Mitral Annular Disjunction Assessed Using Cardiac MR Imaging in Pediatric Patients
Abstract
1. Introduction
2. Materials/Methods
2.1. Patient Selection
2.2. Echocardiographic Evaluation
2.3. Cardiac Magnetic Resonance Imaging Protocol
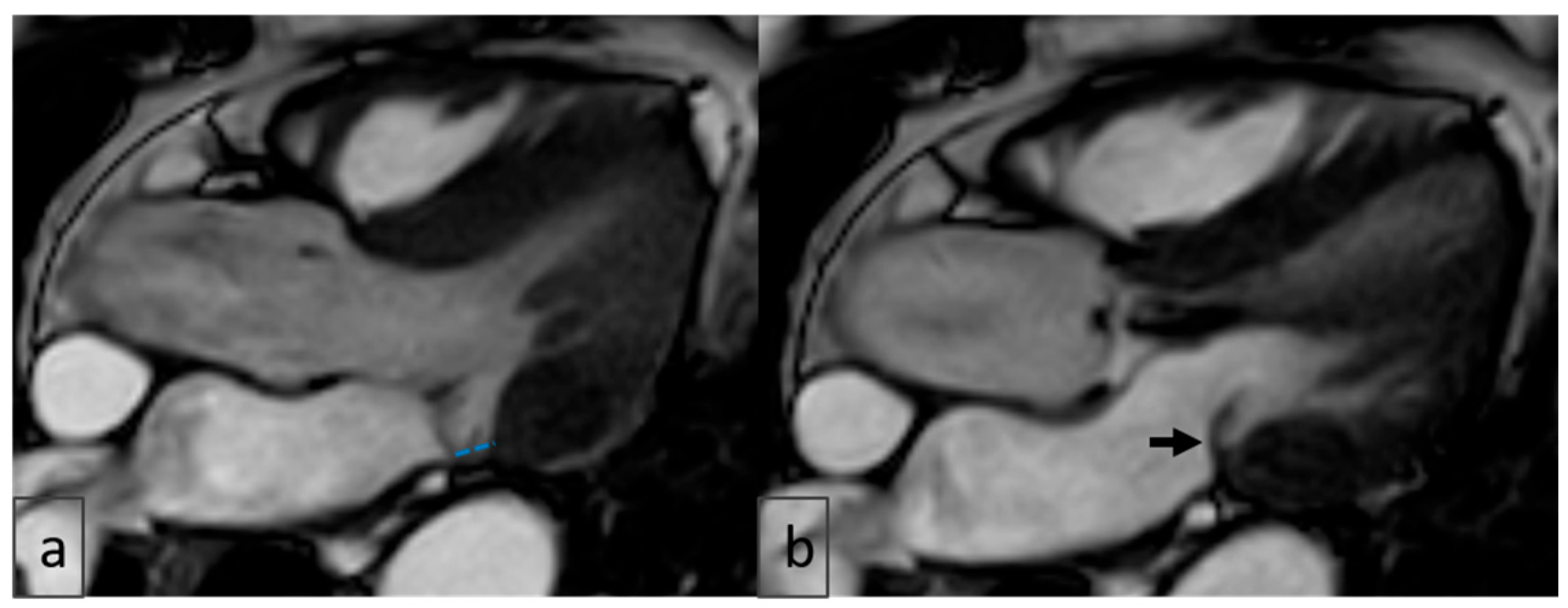
2.4. Image Analysis
3. Results
3.1. Study Population
3.2. Echocardiographic Findings
3.3. Agreement Between Echocardiography and Cardiac MRI for MAD
3.4. Agreement Between Echocardiography and Cardiac MRI for MVP
3.5. Agreement Between Echocardiography and Cardiac MRI for MR
3.6. Cardiac MRI Findings in MAD
3.7. Association of MAD with Arrhythmia
| Univariable Logistic Regression Analysis | Multivariable Logistic Regression Analysis a | |||
|---|---|---|---|---|
| Variable | OR (95% CI) for Mitral Annular Disjunction MAD Presence | p Value | OR (95% CI) for Mitral Annular Disjunction MAD Presence | p Value |
| MVP | 18.25 (8.39–39.66) | <0.001 | 20.09 (8.39–48.07) | <0.001 |
| MR | 2.88 (1.36–6.07) | 0.006 | 1.67 (0.66–4.26) | 0.282 |
| Congenital cardiac disease | 0.73 (0.26–2.02) | 0.541 | 0.41 (0.12–1.42) | 0.159 |
| LVTA | 0.45 (0.18–1.13) | 0.088 | 0.44 (0.15–1.31) | 0.14 |
| Age (years) | 1.09 (0.99–1.22) | 0.086 | 1.08 (0.94–1.24) | 0.283 |
| Leaflet curling | 1.96 (1.03–3.72) | 0.040 | 1.9 (0.86–4.22) | 0.115 |
| LGE | 1.70 (0.49–5.90) | 0.399 | 3.68 (0.66–20.48) | 0.136 |
| Female sex | 1.73 (0.95–3.19) | 0.075 | 0.81 (0.36–1.79) | 0.60 |
