Global Myocardial Wall Thickness in Transfusion-Dependent Thalassemia: A Cross-Sectional MRI Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MRI
2.3. Biochemical Assays
2.4. Diagnostic Criteria
2.5. Statistical Analysis
3. Results
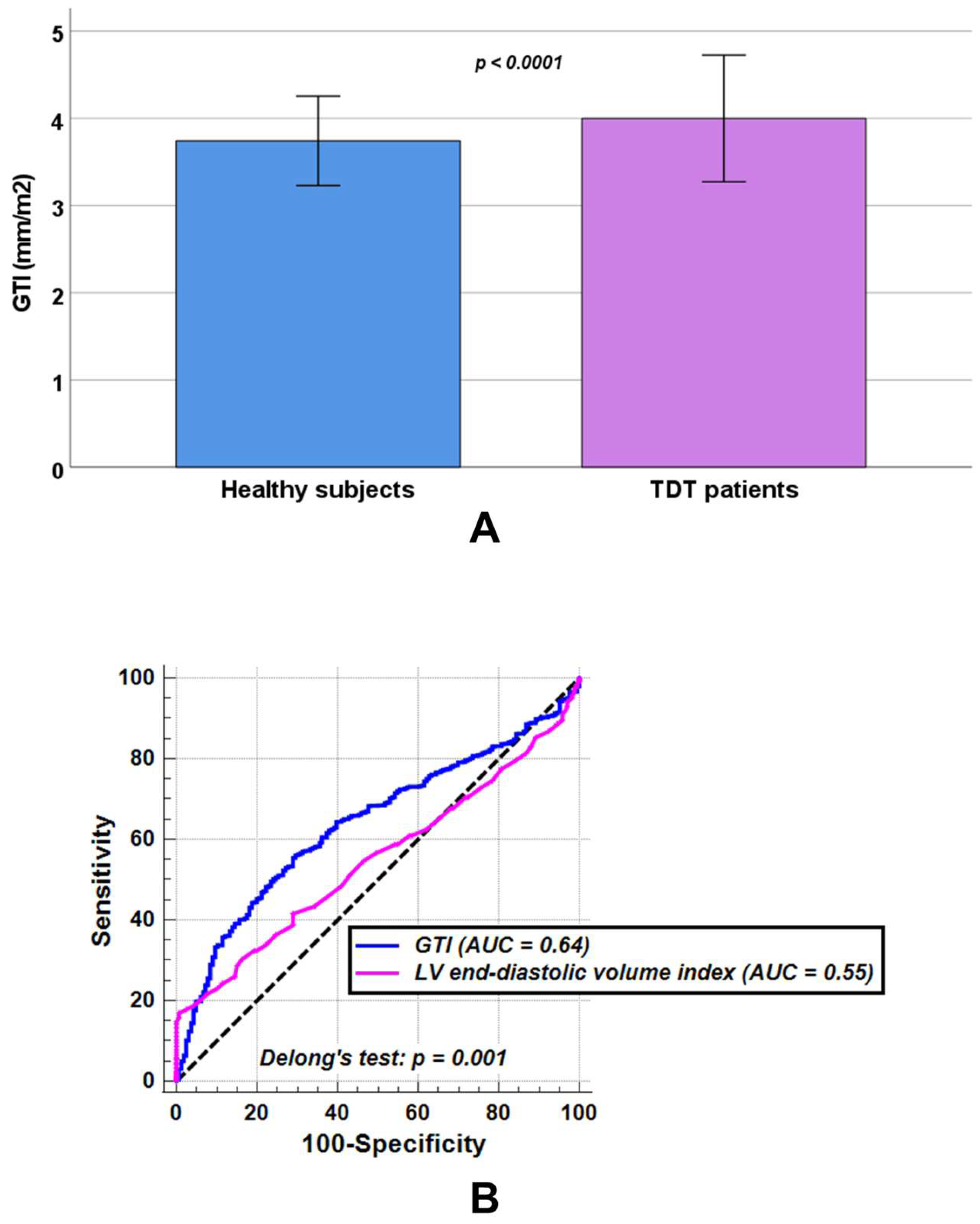
3.1. Comparison Between TDT Patients and Healthy Subjects
3.2. Associations Between Demographic/Clinical Characteristics and GTI in TDT
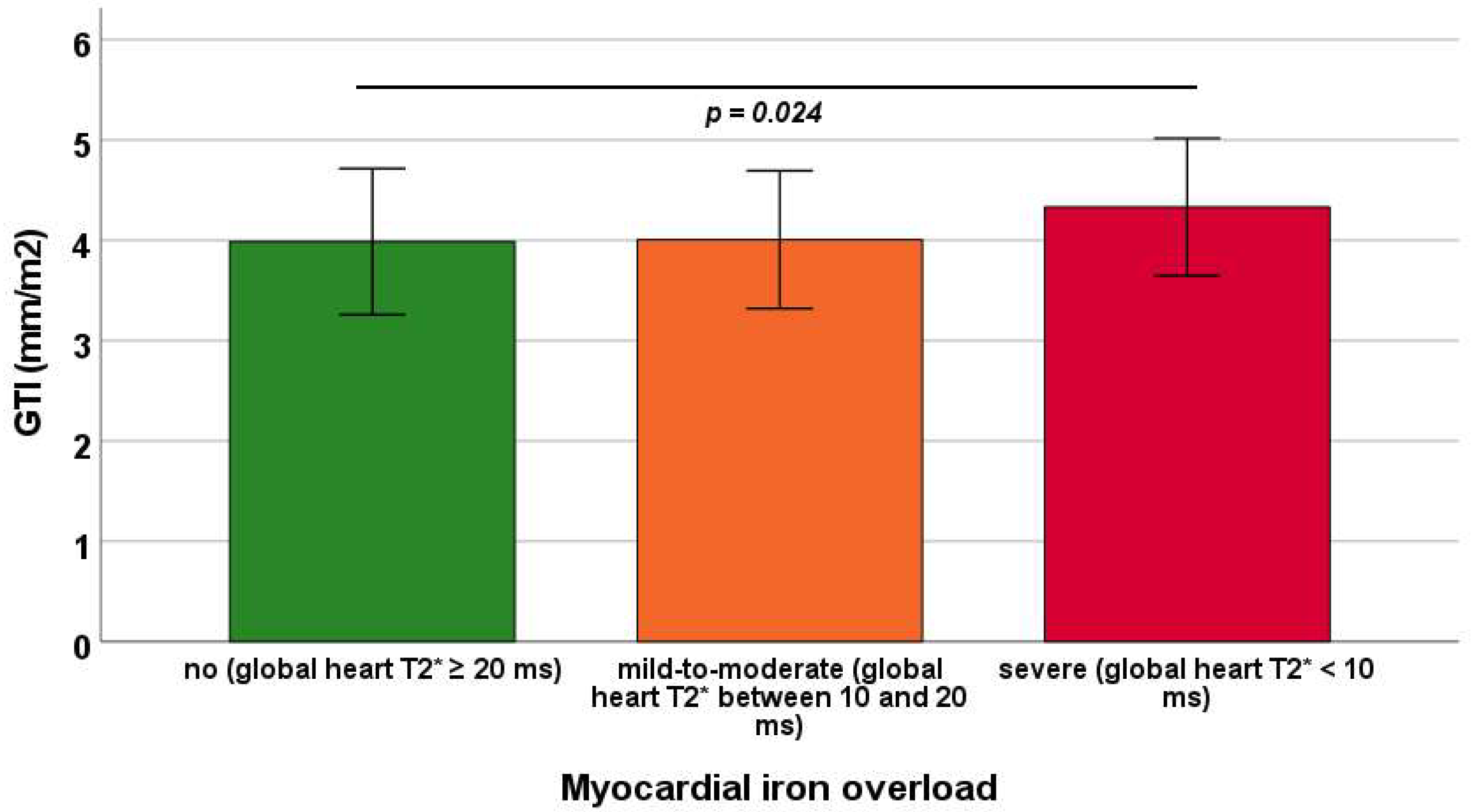
3.3. Associations Between GTI and Iron Overload in TDT
3.4. Predictors of GTI
3.5. Association of GTI with LV Function and Fibrosis
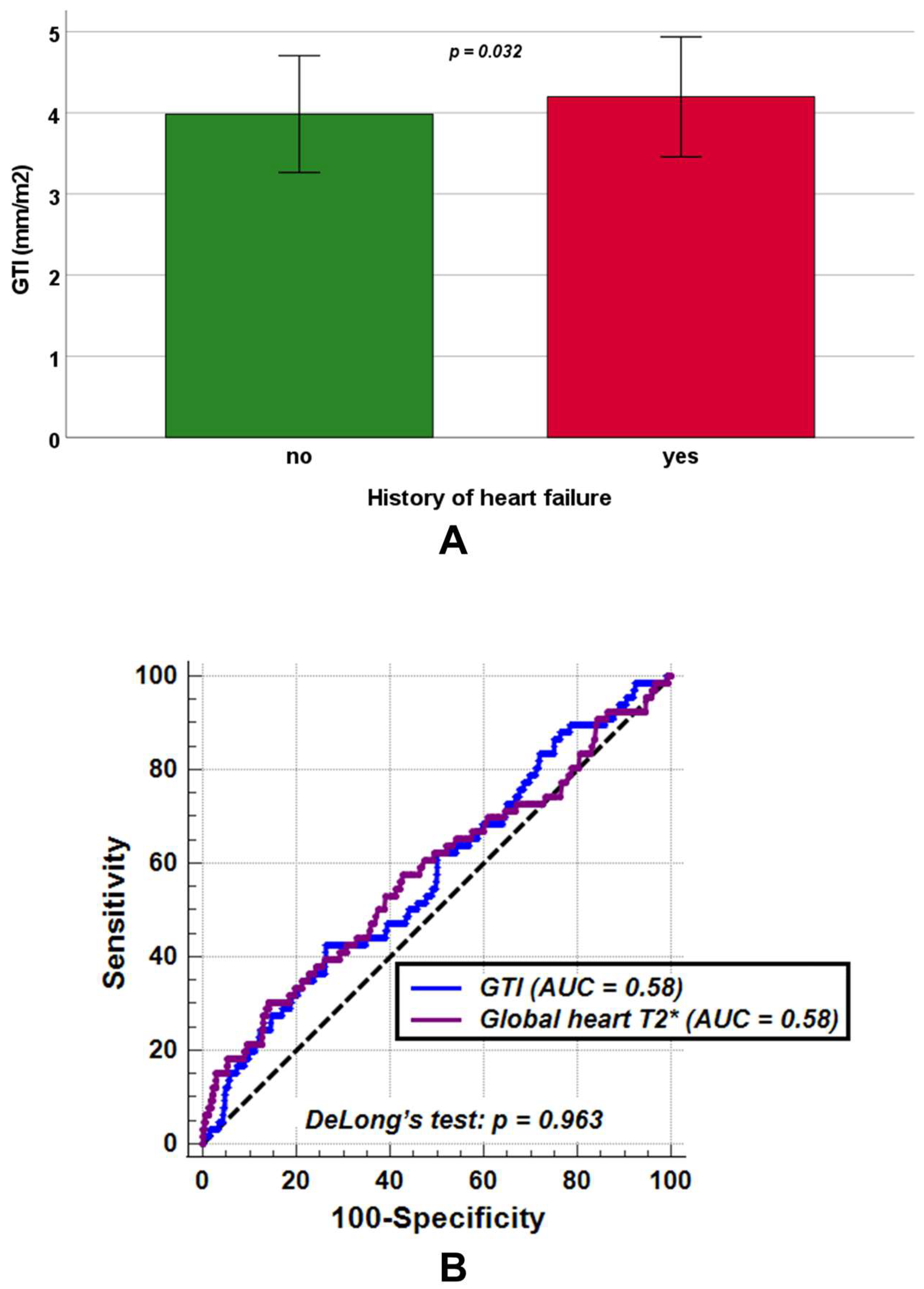
3.6. Association Between GTI and History of Heart Failure
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angastiniotis, M.; Lobitz, S. Thalassemias: An Overview. Int. J. Neonatal Screen. 2019, 5, 16. [Google Scholar] [CrossRef]
- Weatherall, D.J. Thalassemia as a global health problem: Recent progress toward its control in the developing countries. Ann. N. Y. Acad. Sci. 2010, 1202, 17–23. [Google Scholar] [CrossRef]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. beta-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef]
- El-Beshlawy, A.; Dewedar, H.; Hindawi, S.; Alkindi, S.; Tantawy, A.A.; Yassin, M.A.; Taher, A.T. Management of transfusion-dependent β-thalassemia (TDT): Expert insights and practical overview from the Middle East. Blood Rev. 2024, 63, 101138. [Google Scholar] [CrossRef]
- Forni, G.L.; Grazzini, G.; Boudreaux, J.; Agostini, V.; Omert, L. Global burden and unmet needs in the treatment of transfusion-dependent β-thalassemia. Front. Hematol. 2023, 2, 1187681. [Google Scholar] [CrossRef]
- Taher, A.T.; Farmakis, D.; Porter, J.B.; Cappellini, M.D.; Musallam, K.M. Guidelines for the Management of Transfusion-Dependent β-Thalassaemia, 5th ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2025. [Google Scholar]
- Origa, R. β-Thalassemia. Genet. Med. 2017, 19, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Giakoumis, A.; Angastiniotis, M.; Eleftheriou, A. The changing epidemiology of the ageing thalassaemia populations: A position statement of the Thalassaemia International Federation. Eur. J. Haematol. 2020, 105, 16–23. [Google Scholar] [CrossRef]
