Associations Between BioFire FilmArray Gastrointestinal Panel Results and Clinical Outcomes in Infectious Gastroenteritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. BioFire FilmArray
2.3. Stool Culture
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Distribution of Detected Pathogens in FA-GIP
3.3. Coinfections
3.4. Overview of FA-GIP Detection Results
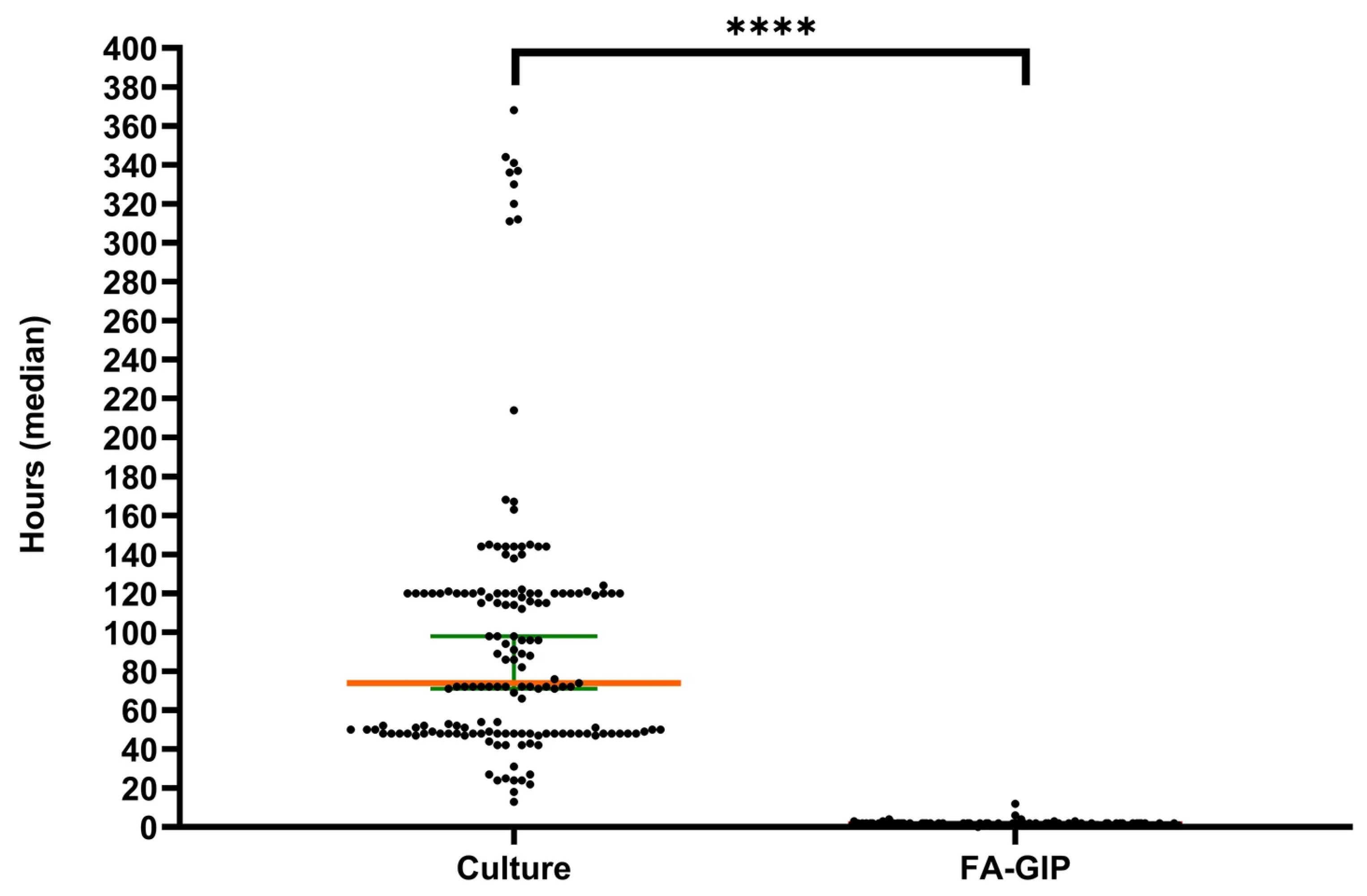
3.5. Turnaround Time
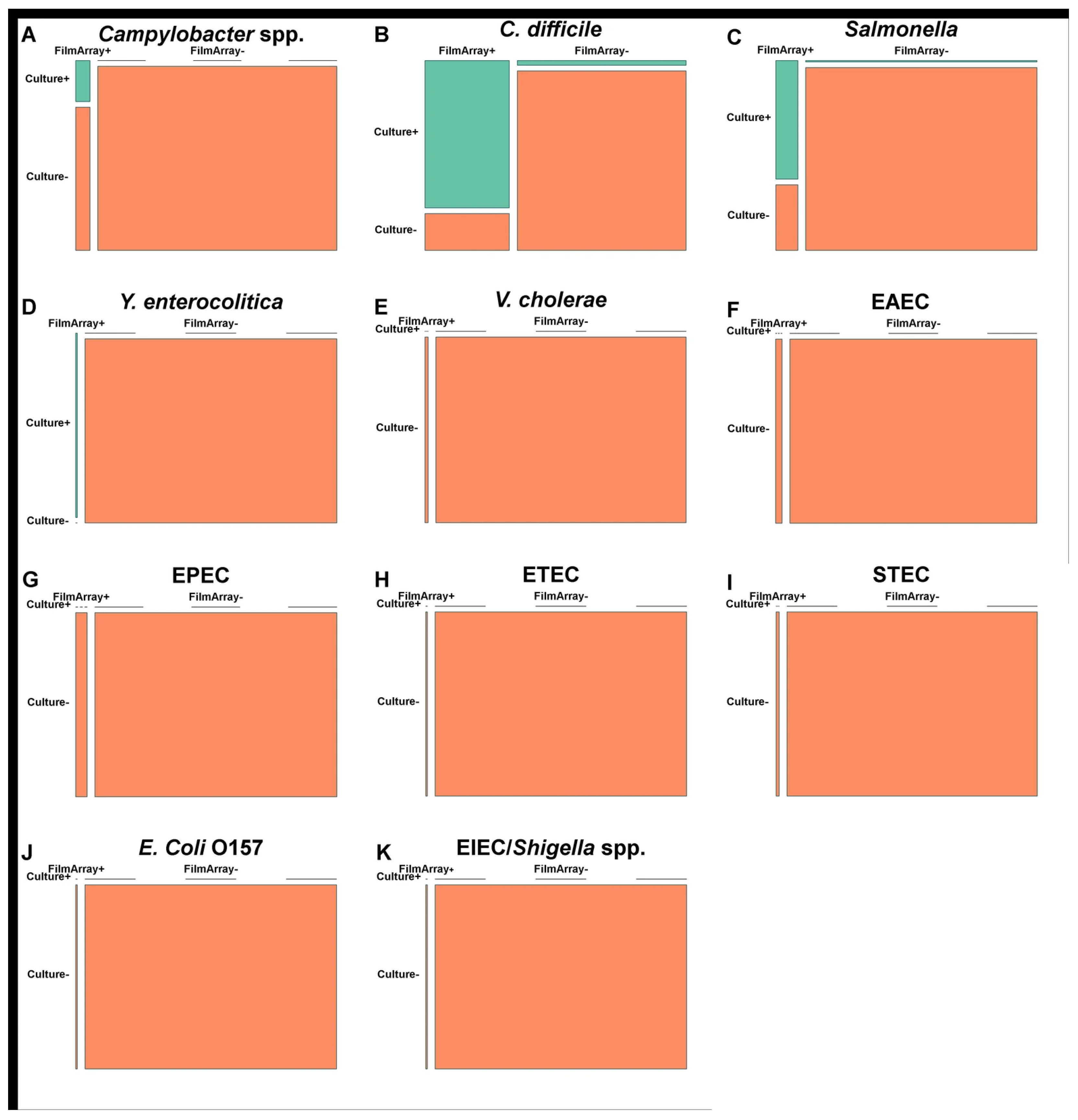
3.6. Comparison of FA-GIP and Stool Culture Performance
3.7. Patient Characteristics and Clinical Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musher, D.M.; Musher, B.L. Contagious acute gastrointestinal infections. N. Engl. J. Med. 2004, 351, 2417–2427. [Google Scholar] [CrossRef]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Walker, C.L.F.; Rudan, I.; Liu, L.; Nair, H.; Theodoratou, E.; Bhutta, Z.A.; O’Brien, K.L.; Campbell, H.; Black, R.E. Global burden of childhood pneumonia and diarrhoea. Lancet 2013, 381, 1405–1416. [Google Scholar] [CrossRef]
- Ogilvie, I.; Khoury, H.; Goetghebeur, M.M.; El Khoury, A.C.; Giaquinto, C. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: A scoping review. BMC Infect. Dis. 2012, 12, 62. [Google Scholar] [CrossRef]
- Hodges, K.; Gill, R. Infectious diarrhea: Cellular and molecular mechanisms. Gut Microbes 2010, 1, 4–21. [Google Scholar] [CrossRef]
- da Cruz Gouveia, M.A.C.; Lins, M.T.C.; da Silva, G.A.P. Acute diarrhea with blood: Diagnosis and drug treatment. J. Pediatr. 2020, 96, 20–28. [Google Scholar] [CrossRef]
- Buss, S.N.; Leber, A.; Chapin, K.; Fey, P.D.; Bankowski, M.J.; Jones, M.K.; Rogatcheva, M.; Kanack, K.J.; Bourzac, K.M. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J. Clin. Microbiol. 2015, 53, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, E.; Giannattasio, A.; Guarino, A. Antibiotic treatment of acute gastroenteritis in children. F1000Research 2018, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Paterson, D.L. Multidrug-resistant bacteria in the community: An update. Infect. Dis. Clin. N. Am. 2020, 34, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.H.; Pancholi, P. Appropriate use and future directions of molecular diagnostic testing. Curr. Infect. Dis. Rep. 2020, 22, 5. [Google Scholar] [CrossRef]