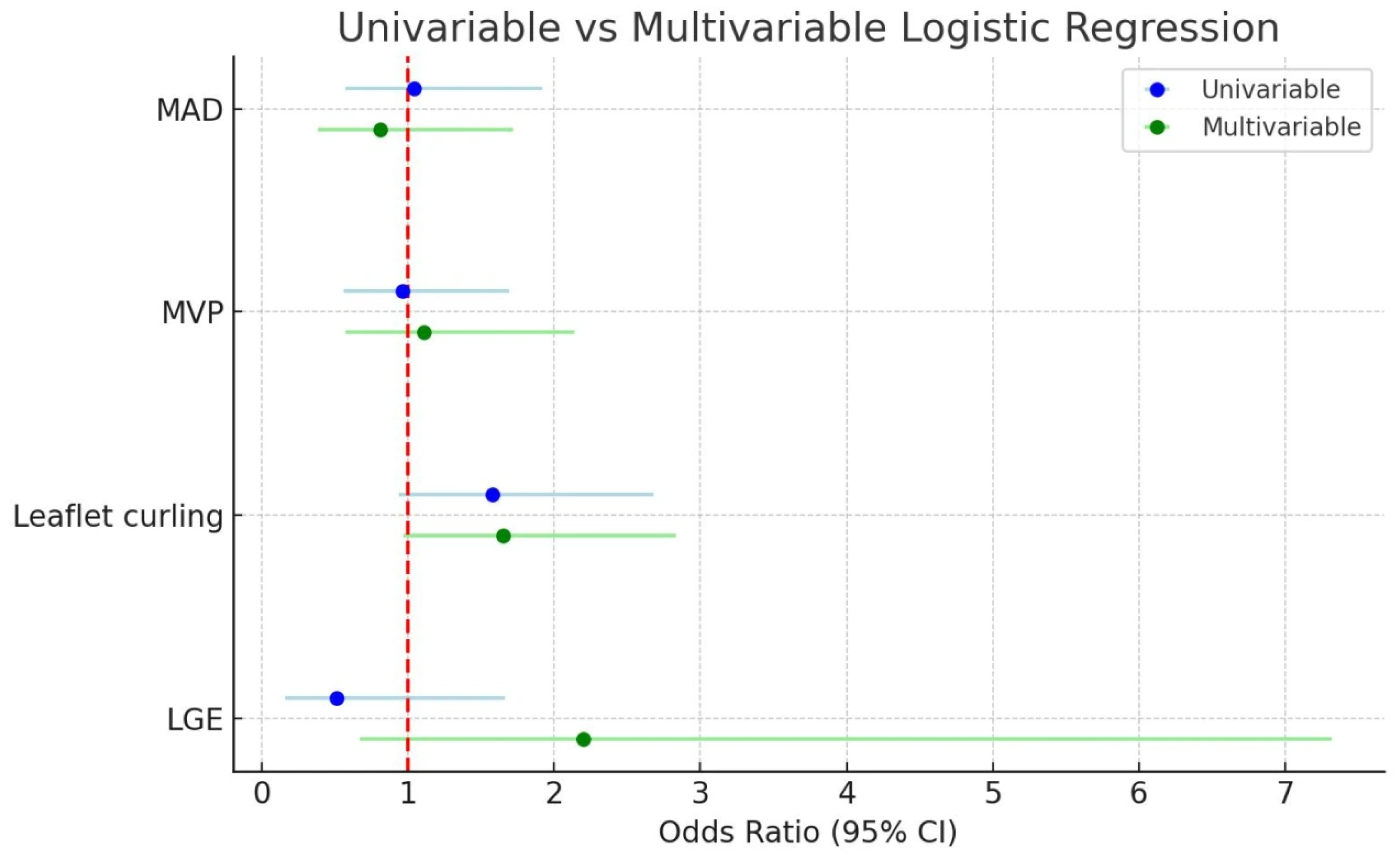
3.8. MAD and Its Association with Other Cardiac Conditions
| Univariable Logistic Regression Analysis | Multivariable Logistic Regression Analysis a | |||
|---|---|---|---|---|
| Variable | OR (95% CI) for Mitral Annular Disjunction MAD Presence | p Value | OR (95% CI) for Mitral Annular Disjunction MAD Presence | p Value |
| MVP | 18.25 (8.39–39.66) | <0.001 | 20.09 (8.39–48.07) | <0.001 |
| MR | 2.88 (1.36–6.07) | 0.006 | 1.67 (0.66–4.26) | 0.282 |
| Congenital cardiac disease | 0.73 (0.26–2.02) | 0.541 | 0.41 (0.12–1.42) | 0.159 |
| LVTA | 0.45 (0.18–1.13) | 0.088 | 0.44 (0.15–1.31) | 0.14 |
| Age (years) | 1.09 (0.99–1.22) | 0.086 | 1.08 (0.94–1.24) | 0.283 |
| Leaflet curling | 1.96 (1.03–3.72) | 0.040 | 1.9 (0.86–4.22) | 0.115 |
| LGE | 1.70 (0.49–5.90) | 0.399 | 3.68 (0.66–20.48) | 0.136 |
| Female sex | 1.73 (0.95–3.19) | 0.075 | 0.81 (0.36–1.79) | 0.60 |
3.9. The Assessment of Interobserver Agreement
| Variable | κ | Interobserver Agreement |
|---|---|---|
| MR | 0.79 | 94.1 |
| MVP | 0.85 | 93.2 |
| MAD | 0.77 | 91.5 |
3.10. Additional Imaging Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAD | Mitral annular disjunction |
| MVP | Mitral annular Prolapses |
| MR | Mitral Regurgitation |
| CMR | Cardiac Magnetic Resonance |
| LVOT | Left Ventricular Out Flow Tract |
| LGE | Late gadolinium enhancement |
| ECHO | Echocardiography |
| ECG | Electrocardiography |