- Shander, A.; Cappellini, M.D.; Goodnough, L.T. Iron overload and toxicity: The hidden risk of multiple blood transfusions. Vox Sang. 2009, 97, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.T.; Sayani, F.; Trompeter, S.; Drasar, E.; Piga, A. Challenges of blood transfusions in β-thalassemia. Blood Rev. 2019, 37, 100588. [Google Scholar] [CrossRef]
- Yadav, P.K.; Singh, A.K. A Review of Iron Overload in Beta-Thalassemia Major, and a Discussion on Alternative Potent Iron Chelation Targets. Plasmatology 2022, 16, 26348535221103560. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Rugolotto, S.; De Stefano, P.; Zhao, H.; Cappellini, M.D.; Del Vecchio, G.C.; Romeo, M.A.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R.; et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004, 89, 1187–1193. [Google Scholar]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef]
- Pepe, A.; Pistoia, L.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Cecinati, V.; Maggio, A.; Sorrentino, F.; Filosa, A.; Rosso, R.; et al. National networking in rare diseases and reduction of cardiac burden in thalassemia major. Eur. Heart J. 2022, 43, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Recalcati, S.; Rybinska, I.; Buratti, P.; Cairo, G. Iron-induced damage in cardiomyopathy: Oxidative-dependent and independent mechanisms. Oxid. Med. Cell Longev. 2015, 2015, 230182. [Google Scholar] [CrossRef]
- Ahmed, S.; Peterson, S.J.; Parikh, M.A.; Frishman, W.H. Cardiovascular Manifestations of Hemochromatosis: A Review of Pathophysiology, Mechanisms, and Treatment Options. Cardiol. Rev. 2025, 33, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; El Dahdah, J.; Haroun, E.; Arockiam, A.D.; Safdar, A.; Sorathia, S.; Dong, T.; Griffin, B.; Wang, T.K.M. A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload. Hearts 2025, 6, 17. [Google Scholar] [CrossRef]
