Correction: Chen et al. The Efficacy and Safety of Tandem Transplant Versus Single Stem Cell Transplant for Multiple Myeloma Patients: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 1030
- Abstract and Methods Correction
- (1)
- A correction has been made to the Abstract, Methods:
- (2)
- A correction has been made to the Abstract, Result:
- (3)
- A correction has been made to the Materials and Methods, 2.2. Inclusion and exclusion criteria, Paragraph 2:
- (4)
- A correction has been made to the Materials and Methods, 2.5. Statistical analysis, Paragraph 1 and Paragraph 2:
- Missing Citation
- Introduction Correction
- Results Correction
- (1)
- A correction has been made to Result, 3.1. Characteristics of studies, Paragraph 1–4:
- (2)
- A correction has been made to Result, 3.3.1. Progression-Free Survival:
- (3)
- A correction has been made to Result, 3.3.2. Overall Survival:
- (4)
- A correction has been made to Result, 3.3.3. Overall Response Rate:
- (5)
- A correction has been made to Result, 3.3.4. Complete Response Rate:
- (6)
- A correction has been made to Result, 3.3.5. Treatment-Related Mortality
- Table Correction
- Figure Correction
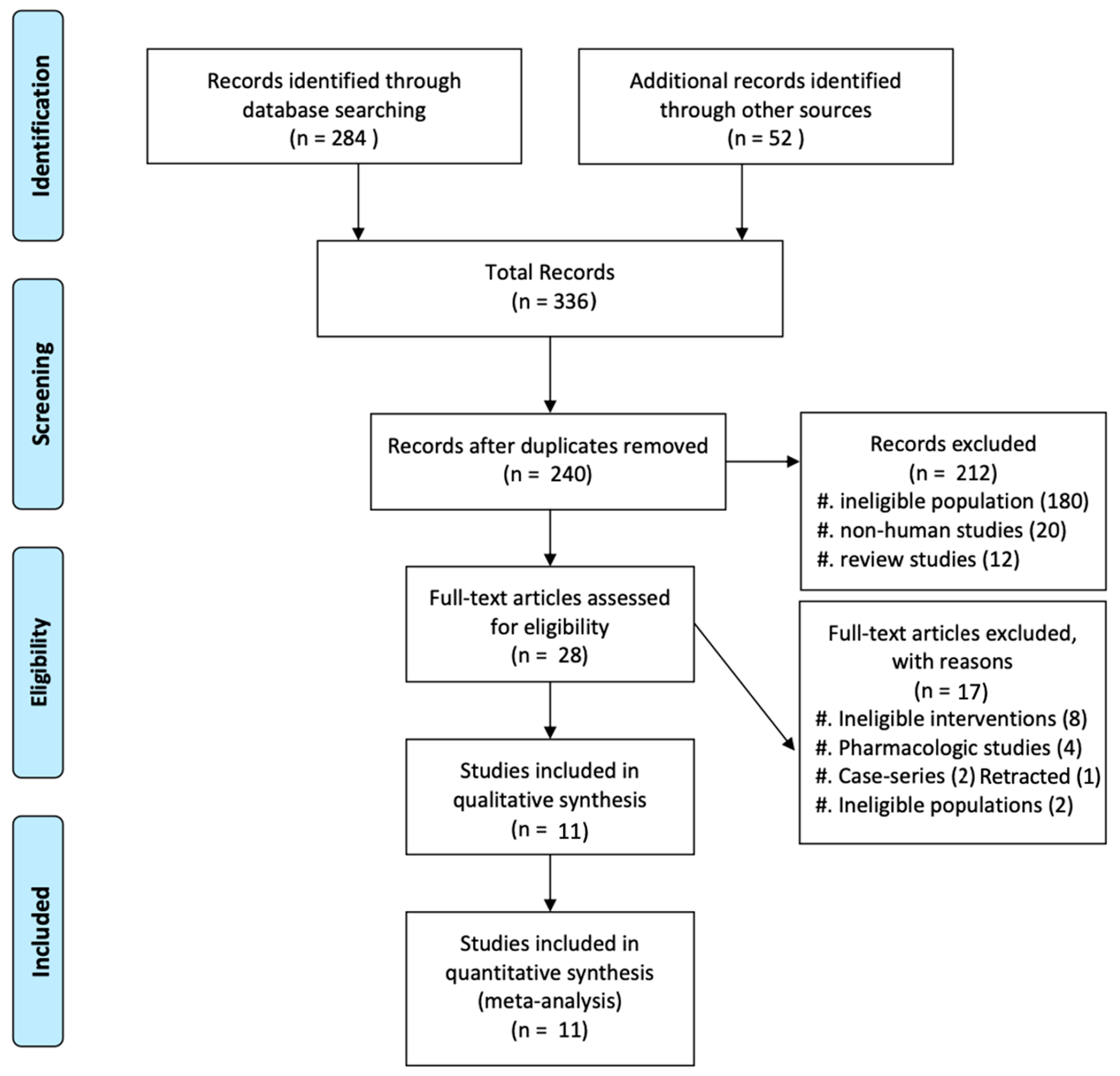
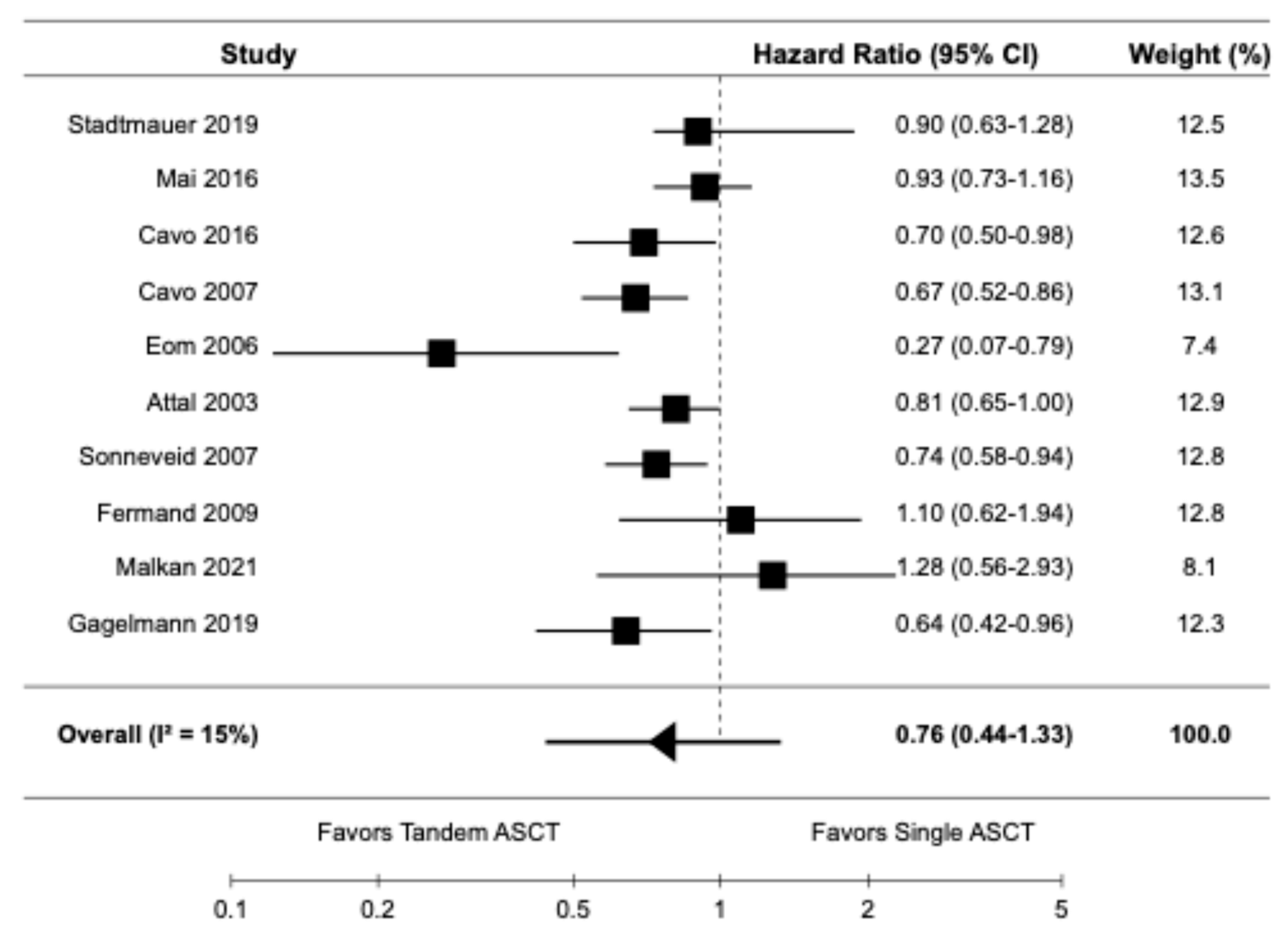
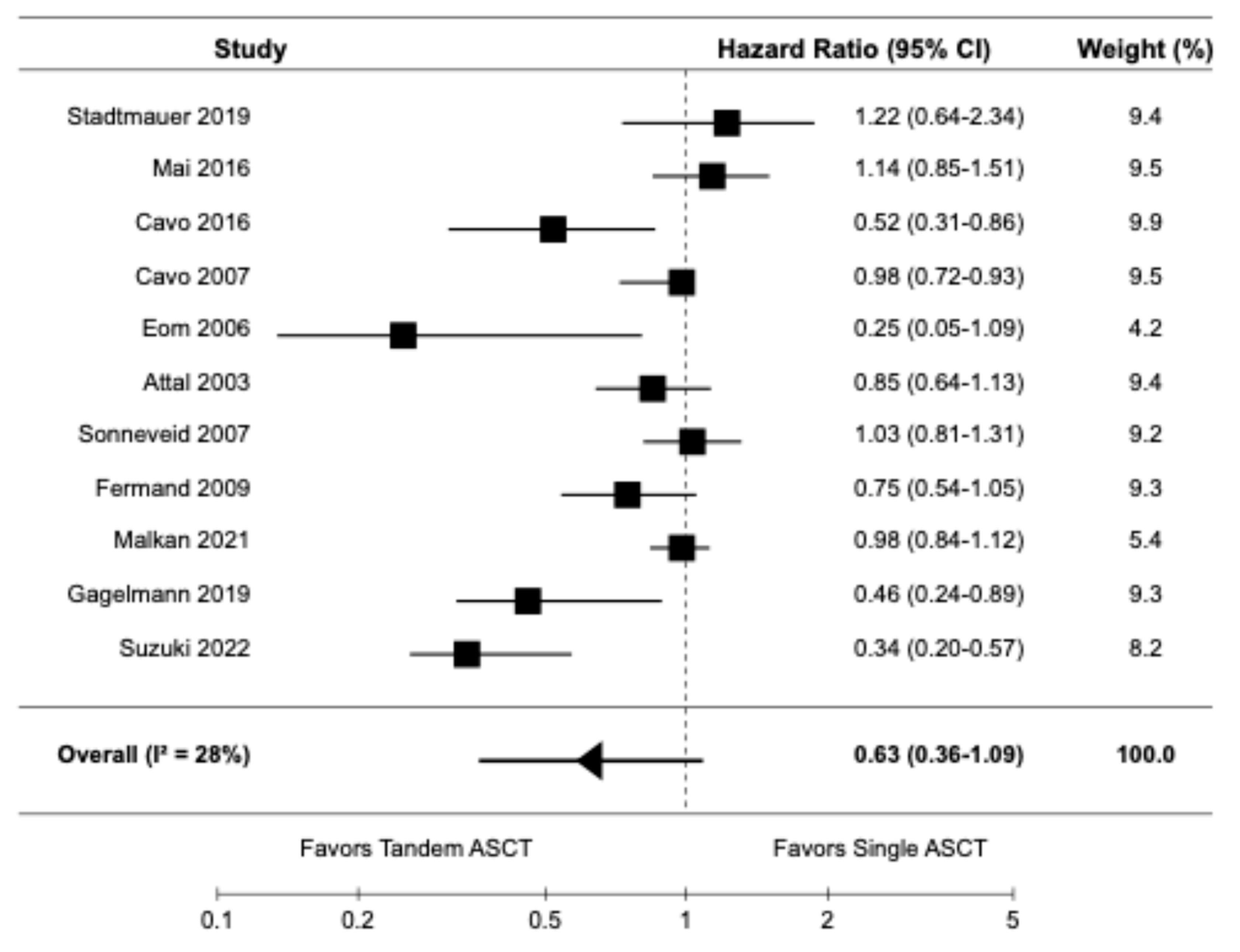
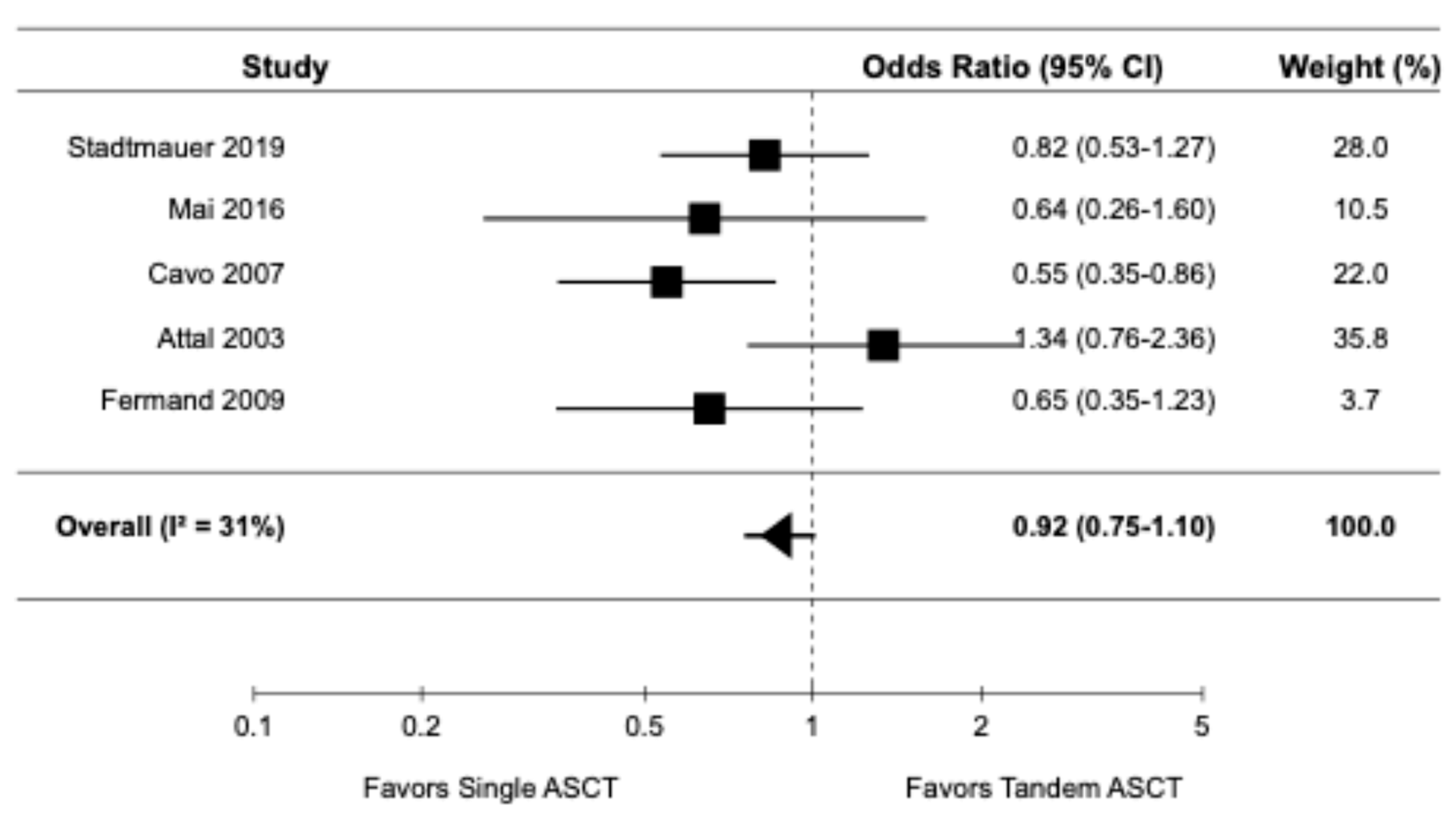
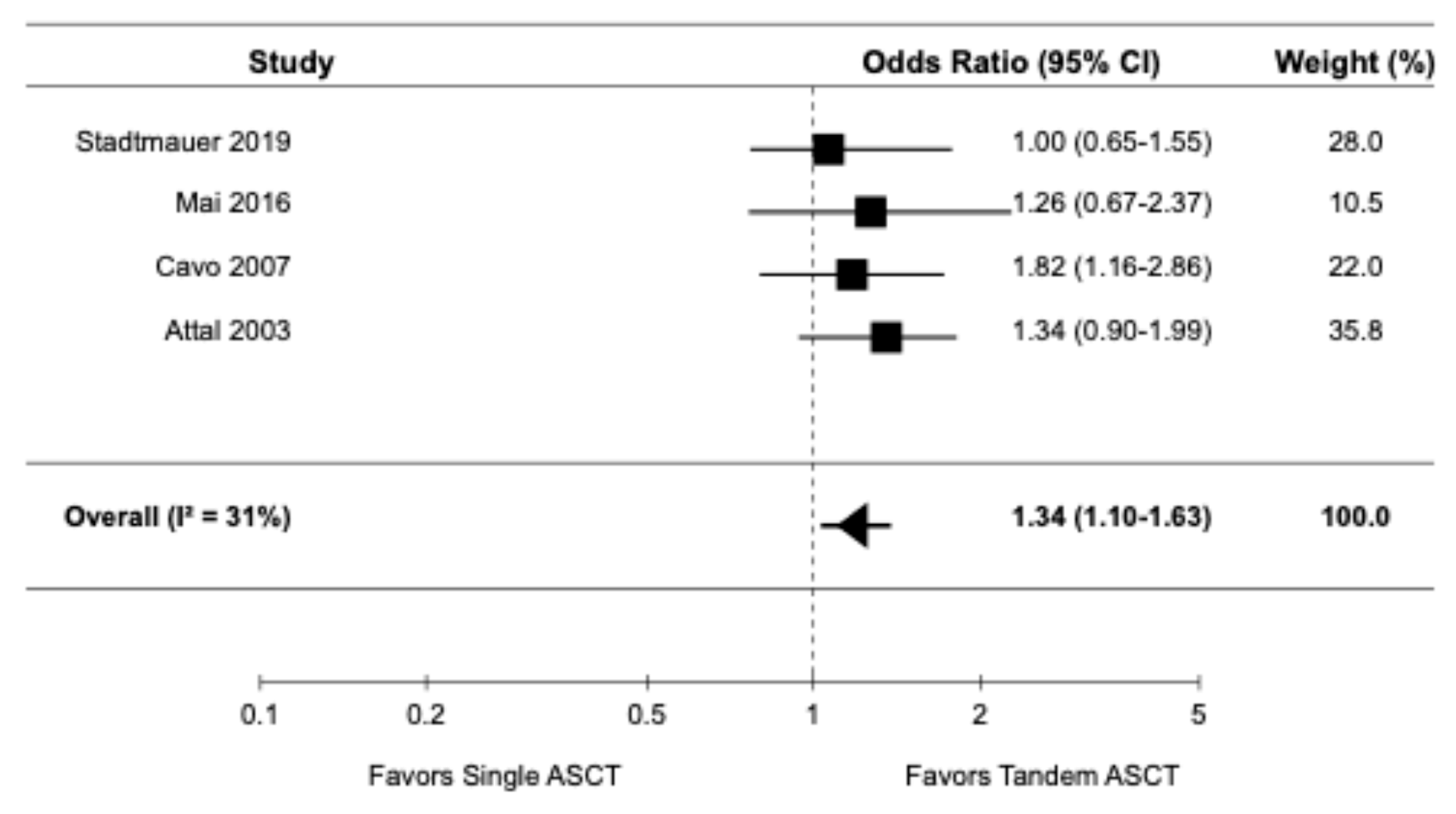
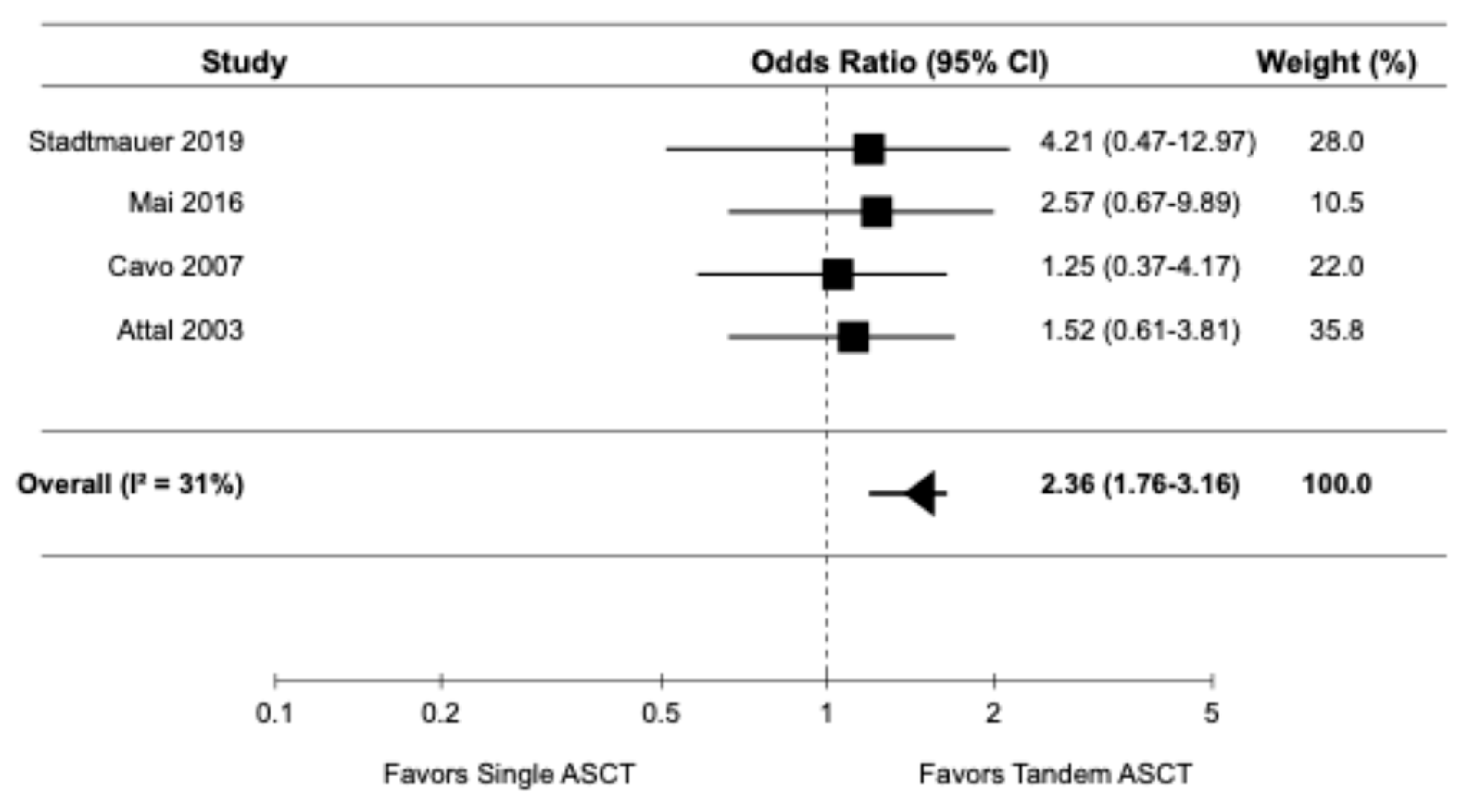
- Discussion and Conclusion Correction
- (1)
- Discussion, Paragraph 1:
- (2)
- Conclusions, Paragraph 1
- Language Correction
- References Correction
References
- Chen, Y.-H.; Fogel, L.; Sun, A.Y.-E.; Yang, C.; Patel, R.; Chang, W.-C.; Chen, P.-H.; Jhou, H.-J.; Chen, Y.-C.; Dai, M.-S.; et al. The Efficacy and Safety of Tandem Transplant Versus Single Stem Cell Transplant for Multiple Myeloma Patients: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 1030. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelkefi, A.; Ladeb, S.; Torjman, L.; Othman, T.B.; Lakhal, A.; Romdhane, N.B.; Omri, H.E.; Elloumi, M.; Belaaj, H.; Jeddi, R.; et al. Single autologous stem-cell transplantation followed by maintenance therapy with thalidomide is superior to double autologous transplantation in multiple myeloma: Results of a multicenter randomized clinical trial. Blood 2008, 111, 1805–1810, Retraction in Blood 2009, 113, 6265. https://doi.org/10.1182/blood-2009-05-222927. [Google Scholar] [CrossRef] [PubMed]
| Author Year (Trial Name) | Design (Country) | Intervention Vs Comparison | ISS III % (High-Risk Cytogenetics %) | Number of Patients | Mean Age | Disease Condition % (at Least PR) | Condition Regimen | Follow Up (Quality *) |
|---|---|---|---|---|---|---|---|---|
| Mai 2016 [9] (GMMG-HD2) | Phase III RCT, (Europe, muti-centers) | Tandem vs. Single ASCT | NA (NA) | 358 | 55.4 | 83 | High-dose melphalan (200 mg/m2) | 24 months (5) |
| Attal 2003 [14] | RCT (France, multi-centers) | Tandem vs. Single ASCT | 78.7 (NA) | 399 | 52 | 84 | Melphalan (140 mg/m2) + total body irradiation | 29 months (6) |