| BMI | Body Mass Index |
| EF | Ejection Fraction |
References
- Koo, H.J.; Yang, D.H.; Oh, S.Y.; Kang, J.-W.; Kim, D.-H.; Song, J.-K.; Lee, J.W.; Chung, C.H.; Lim, T.-H. Demonstration of mitral valve prolapse with CT for planning of mitral valve repair. RadioGraphics 2014, 34, 1537–1552. [Google Scholar] [CrossRef]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic mitral valve prolapses and sudden cardiac death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Garg, P.; Swift, A.J.; Zhong, L.; Carlhäll, C.J.; Ebbers, T.; Westenberg, J.; Hope, M.D.; Bucciarelli-Ducci, C.; Bax, J.J.; Myerson, S.G. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat. Rev. 2020, 17, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Nordhues, B.D.; Siontis, K.C.; Scott, C.G.; Nkomo, V.T.; Ackerman, M.J.; Asirvatham, S.J.; Noseworthy, P.A. Bileafet mitral valve prolapse and risk of ventricular dysrhythmias and death. J. Cardiovasc. Electrophysiol. 2016, 26, 463–468. [Google Scholar] [CrossRef]
- Custódio, P.; de Campos, D.; Moura, A.R.; Shiwani, H.; Savvatis, K.; Joy, G.; Lambiase, P.D.; Moon, J.C.; Khanji, M.Y.; Augusto, J.B.; et al. Mitral Annulus Disjunction: A Comprehensive Cardiovascular Magnetic Resonance Phenotype and Clinical Outcomes Study. J. Magn. Reson. Imaging 2025, 61, 368–1375. [Google Scholar] [CrossRef]