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. Beta-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Triposkiadis, F.; Lekakis, J.; Parissis, J. Heart failure in haemoglobinopathies: Pathophysiology, clinical phenotypes, and management. Eur. J. Heart Fail. 2017, 19, 479–489. [Google Scholar] [CrossRef]
- Akiki, N.; Hodroj, M.H.; Bou-Fakhredin, R.; Matli, K.; Taher, A.T. Cardiovascular Complications in β-Thalassemia: Getting to the Heart of It. Thalass. Rep. 2023, 13, 38–50. [Google Scholar] [CrossRef]
- Aessopos, A.; Berdoukas, V. Cardiac function and iron chelation in thalassemia major and intermedia: A review of the underlying pathophysiology and approach to chelation management. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009002. [Google Scholar] [CrossRef]
- Williams, A.M.; Levine, B.D.; Stembridge, M. A change of heart: Mechanisms of cardiac adaptation to acute and chronic hypoxia. J. Physiol. 2022, 600, 4089–4104. [Google Scholar] [CrossRef] [PubMed]
- Oni, O.O.; Adebiyi, A.A.; Aje, A.; Akingbola, T.S. Left ventricular geometry and electrocardiographic criteria in assessing left ventricular hypertrophy in sickle cell anemia patients. J. Natl. Med. Assoc. 2022, 114, 504–511. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Caruso, V.; Cuccia, L.; Spasiano, A.; Gerardi, C.; Zuccarelli, A.; D’Ascola, D.G.; Grimaldi, S.; et al. Cardiac complications and diabetes in thalassaemia major: A large historical multicentre study. Br. J. Haematol. 2013, 163, 520–527. [Google Scholar] [CrossRef]
- Barbero, U.; Ajassa, M.; Gaglioti, C.M.; Piga, A.; Ferrero, G.B.; Longo, F. The Influence of Cardiovascular Risk Factors and Hypogonadism on Cardiac Outcomes in an Aging Population of Beta-Thalassemia Patients. J. Cardiovasc. Dev. Dis. 2021, 9, 3. [Google Scholar] [CrossRef]
- Wood, J.C. Cardiac complications in thalassemia throughout the lifespan: Victories and challenges. Ann. N. Y. Acad. Sci. 2023, 1530, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A.; et al. Cardiovascular function and treatment in beta-thalassemia major: A consensus statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.I.; Kallifatidis, A.; Kourtidou, S.; Lama, N.; Christidi, A.; Detorakis, E.; Chatzantonis, G.; Vrachliotis, T.; Karamitsos, T.; Kouskouras, K.; et al. Cardiovascular magnetic resonance for the evaluation of patients with cardiovascular disease: An overview of current indications, limitations, and procedures. Hell. J. Cardiol. 2023, 70, 53–64. [Google Scholar] [CrossRef]
- Liu, C.; Ferrari, V.A.; Han, Y. Cardiovascular Magnetic Resonance Imaging and Heart Failure. Curr. Cardiol. Rep. 2021, 23, 35. [Google Scholar] [CrossRef]
- Merlo, M.; Gagno, G.; Baritussio, A.; Bauce, B.; Biagini, E.; Canepa, M.; Cipriani, A.; Castelletti, S.; Dellegrottaglie, S.; Guaricci, A.I.; et al. Clinical application of CMR in cardiomyopathies: Evolving concepts and techniques: A position paper of myocardial and pericardial diseases and cardiac magnetic resonance working groups of Italian society of cardiology. Heart Fail. Rev. 2023, 28, 77–95. [Google Scholar] [CrossRef]
- Azpiroz Franch, M.J.; Casas Masnou, G.; Romero, A.; Gonzalez Del Hoyo, M.I.; Larranaga Moreira, J.M.; Escalona Silva, R.; Guala, A.; Limeres Freire, J.; Bayes De Luna, A.; Zorio Grima, E.; et al. Prognostic role of cardiac magnetic resonance in left ventricular non compaction. Eur. Heart J. 2022, 43, ehac544.254. [Google Scholar] [CrossRef]
- Klem, I.; Shah, D.J.; White, R.D.; Pennell, D.J.; van Rossum, A.C.; Regenfus, M.; Sechtem, U.; Schvartzman, P.R.; Hunold, P.; Croisille, P.; et al. Prognostic Value of Routine Cardiac Magnetic Resonance Assessment of Left Ventricular Ejection Fraction and Myocardial Damage. Circ. Cardiovasc. Imaging 2011, 4, 610–619. [Google Scholar] [CrossRef]
- Boretto, P.; Patel, N.H.; Patel, K.; Rana, M.; Saglietto, A.; Soni, M.; Ahmad, M.; Sin Ying Ho, J.; De Filippo, O.; Providencia, R.A.; et al. Prognosis prediction in cardiac amyloidosis by cardiac magnetic resonance imaging: A systematic review with meta-analysis. Eur. Heart J. Open 2023, 3, oead092. [Google Scholar] [CrossRef]