| Cavo 2007 [16] (Bologna 96 Clinical Study) | RCT (Globally, muti-centers) | Tandem vs. Single ASCT | 64.0 (19.6) | 321 | 53.1 | NA | High-dose melphalan (200 mg/m2) | 55 months (5) |
| Cavo 2016 [10] (EMN02/HO95 Study) | Phase III RCT, (Globally, muti-centers) | Tandem + Len vs. Single ASCT + Len | 19.0 (23.5) | 415 | 57.5 | NA | High-dose melphalan (200 mg/m2) | 38 months (5) |
| Eom 2006 [19] | Retrospective study (Korea, Single-center) | Tandem vs. Single ASCT | 92.5 (20.7) | 53 | 51 | NA | Melphalan (140 mg/m2) + TBI | 32 months (7/9) # |
| Fermand 2009 [20] | RCT (France, multi-centers) | Tandem vs. Single ASCT | NA | 225 | NA | NA | Melphalan (140 mg/m2) + TBI | 123 months (5) |
| Sonneveid 2007 [21] (HOVON 24 trial) | Phase III RCT, (Dutch, muti-centers) | Tandem vs. Single ASCT | 74.9 (NA) | 303 | 56 | CR 14 | Melphalan (140 mg/m2) | 52 months (4) |
| Stadtmauer 2019 [22] (BMT CTN 0702 Trial) | Phase III RCT, (US, muti-centers) | Tandem + Len vs. Single ASCT + Len | NA (29.0) | 504 | 56 | 91 | High dose melphalan (200 mg/m2) | 38 months (6) |
| Gagelmann 2019 [6] | Retrospective study (Europe, muti-centers) | Tandem vs. Single ASCT | 30.0 (41.0) | 488 | 59 | 90 | Melphalan (200 mg/m2 for most, some 140 mg/m2) | 49 months (6/9) # |
| Malkan 2021 [23] | Retrospective study | Tandem vs. Single ASCT | 20.0 (NA) | 228 | 55 | 89 | Melphalan (200 mg/m2) | Year 2003 to 2020 (7/9) # |
| Suzuki 2022 [24] | Multicenter retrospective study | Elderly patients with tandem ASCT vs. Elderly patients with single ASCT vs Young patients received tandem ASCR | 22.0 (9.90) | 1568 | 68 vs. 55 | 85 | High-dose melphalan (200 mg/m2) | Year 1994 to 2019 (6/9) # |
| Cochrane Risk of Bias Assessment (RoB) for Randomized Control Trials | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RCT Author Year | Random Sequence Generation | Allocation Concealment | Blinding of Participant and Personnel | Blinding of Outcome Assessment (Subjective) | Blinding of Outcome Assessment (Objective) | Incomplete Outcome Date | Selective Reporting | Other Bias | |
| Attal 2003 [14] | L | L | H | H | L | L | L | L | |
| Cavo 2007 [16] | L | L | H | H | L | L | L | H | |
| Cavo 2016 [10] | L | L | H | H | L | H | L | L | |
| Fermand 2009 [20] | L | L | H | H | L | L | L | U | |
| Mai 2016 [9] | L | L | H | H | L | L | L | H | |
| Sonneveid 2007 [21] | L | U | H | H | L | U | L | L | |
| Stadtmauer 2019 [22] | L | L | H | H | L | L | L | L | |
| Newcastle-Ottawa Quality Assessment Scale (NOS) for Cohort studies | |||||||||
| Cohort Author Year | Selection | Comparability | Outcome | Total Score | |||||
| Represen-Tativeness of the Exposed Cohort | Selection of External Control | Ascertainment of Exposure | Outcome of Interested Not present at the Start | Comparability of Cohorts on the Basis of the Design of Analysis | Assessment of the Outcome | Follow-Up Long Enough for Outcomes Occur | Adequacy of Folllow-Up of Cohorts | ||
| Eom 2006 [19] | * | 0 | * | * | * | * | * | * | 7/9 |
| Gageimann 2019 [6] | * | 0 | * | * | 0 | * | * | * | 6/9 |
| Malkan 2021 [23] | * | * | * | * | 0 | * | * | * | 7/9 |
| Suzuki 2022 [24] | 0 | * | * | * | 0 | * | * | * | 6/9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Fogel, L.; Sun, A.Y.-E.; Yang, C.; Patel, R.; Chang, W.-C.; Chen, P.-H.; Jhou, H.-J.; Chen, Y.-C.; Dai, M.-S.; et al. Correction: Chen et al. The Efficacy and Safety of Tandem Transplant Versus Single Stem Cell Transplant for Multiple Myeloma Patients: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 1030. Diagnostics 2025, 15, 2942. https://doi.org/10.3390/diagnostics15232942
Chen Y-H, Fogel L, Sun AY-E, Yang C, Patel R, Chang W-C, Chen P-H, Jhou H-J, Chen Y-C, Dai M-S, et al. Correction: Chen et al. The Efficacy and Safety of Tandem Transplant Versus Single Stem Cell Transplant for Multiple Myeloma Patients: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 1030. Diagnostics. 2025; 15(23):2942. https://doi.org/10.3390/diagnostics15232942
Chicago/Turabian StyleChen, Yu-Han, Lindsay Fogel, Andrea Yue-En Sun, Chieh Yang, Rushin Patel, Wei-Cheng Chang, Po-Huang Chen, Hong-Jie Jhou, Yeu-Chin Chen, Ming-Shen Dai, and et al. 2025. "Correction: Chen et al. The Efficacy and Safety of Tandem Transplant Versus Single Stem Cell Transplant for Multiple Myeloma Patients: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 1030" Diagnostics 15, no. 23: 2942. https://doi.org/10.3390/diagnostics15232942
APA StyleChen, Y.-H., Fogel, L., Sun, A. Y.-E., Yang, C., Patel, R., Chang, W.-C., Chen, P.-H., Jhou, H.-J., Chen, Y.-C., Dai, M.-S., & Lee, C.-H. (2025). Correction: Chen et al. The Efficacy and Safety of Tandem Transplant Versus Single Stem Cell Transplant for Multiple Myeloma Patients: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 1030. Diagnostics, 15(23), 2942. https://doi.org/10.3390/diagnostics15232942






