Clinical Relevance of Trace-Positive Results in Xpert MTB/RIF Ultra for Tuberculosis Diagnosis in a High-Burden Setting: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Patient and Sample Selection
- •
- They were evaluated as presumptive TB patients at the time of diagnostic workup.
- •
- At least one clinical specimen was tested using Xpert MTB/RIF Ultra and returned a “trace” result.
- •
- Comprehensive clinical records, imaging data, and microbiological workup results were available for retrospective review.
2.3. Diagnostic Methods
2.3.1. Bacteriological Examination
- •
- Microscopy using Ziehl–Neelsen staining
- •
- Culture on Löwenstein–Jensen medium or liquid medium culture
- •
- Xpert MTB/RIF Ultra testing as per manufacturer protocol
2.3.2. Clinical and Radiological Evaluation
- •
- Respiratory and systemic symptoms (cough, dyspnea, sputum, hemoptysis, fever, night sweats, fatigue, weight loss)
- •
- Comorbidities (oncological, immunological, pulmonary, cardiovascular)
- •
- TB history (prior treatment, default, or relapse)
- •
- Radiological findings (chest X-ray or CT)—Images were interpreted by radiologists blinded to culture results and categorized as either suggestive or non-suggestive for TB.
2.3.3. Composite Reference Standard (CRS)
- •
- Confirmed TB: positive culture, both solid or liquid (smear positive/negative);
- •
- Probable TB: negative or unavailable culture but strong clinical and radiological evidence of active TB, as determined by an independent clinician;
- •
- Not TB: absence of microbiological, clinical, or radiological findings compatible with TB.
2.3.4. Ethics
3. Results
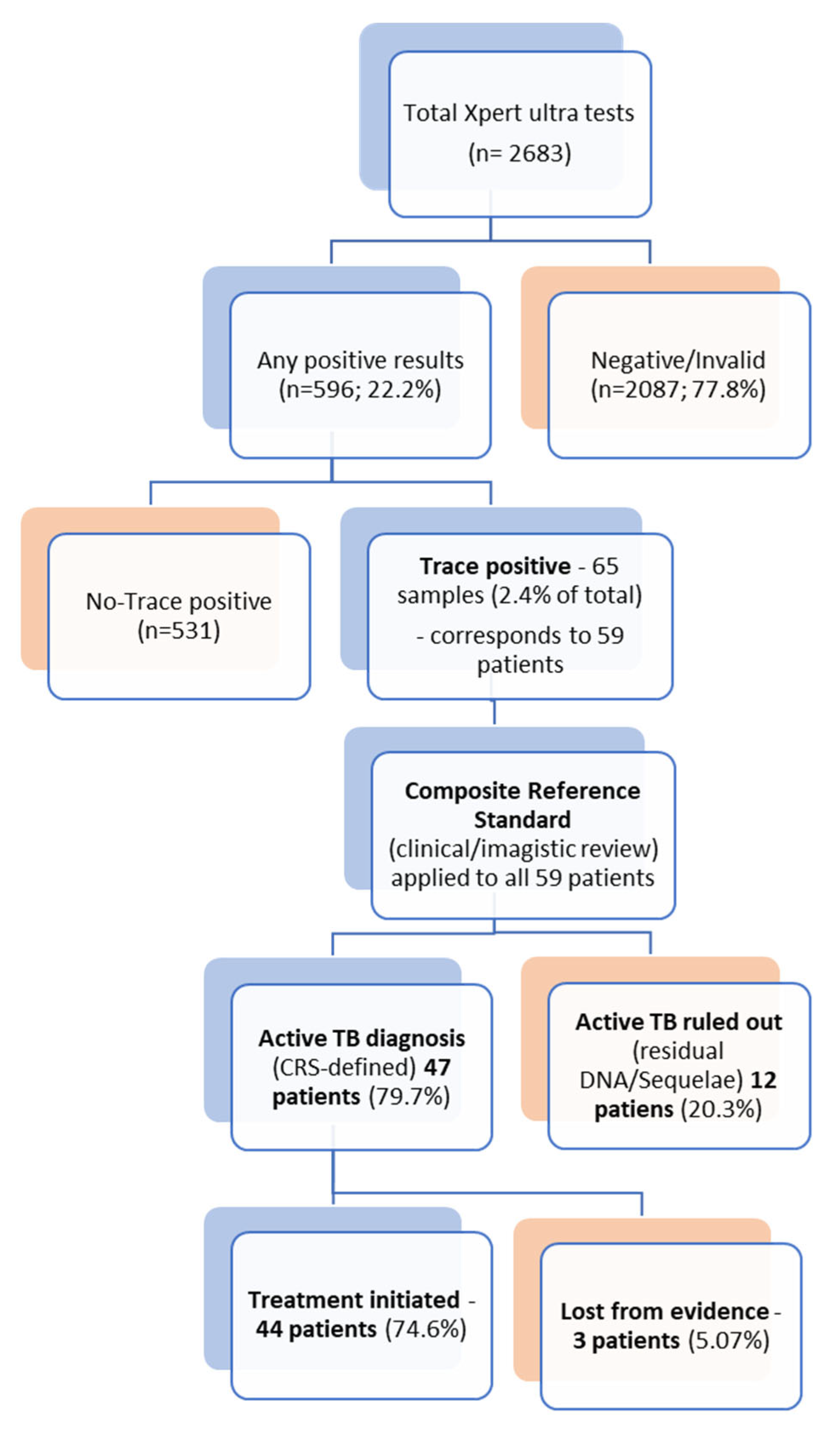
3.1. Study Cohort, Trace Incidence, and Specimen Characteristics
3.2. Demographic and Clinical Profile
3.3. Microbiological Correlation and Diagnostic Yield
3.4. Results of Imaging Studies
3.5. Final Diagnosis and Treatment Decisions
4. Discussion
4.1. Diagnostic Yield and Correlation with Culture
4.2. Clinical and Radiological Correlation
4.3. Interpretation in Specific Patient Groups
- •
