Preoperative Predictors of Subsequent Breast Cancer Events Detected on Abbreviated MRI in Patients with Early-Stage Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
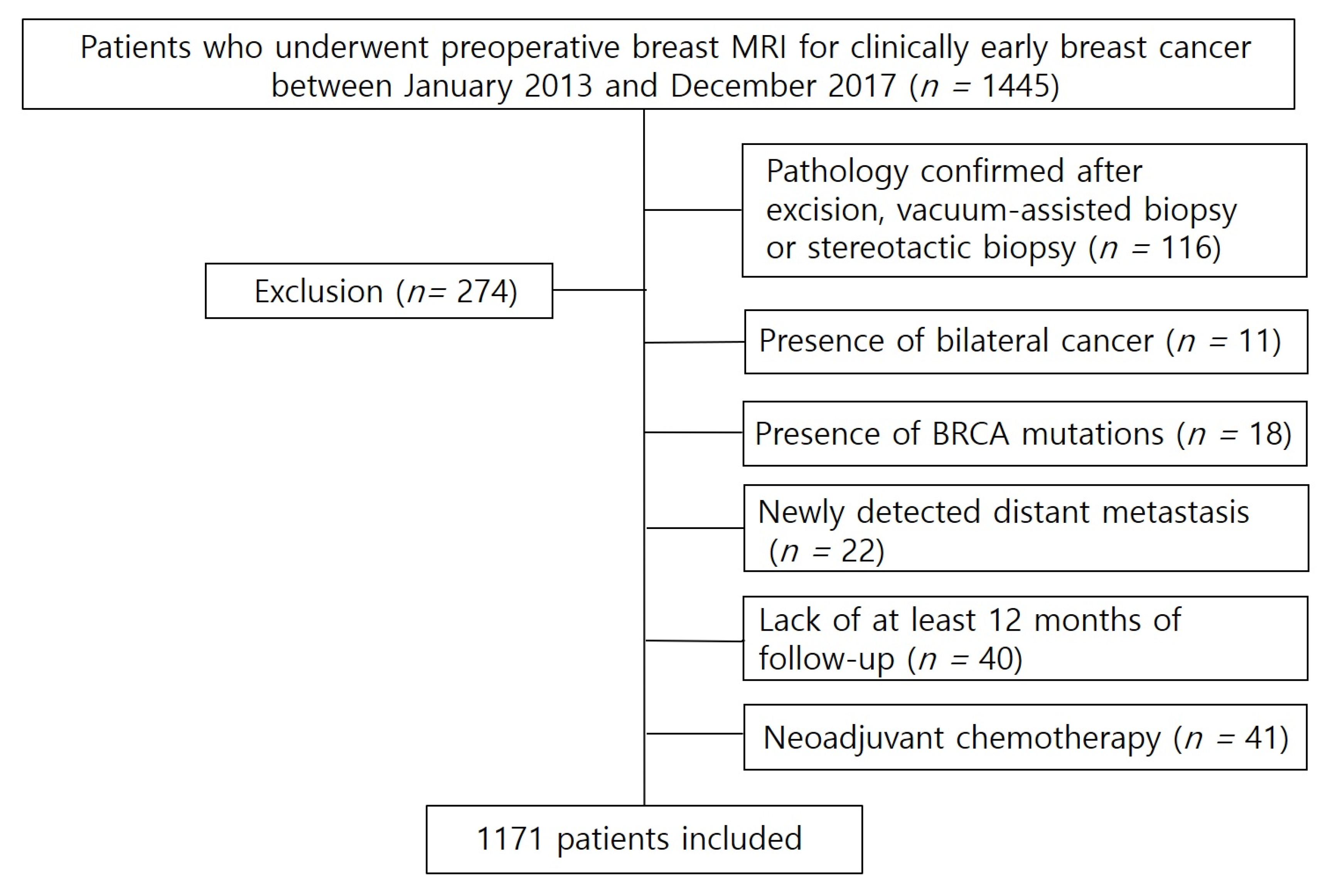
2.1. Study Population
2.2. MRI Technique
2.3. Image Analysis
2.4. Histopathologic Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPE | Background Parenchymal Enhancement |
| CI | Confidence Interval |
| ER | Estrogen receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| IHC | I Immunohistochemistry |
| LVI | Lymphovascular invasion |
| MRI | Magnetic resonance imaging |
| PHBC | Personal history of breast cancer |
| PR | Progesterone receptor |
References
- Haffty, B.G.; Reiss, M.; Beinfield, M.; Fischer, D.; Ward, B.; McKhann, C. Ipsilateral breast tumor recurrence as a predictor of distant disease: Implications for systemic therapy at the time of local relapse. J. Clin. Oncol. 1996, 14, 52–57. [Google Scholar] [CrossRef]
- Fortin, A.; Larochelle, M.; Laverdière, J.; Lavertu, S.; Tremblay, D. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J. Clin. Oncol. 1999, 17, 101–109. [Google Scholar] [CrossRef]
- Anderson, S.J.; Wapnir, I.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Jeong, J.H.; Geyer, C.E., Jr.; Wickerham, D.L.; Costantino, J.P.; Wolmark, N. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five national surgical adjuvant breast and bowel project protocols of node-negative breast cancer. J. Clin. Oncol. 2009, 27, 2466–2473. [Google Scholar] [CrossRef]
- Houssami, N.; Abraham, L.A.; Miglioretti, D.L.; Sickles, E.A.; Kerlikowske, K.; Buist, D.S.; Geller, B.M.; Muss, H.B.; Irwig, L. Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA 2011, 305, 790–799. [Google Scholar] [CrossRef]
- Lawson, M.B.; Herschorn, S.D.; Sprague, B.L.; Buist, D.S.M.; Lee, S.J.; Newell, M.S.; Lourenco, A.P.; Lee, J.M. Imaging surveillance options for individuals with a personal history of breast cancer: Ajr expert panel narrative review. AJR Am. J. Roentgenol. 2022, 219, 854–868. [Google Scholar] [CrossRef]
- Lee, J.M.; Ichikawa, L.E.; Wernli, K.J.; Bowles, E.; Specht, J.M.; Kerlikowske, K.; Miglioretti, D.L.; Lowry, K.P.; Tosteson, A.N.A.; Stout, N.K.; et al. Digital mammography and breast tomosynthesis performance in women with a personal history of breast cancer, 2007–2016. Radiology 2021, 300, 290–300. [Google Scholar] [CrossRef]