- Dumkow, L.E.; Worden, L.J.; Rao, S.N. Syndromic diagnostic testing: A new way to approach patient care in the treatment of infectious diseases. J. Antimicrob. Chemother. 2021, 76, iii4–iii11. [Google Scholar] [CrossRef]
- Murphy, C.N.; Fowler, R.C.; Iwen, P.C.; Fey, P.D. Evaluation of the BioFire FilmArray® GastrointestinalPanel in a midwestern academic hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.J.; Kang, H.M.; Kim, J.O.; Cho, H.; Heo, W.; Yoo, I.Y.; Park, Y.-J. Evaluation of the BioFire gastrointestinal panel to detect diarrheal pathogens in pediatric patients. Diagnostics 2021, 12, 34. [Google Scholar] [CrossRef]
- Torres-Miranda, D.; Akselrod, H.; Karsner, R.; Secco, A.; Silva-Cantillo, D.; Siegel, M.O.; Roberts, A.D.; Simon, G.L. Use of BioFire FilmArray gastrointestinal PCR panel associated with reductions in antibiotic use, time to optimal antibiotics, and length of stay. BMC Gastroenterol. 2020, 20, 246. [Google Scholar] [CrossRef]
- Burke, K.E.; Lamont, J.T. Clostridium difficile infection: A worldwide disease. Gut Liver 2014, 8, 1–6. [Google Scholar] [CrossRef]
- Kim, Y.S.; Han, D.S.; Kim, Y.H.; Kim, W.H.; Kim, J.S.; Kim, H.S.; Kim, H.S.; Park, Y.S.; Song, H.J.; Shin, S.J.; et al. Incidence and clinical features of Clostridium difficile infection in Korea: A nationwide study. Epidemiol. Infect. 2013, 141, 189–194. [Google Scholar] [CrossRef]
- Ray, L.C.; Collins, J.P.; Griffin, P.M.; Shah, H.J.; Boyle, M.M.; Cieslak, P.R.; Dunn, J.; Lathrop, S.; McGuire, S.; Rissman, T.; et al. Decreased incidence of infections caused by pathogens transmitted commonly through food during the COVID-19 pandemic —foodborne diseases active surveillance network, 10 U.S. sites, 2017–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, L.; Couturier, J.; Eckert, C.; Delannoy, J.; Barbut, F.; Butel, M.-J.; Aires, J. Carriage and colonization of C. difficile in preterm neonates: A longitudinal prospective study. PLoS ONE 2019, 14, e0212568. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O. Persistent infection and long-term carriage of typhoidal and nontyphoidal salmonellae. Clin. Microbiol. Rev. 2018, 32, e00088-18. [Google Scholar] [CrossRef]