- Pediatric and EPTB: Findings reinforce high clinical significance in these groups. All four children with trace results were diagnosed and treated. Trace detection in EP samples (18.5% of all trace-positive specimens) was clinically meaningful, with pleural fluid showing 100% culture positivity. This supports the WHO recommendation to regard trace as bacteriological confirmation in children and EPTB, where conventional methods have limited sensitivity [5,18,22,27].
- •
- Patients with Prior Tuberculosis: Interpretation is more complex. Twelve patients with trace results were ultimately classified as non-active TB, with 11 having a documented prior history. This suggests trace may reflect residual DNA in this group [16,25]. WHO guidance advises that re-treatment should rely on clear clinical and radiological evidence of new activity [5]. Our data support this: patients with trace and prior TB required corroborating evidence, while trace positivity was almost always clinically significant in new TB suspects.
4.4. The Utility of a Composite Reference Standard
4.5. Interpretation Framework in Practice
- •
- Trace + Active symptoms + Suggestive imaging + No prior TB → Strong justification for immediate treatment.
- •
- Trace + Immunocompromised host (e.g., HIV) + Compatible findings → Consider empirical treatment even if culture-negative.
- •
- Trace + Prior TB with stable sequelae and no new symptoms → Close monitoring and repeat testing before re-treatment.
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRS | composite reference standard |
| CSF | cerebrospinal fluid |
| EU/EEA | European Union and European Economic Area |
| EPTB | extrapulmonary tuberculosis |
| MTBC | Mycobacterium tuberculosis complex |
| PLHIV | people living with HIV |
| TB | Tuberculosis |
| WHO | World Health Organization |
References
- Global Tuberculosis Report 2024, 1st ed.; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-010153-1.
- Tuberculosis Incidence (per 100 000 Population per Year). Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/incidence-of-tuberculosis-(per-100-000-population-per-year) (accessed on 22 June 2025).