- Dohy, Z.; Szabo, L.; Toth, A.; Czimbalmos, C.; Horvath, R.; Horvath, V.; Suhai, F.I.; Geller, L.; Merkely, B.; Vago, H. Prognostic significance of cardiac magnetic resonance-based markers in patients with hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2021, 37, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Kasa, G.; Teis, A.; Juncà, G.; Aimo, A.; Lupón, J.; Cediel, G.; Santiago-Vacas, E.; Codina, P.; Ferrer-Sistach, E.; Vallejo-Camazón, N.; et al. Clinical and prognostic implications of left ventricular dilatation in heart failure. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 849–856. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Midiri, M.; Missere, M.; Valeri, G.; Sorrentino, F.; D’Ascola, D.G.; Spasiano, A.; Filosa, A.; et al. Prediction of cardiac complications for thalassemia major in the widespread cardiac magnetic resonance era: A prospective multicentre study by a multi-parametric approach. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, P.L.M.; van de Ven, P.M.; Yoo, B.; Peace, R.A.; Heyndrickx, G.R.; Handly, N. Ejection fraction as related to basic components in the left and right ventricular volume domains. Int. J. Cardiol. 2018, 255, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. Ejection Fraction Pros and Cons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2360–2379. [Google Scholar] [CrossRef]
- Ojha, V.; Ganga, K.P.; Seth, T.; Roy, A.; Naik, N.; Jagia, P.; Gulati, G.S.; Kumar, S.; Sharma, S. Role of CMR feature-tracking derived left ventricular strain in predicting myocardial iron overload and assessing myocardial contractile dysfunction in patients with thalassemia major. Eur. Radiol. 2021, 31, 6184–6192. [Google Scholar] [CrossRef]
- Meloni, A.; Saba, L.; Positano, V.; Pistoia, L.; Campanella, A.; Spasiano, A.; Putti, M.C.; Fotzi, I.; Cossu, A.; Corigliano, E.; et al. Global longitudinal strain by cardiac magnetic resonance is associated with cardiac iron and complications in beta-thalassemia major patients. Int. J. Cardiol. 2024, 413, 132319. [Google Scholar] [CrossRef]