- Miyoshi, T.; Uchino, K.; Yoshida, H.; Motomura, K.; Takeda, N.; Matsuura, Y.; Tanaka, T. Long-term viral shedding and viral genome mutation in norovirus infection. J. Med. Virol. 2015, 87, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, S.K.; Yoon, J.W. Pathophysiology of enteropathogenic Escherichia coli during a host infection. J. Vet. Sci. 2022, 23, e28. [Google Scholar] [CrossRef]
- Jump, R.L. Clostridium difficile infection in older adults. Aging Health 2013, 9, 403–414. [Google Scholar] [CrossRef]
- Cybulski, R.J., Jr.; Bateman, A.C.; Bourassa, L.; Bryan, A.; Beail, B.; Matsumoto, J.; Cookson, B.T.; Fang, F.C. Clinical impact of a multiplex gastrointestinal polymerase chain reaction panel in patients with acute gastroenteritis. Clin. Infect. Dis. 2018, 67, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.D.; Cremers, A.J.H.; van Bergen-Verkuyten, M.C.G.T.; Paardekoper-Strijbosch, S.J.M.; Frijns, K.C.J.; Wertheim, H.F.L.; Rahamat-Langendoen, J.; Melchers, W.J.G. Impact of the BioFire FilmArray gastrointestinal panel on patient care and infection control. PLoS ONE 2020, 15, e0228596. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.H.; Kang, H.M.; Suh, W.; Cho, H.; Yoo, I.Y.; Jo, S.J.; Park, Y.J.; Jeong, D.C. Quality improvements in management of children with acute diarrhea using a multiplex-PCR-based gastrointestinal pathogen panel. Diagnostics 2021, 11, 1175. [Google Scholar] [CrossRef]
- Keske, Ş.; Zabun, B.; Aksoy, K.; Can, F.; Palaoğlu, E.; Ergönül, Ö. Rapid molecular detection of gastrointestinal pathogens and its role in antimicrobial stewardship. J. Clin. Microbiol. 2018, 56, e00148-18. [Google Scholar] [CrossRef] [PubMed]

| Patient Subset | Patient Sex and Age Data (Years) a: | Total No. (%) of Specimens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 | 1–5 | 6–12 | 13–21 | 22–64 | ≥65 | ||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | ||
| Emergency Room | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 3 | 3 | 4 | 15 (9.3) |
| Outpatient | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (1.9) |
| Hospitalized | 18 | 9 | 13 | 16 | 12 | 7 | 2 | 8 | 9 | 5 | 17 | 27 | 143 (88.8) |