- European Centre for Disease Prevention and Control; World Health Organization. Tuberculosis Surveillance and Monitoring in Europe 2024: 2022 Data; Publications Office: Luxembourg, 2024. [Google Scholar]
- WHO Operational Handbook on Tuberculosis. Module 3: Diagnosis-Rapid Diagnostics for Tuberculosis Detection, 3rd ed.; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-008950-1. [Google Scholar]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis. Module 3: Diagnosis. Web Annex B. Evidence Synthesis and Analysis; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Berhanu, R.H.; David, A.; Da Silva, P.; Shearer, K.; Sanne, I.; Stevens, W.; Scott, L. Performance of Xpert MTB/RIF, Xpert Ultra, and Abbott RealTime MTB for Diagnosis of Pulmonary Tuberculosis in a High-HIV-Burden Setting. J. Clin. Microbiol. 2018, 56, E00560-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, M.; Jing, H.; Jia, J.; Dong, L.; Zhao, L.; Wang, F.; Xue, Y.; Deng, Y.; Jiang, G.; et al. The Practical Value of Xpert MTB/RIF Ultra for Diagnosis of Pulmonary Tuberculosis in a High Tuberculosis Burden Setting: A Prospective Multicenter Diagnostic Accuracy Study. Microbiol. Spectr. 2022, 10, e00949-22. [Google Scholar] [CrossRef]
- Iuhas, A.; Jurca, C.; Kozma, K.; Riza, A.-L.; Streață, I.; Petcheși, C.; Dan, A.; Sava, C.; Balmoș, A.; Marinău, C.; et al. PAH Pathogenic Variants and Clinical Correlations in a Group of Hyperphenylalaninemia Patients from North-Western Romania. Diagnostics 2023, 13, 1483. [Google Scholar] [CrossRef]
- Sava, C.N.; Bodog, T.-M.; Niulas, L.R.; Iuhas, A.R.; Marinau, C.P.; Negrut, N.; Balmos, A.B.; Pasca, B.; Roman, N.A.; Delia Nistor-Cseppento, C. Biomarker Changes in Pediatric Patients With COVID-19: A Retrospective Study from a Single Center Database. In Vivo 2022, 36, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Patel, H.; Halliwell, R.; Free, R.C.; Glimour-Caunt, A.; Pareek, M.; Woltmann, G.; Verma, R.; Perera, N.; Haldar, P. Real-World Clinical Utility of Xpert MTB/RIF Ultra in the Assessment of Tuberculosis in a Low-TB-Incidence, High-Resource Setting. BMJ Open Respir. Res. 2025, 12, e002624. [Google Scholar] [CrossRef]
- Sava, C.; Sava, M.; Drăgan, A.-M.; Iuhas, A.; Niulaș, L.; Marinău, C.; Balmoș, A. The Use of Xpert MTB/RIF Ultra Testing for Early Diagnosis of Tuberculosis: A Retrospective Study from a Single-Center Database. Genes 2023, 14, 1231. [Google Scholar] [CrossRef]
- World Health Organization. Xpert MTB/RIF Implementation Manual: Technical and Operational ‘How-to’Practical Considerations; World Health Organization: Geneva, Switzerland, 2014; ISBN 978-92-4-150670-0. [Google Scholar]
- Opota, O.; Mazza-Stalder, J.; Greub, G.; Jaton, K. The Rapid Molecular Test Xpert MTB/RIF Ultra: Towards Improved Tuberculosis Diagnosis and Rifampicin Resistance Detection. Clin. Microbiol. Infect. 2019, 25, 1370–1376. [Google Scholar] [CrossRef]