- Lowry, K.P.; Coley, R.Y.; Miglioretti, D.L.; Kerlikowske, K.; Henderson, L.M.; Onega, T.; Sprague, B.L.; Lee, J.M.; Herschorn, S.; Tosteson, A.N.A.; et al. Screening performance of digital breast tomosynthesis vs. digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw. Open 2020, 3, e2011792. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C. The current status of breast mr imaging. Part i. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007, 244, 356–378. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.H.; Borel Rinkes, I.H.; Zuithoff, N.P.; Mali, W.P.; Moons, K.G.; Peeters, P.H. Meta-analysis of mr imaging in the diagnosis of breast lesions. Radiology 2008, 246, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Morakkabati-Spitz, N.; Wardelmann, E.; Fimmers, R.; Kuhn, W.; Schild, H.H. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.; Liberman, L.; Dershaw, D.D.; Morris, E. Breast MRI screening of women with a personal history of breast cancer. AJR Am. J. Roentgenol. 2010, 195, 510–516. [Google Scholar] [CrossRef]
- Gweon, H.M.; Cho, N.; Han, W.; Yi, A.; Moon, H.G.; Noh, D.Y.; Moon, W.K. Breast mr imaging screening in women with a history of breast conservation therapy. Radiology 2014, 272, 366–373. [Google Scholar] [CrossRef]
- Cho, N.; Han, W.; Han, B.K.; Bae, M.S.; Ko, E.S.; Nam, S.J.; Chae, E.Y.; Lee, J.W.; Kim, S.H.; Kang, B.J.; et al. Breast cancer screening with mammography plus ultrasonography or magnetic resonance imaging in women 50 years or younger at diagnosis and treated with breast conservation therapy. JAMA Oncol. 2017, 3, 1495–1502. [Google Scholar] [CrossRef]
- Rahbar, H.; Lee, J.M.; Lee, C.I. Optimal screening in breast cancer survivors with dense breasts on mammography. J. Clin. Oncol. 2020, 38, 3833–3840. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.R.; Cho, N.; Kim, S.Y.; Han, W.; Moon, W.K. Interval cancers after negative supplemental screening breast MRI results in women with a personal history of breast cancer. Radiology 2021, 300, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Park, V.Y.; Kim, M.J.; Kim, G.R.; Yoon, J.H. Outcomes following negative screening MRI results in korean women with a personal history of breast cancer: Implications for the next MRI interval. Radiology 2021, 300, 303–311. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast cancer screening in women at higher-than-average risk: Recommendations from the acr. J. Am. Coll. Radiol. 2018, 15, 408–414. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.D.; Bieling, H.B. Abbreviated breast magnetic resonance imaging (MRI): First postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef]
- Choi, B.H.; Choi, N.; Kim, M.Y.; Yang, J.H.; Yoo, Y.B.; Jung, H.K. Usefulness of abbreviated breast MRI screening for women with a history of breast cancer surgery. Breast Cancer Res. Treat. 2018, 167, 495–502. [Google Scholar] [CrossRef]
- An, Y.Y.; Kim, S.H.; Kang, B.J.; Suh, Y.J.; Jeon, Y.W. Feasibility of abbreviated magnetic resonance imaging (ab-MRI) screening in women with a personal history (ph) of breast cancer. PLoS ONE 2020, 15, e0230347. [Google Scholar] [CrossRef]
- Kwon, M.R.; Choi, J.S.; Won, H.; Ko, E.Y.; Ko, E.S.; Park, K.W.; Han, B.K. Breast cancer screening with abbreviated breast MRI: 3-year outcome analysis. Radiology 2021, 299, 73–83. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, N.; Hong, H.; Lee, Y.; Yoen, H.; Kim, Y.S.; Park, A.R.; Ha, S.M.; Lee, S.H.; Chang, J.M.; et al. Abbreviated screening MRI for women with a history of breast cancer: Comparison with full-protocol breast MRI. Radiology 2022, 305, 36–45. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Woo, O.H. Abbreviated breast magnetic resonance imaging: Background, evidence from studies, and future considerations. Investig. Magn. Reson. Imaging 2025, 29, 14–22. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef]
- Bae, J.M.; Kim, E.H. Breast density and risk of breast cancer in asian women: A meta-analysis of observational studies. J. Prev. Med. Public Health 2016, 49, 367–375. [Google Scholar] [CrossRef]
- Jo, H.M.; Lee, E.H.; Ko, K.; Kang, B.J.; Cha, J.H.; Yi, A.; Jung, H.K.; Jun, J.K. Prevalence of women with dense breasts in korea: Results from a nationwide cross-sectional study. Cancer Res. Treat. 2019, 51, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Choi, H.; Choi, S.A.; Youk, J.H. Dynamic contrast-enhanced breast magnetic resonance imaging for the prediction of early and late recurrences in breast cancer. Medicine 2016, 95, e5330. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, E.S.; Lim, Y.; Lee, K.S.; Han, B.K.; Ko, E.Y.; Hahn, S.Y.; Nam, S.J. Breast cancer heterogeneity: Mr imaging texture analysis and survival outcomes. Radiology 2017, 282, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.H.; Kang, B.J. Prognostic factors of disease recurrence in breast cancer using quantitative and qualitative magnetic resonance imaging (MRI) parameters. Sci. Rep. 2020, 10, 7598. [Google Scholar] [CrossRef]
- Song, W.J.; Kim, K.I.; Park, S.H.; Kwon, M.S.; Lee, T.H.; Park, H.K.; An, J.S. The risk factors influencing between the early and late recurrence in systemic recurrent breast cancer. J. Breast Cancer 2012, 15, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Kurosumi, M.; Akiyama, F.; Ohno, S.; Saji, S.; Masuda, N.; Shimomura, A.; Sato, N.; Takao, S.; Ohsumi, S.; et al. Validation of a nuclear grading system for resected stage i-iiia, high-risk, node-negative invasive breast carcinoma in the n·sas-bc 01 trial. Breast Cancer 2022, 29, 720–729. [Google Scholar] [CrossRef]
- He, X.-M.; Zou, D.-H. The association of young age with local recurrence in women with early-stage breast cancer after breast-conserving therapy: A meta-analysis. Sci. Rep. 2017, 7, 11058. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.; Freedman, R.A.; Partridge, A.H. The impact of young age at diagnosis (age <40 years) on prognosis varies by breast cancer subtype: A U.S. Seer database analysis. Breast 2022, 61, 77–83. [Google Scholar]
- Shin, D.S.; Lee, J.; Kang, E.; Noh, D.; Cheun, J.H.; Lee, J.H.; Son, Y.; Bae, S.J.; Kim, S.W.; Lee, J.E.; et al. Age and late recurrence in young patients with er-positive, erbb2-negative breast cancer. JAMA Netw. Open 2024, 7, e2442663. [Google Scholar] [CrossRef]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Pedersen, R.N.; Esen, B.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J. Natl. Cancer Inst. 2022, 114, 391–399. [Google Scholar] [CrossRef]
- Yaghjyan, L.; Colditz, G.A.; Collins, L.C.; Schnitt, S.J.; Rosner, B.; Vachon, C.; Tamimi, R.M. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics. J. Natl. Cancer Inst. 2011, 103, 1179–1189. [Google Scholar] [CrossRef]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
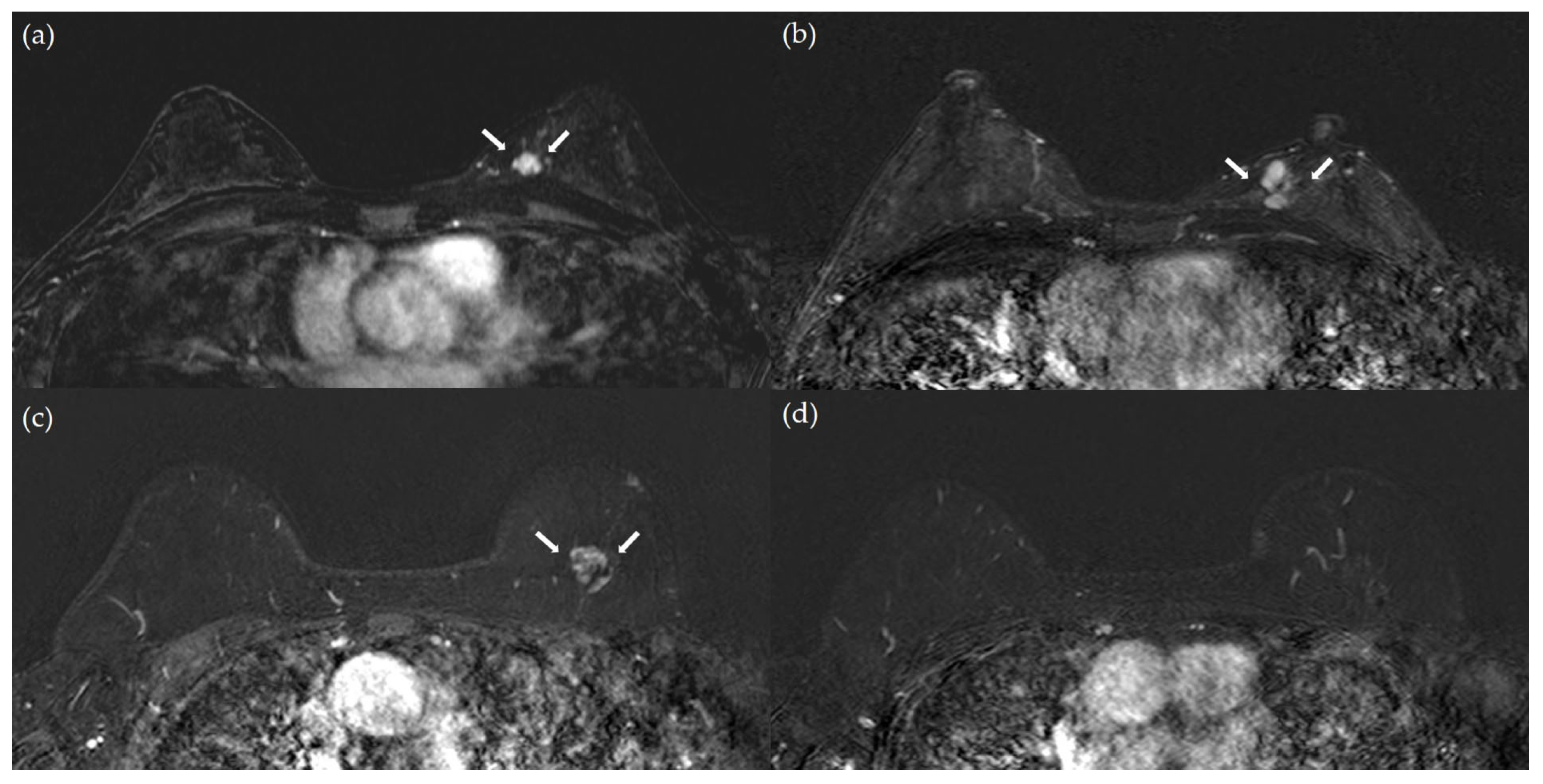