- Bennett, S.; Tafuro, J.; Duckett, S.; Appaji, A.; Khan, J.N.; Heatlie, G.; Cubukcu, A.; Kwok, C.S. Definition, prevalence, and clinical significance of mitral annular disjunction in different patient cohorts: A systematic review. Echocardiography 2022, 39, 514–523. [Google Scholar] [CrossRef]
- Faletra, F.F.; Leo, L.A.; Paiocchi, V.L.; Schlossbauer, S.A.; Pavon, A.G.; Ho, S.Y.; Maisano, F. Morphology of Mitral Annular Disjunction in Mitral Valve Prolapse. J. Am. Soc. Echocardiogr. 2022, 35, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef]
- Stankowski, K.; Catapano, F.; Donia, D.; Bragato, R.M.; Lopes, P.; Abecasis, J.; Ferreira, A.; Slipczuk, L.; Masci, P.-G.; Condorelli, G.; et al. True- and pseudo-mitral annular disjunction in patients undergoing cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2025, 27, 101413. [Google Scholar] [CrossRef]
- Zugwitz, D.; Fung, K.; Aung, N.; Rauseo, E.; McCracken, C.; Cooper, J.; Anderson, R.H.; Piechnik, S.K.; Neubauer, S.; Petersen, S.E.; et al. Mitral Annular Disjunction Assessed Using CMR Imaging: Insights From the UK Biobank Population Study. JACC Cardiovasc. Imaging 2022, 15, 1856–1866. [Google Scholar] [CrossRef]
- Toh, H.; Mori, S.; Izawa, Y.; Fujita, H.; Miwa, K.; Suzuki, M.; Takahashi, Y.; Toba, T.; Watanabe, Y.; Kono, A.K.; et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: Comprehensive 3D analysis using cardiac computed tomography. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 614–622. [Google Scholar] [CrossRef]