- WHO. WHO Meeting Report of a Technical Expert Consultation Non-Inferiority Analysis of Xpert MTFRIF Ultra Compared to Xpert; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Andama, A.; Jaganath, D.; Crowder, R.; Asege, L.; Nakaye, M.; Katumba, D.; Mukwatamundu, J.; Mwebe, S.; Semitala, C.F.; Worodria, W.; et al. The Transition to Xpert MTB/RIF Ultra: Diagnostic Accuracy for Pulmonary Tuberculosis in Kampala, Uganda. BMC Infect. Dis. 2021, 21, 49. [Google Scholar] [CrossRef]
- Guillouzouic, A.; Gaudart, A.; Tessier, E.; Risso, K.; Hamdad, F.; Alauzet, C.; Vaillant, P.; Koebel, C.; Kassegne, L.; Chenouard, R.; et al. Xpert MTB/RIF Ultra Trace Results: Decision Support for the Treatment of Extrapulmonary Tuberculosis in Low TB Burden Countries. J. Clin. Med. 2023, 12, 3148. [Google Scholar] [CrossRef]
- Kay, A.W.; González Fernández, L.; Takwoingi, Y.; Eisenhut, M.; Detjen, A.K.; Steingart, K.R.; Mandalakas, A.M. Xpert MTB/RIF and Xpert MTB/RIF Ultra Assays for Active Tuberculosis and Rifampicin Resistance in Children. Cochrane Database Syst. Rev. 2020, 2020, CD013359. [Google Scholar] [CrossRef]
- Aurilio, R.B.; Ferreira, S.; Parente, A.A.A.I.; Sant’Anna, M.D.F.P.; Pereira, C.S.; Malaquias, T.D.S.S.; Kritski, A.L.; Sant’Anna, C.C. Gene-Xpert Ultra for the Diagnosis of Extrapulmonary Tuberculosis in Children and Adolescents. Rev. Inst. Med. Trop. São Paulo 2022, 64, e12. [Google Scholar] [CrossRef]
- Signorino, C.; Votto, M.; De Filippo, M.; Marseglia, G.L.; Galli, L.; Chiappini, E. Diagnostic Accuracy of Xpert Ultra for Childhood Tuberculosis: A Preliminary Systematic Review and Meta-analysis. Pediatr. Allergy Immunol. 2022, 33, 80–82. [Google Scholar] [CrossRef]
- Slail, M.J.; Booq, R.Y.; Al-Ahmad, I.H.; Alharbi, A.A.; Alharbi, S.F.; Alotaibi, M.Z.; Aljubran, A.M.; Aldossary, A.M.; Memish, Z.A.; Alyamani, E.J.; et al. Evaluation of Xpert MTB/RIF Ultra for the Diagnosis of Extrapulmonary Tuberculosis: A Retrospective Analysis in Saudi Arabia. J. Epidemiol. Glob. Health 2023, 13, 782–793. [Google Scholar] [CrossRef]
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N.; et al. Xpert MTB/RIF Ultra for Detection of Mycobacterium Tuberculosis and Rifampicin Resistance: A Prospective Multicentre Diagnostic Accuracy Study. Lancet Infect. Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef]
- Bahr, N.C.; Nuwagira, E.; Evans, E.E.; Cresswell, F.V.; Bystrom, P.V.; Byamukama, A.; Bridge, S.C.; Bangdiwala, A.S.; Meya, D.B.; Denkinger, C.M.; et al. Diagnostic Accuracy of Xpert MTB/RIF Ultra for Tuberculous Meningitis in HIV-Infected Adults: A Prospective Cohort Study. Lancet Infect. Dis. 2018, 18, 68–75. [Google Scholar] [CrossRef]
- Sung, J.; Nantale, M.; Nalutaaya, A.; Biché, P.; Mukiibi, J.; Kamoga, C.E.; Akampurira, J.; Kayondo, F.; Kiyonga, R.; Mukiibi, M.; et al. Evidence for Tuberculosis in Individuals With Xpert Ultra “Trace” Sputum During Screening of High-Burden Communities. Clin. Infect. Dis. 2024, 78, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Simmons, A.M.; Rowneki, M.; Parmar, H.; Cao, Y.; Ryan, J.; Banada, P.P.; Deshpande, S.; Shenai, S.; Gall, A.; et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium Tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. mBio 2017, 8, e00812-17. [Google Scholar] [CrossRef]