| Total (n = 1171) | Patients Without Subsequent Breast Cancer Events (n = 1114) | Patients With Subsequent Breast Cancer Events (n = 57) | p-Value | |
|---|---|---|---|---|
| Age | 0.025 | |||
| <40 | 75 (6.40) | 67 (6.01) | 8 (14.04) | |
| ≥40 | 1096 (93.60) | 1047 (93.99) | 49 (85.96) | |
| Clinical T stage | 0.063 | |||
| T1 | 673 (57.47) | 647 (58.08) | 26 (45.61) | |
| T2 | 498 (42.53) | 467 (41.92) | 31 (54.39) | |
| Pathology | 0.775 | |||
| Invasive ductal carcinoma | 1013 (86.51) | 962 (86.35) | 51 (89.47) | |
| Invasive lobular carcinoma | 61 (5.21) | 59 (5.30) | 2 (3.51) | |
| Others | 97 (8.28) | 93 (8.35) | 4 (7.02) | |
| Histologic grade | 0.094 | |||
| Low–intermediate | 780 (66.61) | 744 (66.79) | 36 (63.16) | |
| High | 235 (20.07) | 218 (19.57) | 17 (29.82) | |
| Unknown | 156 (13.32) | 152 (16.64) | 4 (7.02) | |
| Nuclear grade | 0.001 | |||
| Low–intermediate | 753 (64.30) | 729 (65.44) | 24 (42.11) | |
| High | 416 (35.53) | 383 (34.38) | 33 (57.89) | |
| Unknown | 2 (0.17) | 2 (0.18) | 0 (0.00) | |
| Lymphovascular invasion | 0.03 | |||
| No | 881 (75.23) | 846 (75.94) | 35 (61.40) | |
| Yes | 248 (21.18) | 228 (20.47) | 20 (35.09) | |
| Unknown | 42 (3.59) | 40 (3.59) | 2 (3.51) | |
| Lymph node metastasis | 0.759 | |||
| No | 884 (75.49) | 840 (75.40) | 44 (77.19) | |
| Yes | 287 (24.51) | 274 (24.60) | 13 (22.81) | |
| ER | 0.558 | |||
| Negative | 251 (21.45) | 237 (21.27) | 14 (24.56) | |
| Positive | 919 (78.55) | 877 (78.73) | 43 (75.44) | |
| PR | 0.122 | |||
| Negative | 403 (34.42) | 378 (33.93) | 25 (43.86) | |
| Positive | 768 (65.58) | 736 (66.07) | 32 (56.14) | |
| p53 | 0.009 | |||
| Negative | 589 (50.30) | 570 (51.17) | 19 (33.33) | |
| Positive | 582 (49.70) | 544 (48.83) | 38 (66.67) | |
| Ki-67 (%) | 0.043 | |||
| <14 | 665 (56.79) | 640 (57.50) | 25 (43.86) | |
| ≥14 | 506 (43.21) | 474 (42.50) | 32 (56.14) | |
| HER2 | 0.154 | |||
| Negative | 880 (75.15) | 842 (75.58) | 38 (66.67) | |
| Positive | 253 (21.60) | 235 (21.10) | 18 (31.58) | |
| Unknown | 38 (3.25) | 37 (3.32) | 1 (1.75) | |
| Subtype | 0.461 | |||
| Hormone-positive | 921 (78.65) | 878 (78.82) | 43 (75.44) | |
| HER2-positive | 118 (10.08) | 109 (9.78) | 9 (15.79) | |
| Triple negative | 125 (10.67) | 120 (10.77) | 5 (8.77) | |
| Unknown | 7 (0.60) | 7 (0.63) | 0 (0.00) |
