2977. https://doi.org/10.3390/diagnostics15232977
Chicago/Turabian StyleMoretti, Giacomo, Francesca Danila Alcaro, Luigi Colacicco, and Andrea Urbani. 2025. "Comprehensive Evaluation of a Point-of-Care Testing Platform for Decentralized Primary Healthcare: Ensuring Analytical Quality Through Central Laboratory Oversight" Diagnostics 15, no. 23: 2977. https://doi.org/10.3390/diagnostics15232977
APA StyleMoretti, G., Alcaro, F. D., Colacicco, L., & Urbani, A. (2025). Comprehensive Evaluation of a Point-of-Care Testing Platform for Decentralized Primary Healthcare: Ensuring Analytical Quality Through Central Laboratory Oversight. Diagnostics, 15(23), 2977. https://doi.org/10.3390/diagnostics15232977