- Sabbag, A.; Essayagh, B.; Barrera, J.D.R.; Basso, C.; Berni, A.; Cosyns, B.; Deharo, J.-C.; Deneke, T.; Di Biase, L.; Enriquez-Sarano, M.; et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed cby the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace 2022, 24, 1981–2003. [Google Scholar] [PubMed]
- Figliozzi, S.; Stankowski, K.; Tondi, L.; Catapano, F.; Gitto, M.; Lisi, C.; Bombace, S.; Olivieri, M.; Cannata, F.; Fazzari, F.; et al. Mitral annulus disjunction in consecutive patients undergoing cardiovascular magnetic resonance: Where is the boundary between normality and disease? J. Cardiovasc. Magn. Reson. 2024, 26, 101056. [Google Scholar] [CrossRef]
- Levine, R.A.; Triulzi, M.O.; Harrigan, P.; Weyman, A.E. The relationship of mitral annular shape to the diagnosis of mitral valve prolapses. Circulation 1987, 75, 756–767. [Google Scholar] [CrossRef]
- Silva Ferreira, M.V.; Soares, C.S.P.; Araujo-Filho, J.A.B.; Dantas, R.N., Jr.; Torres, R.V.A.; Morais, T.C.; Avila, L.F.R.; Ishikawa, W.; Nomura, C.H.; Rajiah, P.S.; et al. Mitral Annular Disease at Cardiac MRI: What to Know and Look For. RadioGraphics 2024, 44, e230156. [Google Scholar] [CrossRef]
- Han, Y.; Peters, D.C.; Salton, C.J.; Bzymek, D.; Nezafat, R.; Goddu, B.; Kissinger, K.V.; Zimetbaum, P.J.; Manning, W.J.; Yeon, S.B. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc. Imaging 2008, 1, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Catapano, F.; Moser, L.J.; Francone, M.; Catalano, C.; Vliegenthart, R.; Budde, R.P.J.; Salgado, R.; Hrabak Paar, M.; Pirnat, M.; Loewe, C.; et al. Competence of radiologists in cardiac CT and MR imaging in Europe: Insights from the ESCR Registry. Eur. Radiol. 2024, 34, 5666–5677. [Google Scholar] [CrossRef]
- Miguel-Dasit, A.; Martí-Bonmatí, L.; Sanfeliu, P.; Aleixandre, R. Cardiac MR imaging: Balanced publication by radiologists and cardiologists. Radiology 2007, 242, 410–416. [Google Scholar] [CrossRef]
- Doan, T.T.; Iturralde Chavez, A.; Valdes, S.O.; Weigand, J.D.; Wilkinson, J.C.; Parthiban, A.; Stephens, S.B.; Pignatelli, R.H.; Morris, S.A. Mitral annular disjunction and its progression during childhood in Marfan syndrome. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1306–1314. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Batista, R.; Yang, L.T.; Maalouf, J.; Thapa, P.; Asirvatham, S.; Michelena, H.I.; et al. The Mitral Annular Disjunction of Mitral Valve Prolapse: Presentation and Outcome. J. Am. Coll. Cardiol. 2020, 4, 2073–2087. [Google Scholar] [CrossRef]
- Niarchou, P.; Prappa, E.; Liatakis, I.; Vlachos, K.; Chatziantoniou, A.; Nyktari, E.; Tse, G.; Efremidis, M.; Letsas, K.P. Mitral Valve Prolapse and Mitral Annular Disjunction Arrhythmic Syndromes: Diagnosis, Risk Stratification and Management. Rev. Cardiovasc. Med. 2022, 5, 295. [Google Scholar] [CrossRef] [PubMed]