| Univariable | ||
|---|---|---|
| Odds Ratio (95% CI) | p-Value | |
| Age | ||
| <40 | Ref | |
| ≥40 | 0.392 (0.178–0.861) | 0.020 |
| Clinical T stage | ||
| T1 | Ref | |
| T2 | 1.652 (0.968–2.819) | 0.066 |
| Pathology | ||
| Invasive ductal carcinoma | Ref | |
| Invasive lobular carcinoma | 0.582 (0.138–2.460) | 0.462 |
| Others | 0.824 (0.291–2.335) | 0.716 |
| Histologic grade | ||
| Low–intermediate | Ref | |
| High | 1.612 (0.888–2.925) | 0.117 |
| Nuclear grade | ||
| Low–intermediate | Ref | |
| High | 2.617 (1.525–4.492) | 0.001 |
| ER | ||
| Negative | Ref | |
| Positive | 0.830 (0.447–1.543) | 0.556 |
| PR | ||
| Negative | Ref | |
| Positive | 0.657 (0.384–1.125) | 0.126 |
| HER2 | ||
| Negative | Ref | |
| Positive | 1.697 (0.951–3.029) | 0.073 |
| Subtype | ||
| Hormone-positive | Ref | |
| HER2-positive | 1.730 (0.814–3.675) | 0.154 |
| Triple-negative | 0.873 (0.337–2.261) | 0.779 |
| Breast density assessed on mammography | ||
| BI-RADS A or B | Ref | |
| BI-RADS C or D | 4.747 (1.147–19.649) | 0.032 |
| Background parenchymal enhancement | ||
| Minimal-mild | Ref | |
| Moderate-marked | 1.264 (0.712–2.244) | 0.424 |
| Shape | ||
| Oval | Ref | |
| Round | 0.622 (0.217–1.786) | 0.378 |
| Irregular | 0.893 (0.464–1.721) | 0.736 |
| Margin | ||
| Circumscribed | Ref | |
| Irregular | 1.604 (0.563–4.571) | 0.377 |
| Spiculated | 0.600 (0.166–2.175) | 0.437 |
| Internal enhancement of mass | ||
| Homogeneous | Ref | |
| Heterogeneous | 0.451 (0.226–0.901) | 0.024 |
| Rim enhancement | 0.493 (0.195–1.247) | 0.135 |
| Dark internal septation | 0.559 (0.068–4.575) | 0.587 |
| T2 hyperintensity | ||
| No | Ref | |
| Yes | 0.693 (0.245–1.962) | 0.49 |
| Peritumoral edema | ||
| No | Ref | |
| Yes | 1.095 (0.505–2.373) | 0.819 |
| Distribution of nonmass enhancement | ||
| Focal | Ref | |
| Linear | 0.910 (0.196–4.216) | 0.904 |
| Segmental | 0.308 (0.068–1.404) | 0.128 |
| Regional | 2.853 (0.770–10.568) | 0.117 |
| Multiple regional | 1.919 (0.061–60.053) | 0.711 |
| Diffuse | 1.017 (0.041–25.302) | 0.992 |
| Internal enhancement of nonmass enhancement | ||
| Homogeneous | Ref | |
| Heterogeneous | 1.352 (0.164–11.137) | 0.779 |
| Clumped | 2.805 (0.326–24.172) | 0.348 |
| Clustered ring | 1.220 (0.107–13.945) | 0.873 |
| Multivariable | ||