| Total | 18 | 9 | 13 | 18 | 15 | 7 | 2 | 9 | 11 | 8 | 20 | 31 | 161 |
| No. of Potential Pathogens in FilmArray GI Panel Result | No. of Specimens (n = 161) | % of Total (% Positives) |
|---|---|---|
| Detected (at least one) | 86 | 53.4 (100) |
| One | 62 | 38.5 (72.1) |
| Two | 18 | 11.2 (20.9) |
| Three | 5 | 3.1 (5.8) |
| Four | 1 | 0.6 (1.2) |
| Potential Pathogen | Total No. | No. (% of Total) Associated with Coinfection | No. Detections in Age Group (Years): | |||||
|---|---|---|---|---|---|---|---|---|
| <1 (n = 9) | 1–5 (n = 32) | 6–12 (n = 23) | 13–21 (n = 9) | 22–64 (n = 7) | ≥65 (n = 37) | |||
| Campylobacter spp. | 14 | 2 (14.3) | 1 | 1 | 2 | 5 | 1 | 4 |
| C. difficile | 36 | 16 (44.4) | 2 | 11 | 3 | 2 | 5 | 13 |
| P. shigelloides | 0 | 0(0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Salmonella | 14 | 6 (42.9) | 0 | 5 | 4 | 1 | 1 | 3 |
| Y. enterocolitica | 1 | 0(0) | 0 | 1 | 0 | 0 | 0 | 0 |
| Vibrio spp. | 2 | 2 (100) | 0 | 0 | 0 | 0 | 0 | 2 |
| V. cholerae | 0 | 0(0) | 0 | 0 | 0 | 0 | 0 | 0 |
| EAEC | 5 | 3 (60) | 1 | 0 | 1 | 0 | 0 | 3 |
| EPEC | 11 | 10 (90.9) | 0 | 3 | 3 | 1 | 0 | 4 |
| ETEC | 1 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 1 |
| STEC | 2 | 2 (100) | 0 | 0 | 0 | 0 | 0 | 2 |
| E. coli O157 | 1 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 1 |
| EIEC/Shigella spp. | 1 | 0(0) | 0 | 0 | 0 | 0 | 0 | 1 |
| Adenovirus F 40/41 | 1 | 1 (100) | 0 | 1 | 0 | 0 | 0 | 0 |
| Astrovirus | 2 | 2 (100) | 0 | 0 | 2 | 0 | 0 | 0 |
| Norovirus GI/GII | 15 | 6 (40) | 3 | 8 | 3 | 0 | 0 | 1 |
| Rotavirus A | 4 | 1 (25) | 2 | 0 | 0 | 0 | 0 | 2 |
| Sapovirus | 7 | 2 (28.6) | 0 | 2 | 5 | 0 | 0 | 0 |
| Cryptosporidium | 0 | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| C. cayetanensis | 0 | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| E. histolytica | 0 | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| G. lamblia | 0 | 0 (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 117 | 55 | 9 | 32 | 23 | 9 | 7 | 37 |
| Characteristics | FA-GIP− (n = 75) n (%) | FA-GIP+ (n = 86) n (%) | Total (n = 161) n (%) | p-Value * |
|---|---|---|---|---|
| Gender, n (%) | 0.490 | |||
| Female | 36 (48.0) | 46 (53.5) | 82 (49.1) | |