- Dowling, W.B.; Whitelaw, A.; Nel, P. Tracing TB: Are There Predictors for Active TB Disease in Patients with Xpert Ultra Trace Results? Int. J. Infect. Dis. 2022, 114, 115–123. [Google Scholar] [CrossRef]
- Prakash, K.A.; Ramakrishnan, G.A.; Vasudevan, A. Evaluation of the Prevalence of Xpert Ultra Trace Calls and Its Clinical Significance in Pulmonary and Extrapulmonary Tuberculosis in High Tuberculosis-Endemic Setting. J. Assoc. Pulmonologist Tamil Nadu 2023, 6, 96–102. [Google Scholar] [CrossRef]
- Muhammad, A.; Myint, Z.; Nyunt, W.W. Xpert MTB/RIF Ultra-Trace Call Results and Pathways for TB Treatment. J. Pulmonol. Res. Rep. 2024, 6, 1–2. [Google Scholar] [CrossRef]
- Van Dinh, L.; Tran, K.T.; Codlin, A.J.; Vo, L.N.Q.; Nguyen, N.T.T.; Nguyen, L.P.; Forse, R.; Nguyen, H.T.; Dang, T.M.H.; Nguyen, L.H.; et al. Prevalence of Xpert MTB/RIF Ultra Trace Call Results and Associated Risk Factors During Active Tuberculosis Case Finding in Viet Nam: A Programmatic Evaluation. Diagnostics 2025, 15, 1006. [Google Scholar] [CrossRef] [PubMed]
- Iuhas, A.; Marinău, C.; Niulaș, L.; Futaki, Z.; Balmoș, A.; Kozma, K.; Indrieș, M.; Sava, C. Familial Mediterranean Fever in Romania: A Case Report and Literature Review. Front. Pediatr. 2024, 12, 1546387. [Google Scholar] [CrossRef] [PubMed]
- Marinău, C.; Csep, A.; Sava, C.; Iuhas, A.; Niulaș, L.; Szilagyi, A.; Ritli, L.; Balmoș, A.; Jurca, C. Difficulties in the Management of an Askin Tumor in a Pediatric Patient with Cystic Fibrosis: Case Report and Literature Review. Front. Pediatr. 2023, 11, 1289256. [Google Scholar] [CrossRef]
- Galis, R.; Trif, P.; Mudura, D.; Murvai, R.; Daina, L.G.; Szasz, F.; Negrini, R.; Hatos, A.; Gyarmati, B.F.; Daly, M.C.; et al. Preterm Birth and Stillbirth during COVID-19 Pandemic in Bihor County/Romania. Front. Reprod. Health 2024, 6, 1286496. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Nikam, C.; Ragte, T.; Shetty, A.; Rodrigues, C. Is a Composite Reference Standard (CRS) an Alternative to Culture in Assessment and Validation of a Single Tube Nested in-House PCR for TB Diagnosis? Egypt. J. Chest Dis. Tuberc. 2013, 62, 805–815. [Google Scholar] [CrossRef]
- Dendukuri, N.; Schiller, I.; de Groot, J.; Libman, M.; Moons, K.; Reitsma, J.; van Smeden, M. Concerns about Composite Reference Standards in Diagnostic Research. BMJ 2018, 360, j5779. [Google Scholar] [CrossRef]
- Chen, X.; Fan, Y.; Tu, H.; Luo, Y. Clinical Efficacy of Different Therapeutic Options for Knee Osteoarthritis: A Network Meta-Analysis Based on Randomized Clinical Trials. PLoS ONE 2025, 20, e0324864. [Google Scholar] [CrossRef]
- Zifodya, J.S.; Kreniske, J.S.; Schiller, I.; Kohli, M.; Dendukuri, N.; Schumacher, S.G.; Ochodo, E.A.; Haraka, F.; Zwerling, A.A.; Pai, M.; et al. Xpert Ultra versus Xpert MTB/RIF for Pulmonary Tuberculosis and Rifampicin Resistance in Adults with Presumptive Pulmonary Tuberculosis. Cochrane Database Syst. Rev. 2021, 2, CD009593. [Google Scholar] [CrossRef]