|---|---|---|
| Odds Ratio (95% CI) | p-Value | |
| Age | ||
| <40 | Ref | |
| ≥40 | 0.448 (0.193–1.039) | 0.061 |
| Clinical T stage | ||
| T1 | Ref | |
| T2 | 1.744 (0.969–3.139) | 0.064 |
| Histologic grade | ||
| Low–intermediate | Ref | |
| High | 0.684 (0.323–1.448) | 0.321 |
| Nuclear grade | ||
| Low–intermediate | Ref | |
| High | 2.821 (1.427–5.577) | 0.003 |
| ER | ||
| Negative | Ref | |
| Positive | 1.556 (0.654–3.702) | 0.317 |
| PR | ||
| Negative | Ref | |
| Positive | 0.786 (0.364–1.699) | 0.541 |
| HER2 | ||
| Negative | Ref | |
| Positive | 0.73 (0.089–5.997) | 0.770 |
| Breast density assessed on mammography | ||
| BI-RADS A or B | Ref | |
| BI-RADS C or D | 4.68 (1.113–19.684) | 0.035 |
| Internal enhancement of mass | ||
| Homogeneous | Ref | |
| Heterogeneous | 0.429 (0.206–0.891) | 0.023 |
| Rim enhancement | 0.488 (0.184–1.295) | 0.150 |
| Dark internal septations | 0.504 (0.058–4.34) | 0.533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eun, N.L.; Youk, J.H.; Kim, J.-A.; Cha, Y.J.; Bae, S.J.; Ahn, S.G.; Jeong, J.; Yang, H.; Lee, H.S.; Son, E.J. Preoperative Predictors of Subsequent Breast Cancer Events Detected on Abbreviated MRI in Patients with Early-Stage Breast Cancer. Diagnostics 2025, 15, 2953. https://doi.org/10.3390/diagnostics15232953
Eun NL, Youk JH, Kim J-A, Cha YJ, Bae SJ, Ahn SG, Jeong J, Yang H, Lee HS, Son EJ. Preoperative Predictors of Subsequent Breast Cancer Events Detected on Abbreviated MRI in Patients with Early-Stage Breast Cancer. Diagnostics. 2025; 15(23):2953. https://doi.org/10.3390/diagnostics15232953
Chicago/Turabian StyleEun, Na Lae, Ji Hyun Youk, Jeong-Ah Kim, Yoon Jin Cha, Soong June Bae, Sung Gwe Ahn, Joon Jeong, Hyejin Yang, Hye Sun Lee, and Eun Ju Son. 2025. "Preoperative Predictors of Subsequent Breast Cancer Events Detected on Abbreviated MRI in Patients with Early-Stage Breast Cancer" Diagnostics 15, no. 23: 2953. https://doi.org/10.3390/diagnostics15232953
APA StyleEun, N. L., Youk, J. H., Kim, J.-A., Cha, Y. J., Bae, S. J., Ahn, S. G., Jeong, J., Yang, H., Lee, H. S., & Son, E. J. (2025). Preoperative Predictors of Subsequent Breast Cancer Events Detected on Abbreviated MRI in Patients with Early-Stage Breast Cancer. Diagnostics, 15(23), 2953. https://doi.org/10.3390/diagnostics15232953