| Male | 39 (52.0) | 40 (46.5) | 79 (50.9) | |
| Age (years) | 0.764 | |||
| Mean (SD) | 34.3 (35.7) | 32.6 (34.8) | 33.4 (35.1) | |
| Range | (0–91) | (0–98) | (0–98) | |
| Length of stay (Days) | <0.001 | |||
| Mean (SD) | 27.4 (32.7) | 7.6 (9.0) | 16.8 (25.2) | |
| Range | (1–174) | (1–53) | (1–174) | |
| Time to discharge from GIP results (Days) | <0.001 | |||
| Mean (SD) | 15.0 (19.1) | 5.0 (6.5) | 9.7 (14.7) | |
| Range | (0–95) | (0–40) | (0–95) | |
| Number of days on antibiotics (Days) | 0.012 | |||
| Mean (SD) | 8.0 (11.0) | 3.4 (7.9) | 5.9 (9.9) | |
| Range | (0–30) | (0–30) | (0–30) | |
| Antibiotic treatment prior to test, n (%) | 0.103 | |||
| Yes | 3 (5.1) | 0 (0) | 3 (2.7) | |
| No | 56(94.9) | 51(100) | 107(97.3) | |
| Antibiotic treatment after test, n (%) | 0.185 | |||
| Yes | 57 (96.6) | 51 (100) | 108 (98.2) | |
| No | 2 (3.4) | 0 (0) | 2 (1.8) | |
| Antibiotic change and discontinuation, n (%) | 0.011 | |||
| Yes | 24 (32.0) | 13 (15.1) | 37 (23.0) | |
| No | 51 (68.0) | 73 (84.9) | 124 (77.0) | |
| Antibiotics not prescribed or discontinued, n (%) | 0.008 | |||
| Yes | 16 (21.3) | 35 (40.7) | 51 (31.7) | |
| No | 59 (78.7) | 51 (59.3) | 110 (68.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.J.; Lee, J.Y.; Kim, S.W. Associations Between BioFire FilmArray Gastrointestinal Panel Results and Clinical Outcomes in Infectious Gastroenteritis. Diagnostics 2025, 15, 2947. https://doi.org/10.3390/diagnostics15232947
Lee MJ, Lee JY, Kim SW. Associations Between BioFire FilmArray Gastrointestinal Panel Results and Clinical Outcomes in Infectious Gastroenteritis. Diagnostics. 2025; 15(23):2947. https://doi.org/10.3390/diagnostics15232947
Chicago/Turabian StyleLee, Myeong Joo, Ju Yeong Lee, and Suhng Wook Kim. 2025. "Associations Between BioFire FilmArray Gastrointestinal Panel Results and Clinical Outcomes in Infectious Gastroenteritis" Diagnostics 15, no. 23: 2947. https://doi.org/10.3390/diagnostics15232947
APA StyleLee, M. J., Lee, J. Y., & Kim, S. W. (2025). Associations Between BioFire FilmArray Gastrointestinal Panel Results and Clinical Outcomes in Infectious Gastroenteritis. Diagnostics, 15(23), 2947. https://doi.org/10.3390/diagnostics15232947