- Santos, A.; Carneiro, S.; Silva, A.; Gomes, J.P.; Macedo, R. Nontuberculous Mycobacteria in Portugal: Trends from the Last Decade. Pulmonology 2024, 30, 337–343. [Google Scholar] [CrossRef]
- Atherton, R.R.; Cresswell, F.V.; Ellis, J.; Kitaka, S.B.; Boulware, D.R. Xpert MTB/RIF Ultra for Tuberculosis Testing in Children: A Mini-Review and Commentary. Front. Pediatr. 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, R.; Uddin, M.K.M.; Kabir, S.N.; Rahman, T.; Biswas, S.; Hossain, A.; Rahman, S.M.M.; Ahmed, S.; Pouzol, S.; Hoffmann, J.; et al. Xpert MTB/RIF Ultra for the Rapid Diagnosis of Extrapulmonary Tuberculosis in a Clinical Setting of High Tuberculosis Prevalence Country and Interpretation of “trace” Results. Tuberc. Edinb. Scotl. 2024, 145, 102478. [Google Scholar] [CrossRef] [PubMed]
| Specimen Type | Number of Samples (n = 65) | Percentage (%) |
|---|---|---|
| Spontaneous Sputum | 30 | 46.2% |
| Induced Sputum | 23 | 35.4% |
| Pleural Fluid | 6 | 9.2% |
| Bronchial Aspirate | 3 | 4.6% |
| Cerebrospinal Fluid (CSF) | 2 | 3.1% |
| Urine | 1 | 1.5% |
| Total | 65 | 100.0% |
| Characteristic | n (%) | |
|---|---|---|
| Total Patients | 59 (100%) | |
| Mean Age (years) | 49.1 | |
| Sex | Male | 44 (74.6%) |
| Female | 15 (25.4%) | |
| Chronic Comorbidities | 11 (18.6%) | |
| Previous TB History | 16 (27.1%) | |
| Therapeutic Decision (Anti-TB Treatment) | Culture Positive | Culture Negative/Contaminated | Total Patients n (%) |
|---|---|---|---|
| Treated | 26 (5 Smear Positive) | 18 * | 44 (74.6%) |
| Not Treated | 3 | 12 | 15 (25.4%) |
| Total (n) | 29 | 30 | 59 (100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sava, C.; Iuhas, A.; Marinău, C.; Galiș, R.; Rus, M.; Sava, M. Clinical Relevance of Trace-Positive Results in Xpert MTB/RIF Ultra for Tuberculosis Diagnosis in a High-Burden Setting: A Retrospective Cohort Study. Diagnostics 2025, 15, 2860. https://doi.org/10.3390/diagnostics15222860
Sava C, Iuhas A, Marinău C, Galiș R, Rus M, Sava M. Clinical Relevance of Trace-Positive Results in Xpert MTB/RIF Ultra for Tuberculosis Diagnosis in a High-Burden Setting: A Retrospective Cohort Study. Diagnostics. 2025; 15(22):2860. https://doi.org/10.3390/diagnostics15222860
Chicago/Turabian StyleSava, Cristian, Alin Iuhas, Cristian Marinău, Radu Galiș, Marius Rus, and Mihaela Sava. 2025. "Clinical Relevance of Trace-Positive Results in Xpert MTB/RIF Ultra for Tuberculosis Diagnosis in a High-Burden Setting: A Retrospective Cohort Study" Diagnostics 15, no. 22: 2860. https://doi.org/10.3390/diagnostics15222860
APA StyleSava, C., Iuhas, A., Marinău, C., Galiș, R., Rus, M., & Sava, M. (2025). Clinical Relevance of Trace-Positive Results in Xpert MTB/RIF Ultra for Tuberculosis Diagnosis in a High-Burden Setting: A Retrospective Cohort Study. Diagnostics, 15(22), 2860. https://doi.org/10.3390/diagnostics15222860