- Alfares, F.A.; Sohn, J.H.; Lee, Y.J.; Farrell, R.; Delling, F.N.; Avasarala, K.; Moon-Grady, A.J.; Anwar, S.; Austin, K.M. Mitral Annular Disjunction: An Under-Recognized Entity in Pediatrics. JACC Case Rep. 2024, 29, 102297. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Bruno, E.; Aquaro, G.D.; De Gori, C.; Barbieri, S.; Adami, M.; Plataroti, D.; Rondi, P.; di Meo, N.; Ravanelli, M.; et al. Prevalence of Mitral Annular Disjunction at Cardiac MRI: Results from a Multicenter Registry. Radiol. Cardiothorac. Imaging 2024, 6, e230428. [Google Scholar] [CrossRef] [PubMed]
- Fiore, G.; Rizza, V.; Ingallina, G.; Ancona, F.; Stella, S.; Biondi, F.; Cunsolo, P.; Gaspardone, C.; Romagnolo, D.; Tavernese, A.; et al. Prevalence of Diastolic and Systolic Mitral Annular Disjunction in Patients With Mitral Valve Prolapse. J. Am. Soc. Echocardiogr. 2025, 38, 1–11. [Google Scholar]
- Gatti, M.; Santonocito, A.; Papa, F.P.; D’aScenzo, F.; De Filippo, O.; Gallone, G.; Palmisano, A.; Pistelli, L.; De Ferrari, G.M.; Esposito, A.; et al. Role of cardiac magnetic resonance in stratifying arrhythmogenic risk in mitral valve prolapse patients: A systematic review and meta-analysis. Eur. Radiol. 2024, 34, 7321–7333. [Google Scholar]
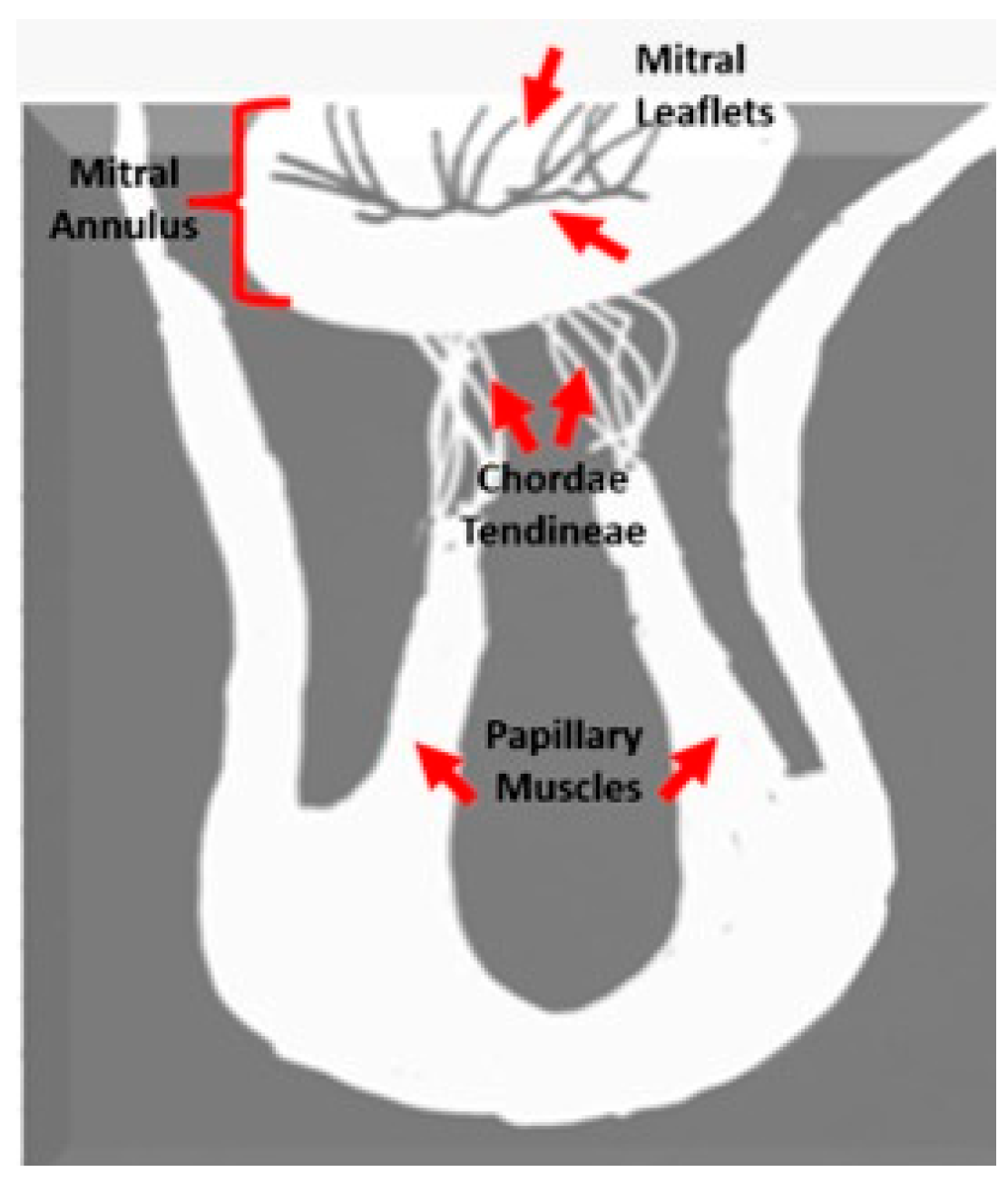



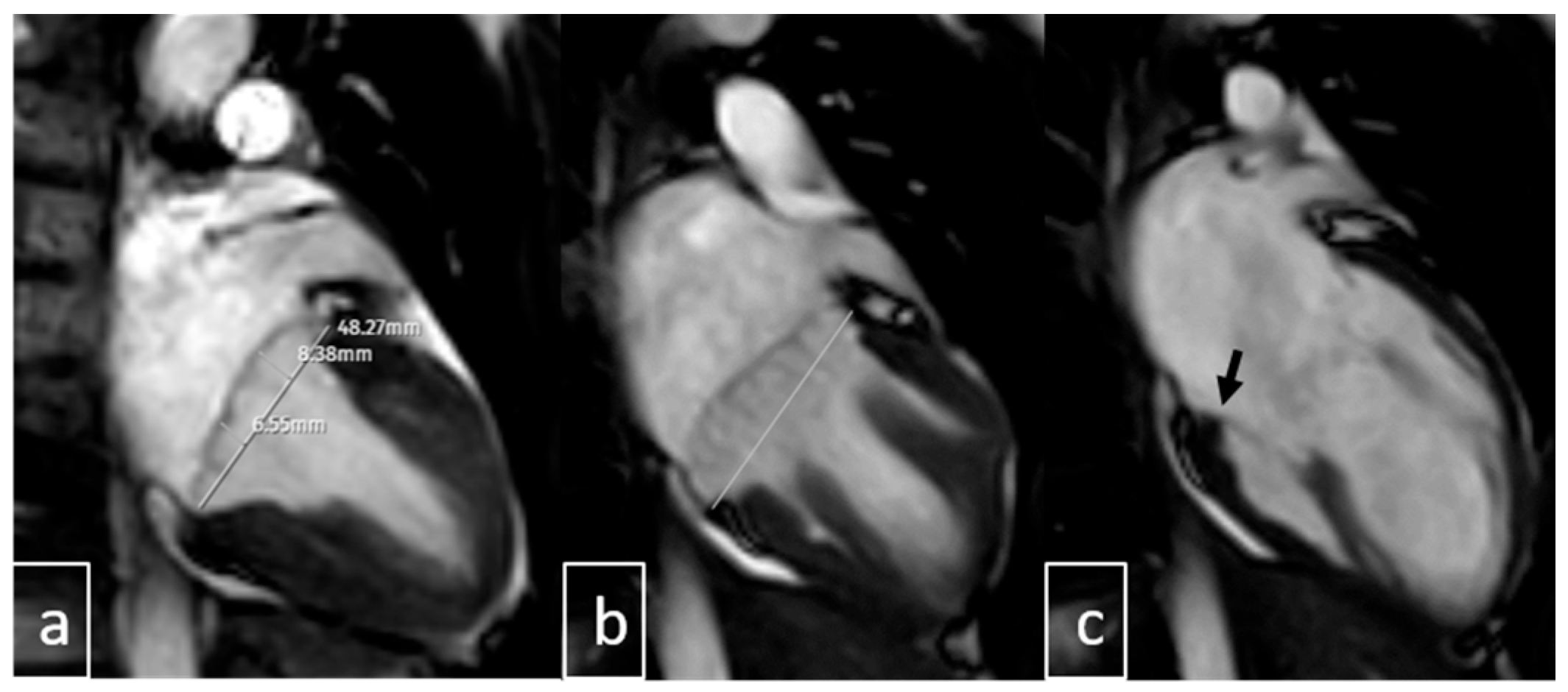
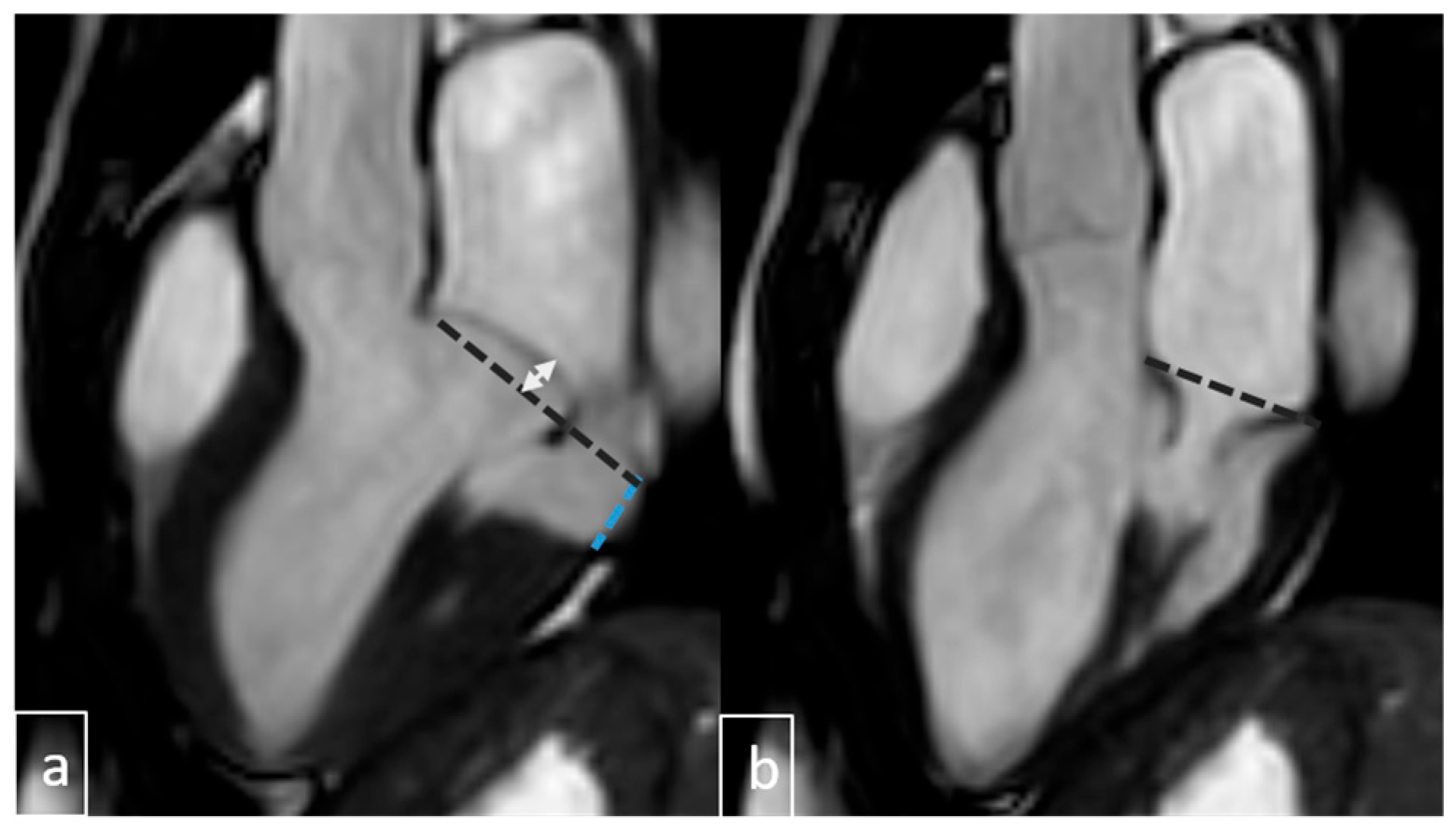
| Variable | Value (n = 237) |
|---|---|
| Age (years), mean ± SD | 14.13 ± 3.16 |
| Sex, n (%) | |
| Female | 96 (41%) |
| Male | 141 (59%) |
| Body surface area (m2), mean ± SD | 1.50 ± 0.28 |
| Presenting symptoms, n (%) | |
| Palpitations | 140 (59%) |
| Chest pain | 90 (38%) |
| Shortness of breath | 7 (3%) |
| Variable | n (%) |
|---|---|
| MAD | 22 (9.3) |
| MVP | 53 (22.4) |
| MR | 74 (31.2) |
| Variable | MAD, n (%) | MVP, n (%) | MR, n (%) |
|---|---|---|---|
| ECHO | 22 (9.3) | 53 (22.4) | 74 (31.2) |
| CMR | 55 (23.2) | 81 (34.2) | 36 (15.2) |
| Cohen’s kappa coefficient | 0.38 | 0.57 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz, Ş.; Ucan, B.; Bulut, H.; Özgür, S.; Yoldaş, T.; Altınbezer, P. Mitral Annular Disjunction Assessed Using Cardiac MR Imaging in Pediatric Patients. Diagnostics 2025, 15, 2857. https://doi.org/10.3390/diagnostics15222857
Yılmaz Ş, Ucan B, Bulut H, Özgür S, Yoldaş T, Altınbezer P. Mitral Annular Disjunction Assessed Using Cardiac MR Imaging in Pediatric Patients. Diagnostics. 2025; 15(22):2857. https://doi.org/10.3390/diagnostics15222857
Chicago/Turabian StyleYılmaz, Şükriye, Berna Ucan, Hasan Bulut, Senem Özgür, Tamer Yoldaş, and Pelin Altınbezer. 2025. "Mitral Annular Disjunction Assessed Using Cardiac MR Imaging in Pediatric Patients" Diagnostics 15, no. 22: 2857. https://doi.org/10.3390/diagnostics15222857
APA StyleYılmaz, Ş., Ucan, B., Bulut, H., Özgür, S., Yoldaş, T., & Altınbezer, P. (2025). Mitral Annular Disjunction Assessed Using Cardiac MR Imaging in Pediatric Patients. Diagnostics, 15(22), 2857. https://doi.org/10.3390/diagnostics15222857