- Arenja, N.; Fritz, T.; Andre, F.; Riffel, J.H.; Aus dem Siepen, F.; Ochs, M.; Paffhausen, J.; Hegenbart, U.; Schönland, S.; Müller-Hennessen, M.; et al. Myocardial contraction fraction derived from cardiovascular magnetic resonance cine images-reference values and performance in patients with heart failure and left ventricular hypertrophy. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1414–1422. [Google Scholar] [CrossRef]
- Lu, D.Y.; Huang, W.M.; Wang, W.T.; Hung, S.C.; Sung, S.H.; Chen, C.H.; Yang, Y.J.; Niu, D.M.; Yu, W.C. Reduced global longitudinal strain as a marker for early detection of Fabry cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 487–495. [Google Scholar] [CrossRef]
- Saran, A.C.; Tormin, S.C.; Vogel, J.; Krakauer, R.; Salles, J.E.N. Left Ventricular Global Longitudinal Strain: An Early Marker of Diabetic Cardiomyopathy. ABC Imagem Cardiovasc. 2025, 38, e20240082. [Google Scholar] [CrossRef]
- Fischer, K.; Obrist, S.J.; Erne, S.A.; Stark, A.W.; Marggraf, M.; Kaneko, K.; Guensch, D.P.; Huber, A.T.; Greulich, S.; Aghayev, A.; et al. Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. JACC Cardiovasc. Imaging 2020, 13, 1891–1901. [Google Scholar] [CrossRef]
- Chadalavada, S.; Fung, K.; Rauseo, E.; Lee, A.M.; Khanji, M.Y.; Amir-Khalili, A.; Paiva, J.; Naderi, H.; Banik, S.; Chirvasa, M.; et al. Myocardial Strain Measured by Cardiac Magnetic Resonance Predicts Cardiovascular Morbidity and Death. J. Am. Coll. Cardiol. 2024, 84, 648–659. [Google Scholar] [CrossRef]
- Lundin, M.; Heiberg, E.; Nordlund, D.; Gyllenhammar, T.; Steding-Ehrenborg, K.; Engblom, H.; Carlsson, M.; Atar, D.; van der Pals, J.; Erlinge, D.; et al. Prognostic utility and characterization of left ventricular hypertrophy using global thickness. Sci. Rep. 2023, 13, 22806. [Google Scholar] [CrossRef]
- Meloni, A.; De Marchi, D.; Pistoia, L.; Grassedonio, E.; Peritore, G.; Preziosi, P.; Restaino, G.; Righi, R.; Riva, A.; Renne, S.; et al. Multicenter validation of the magnetic resonance T2* technique for quantification of pancreatic iron. Eur. Radiol. 2019, 29, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; Martini, N.; Positano, V.; D’Angelo, G.; Barison, A.; Todiere, G.; Grigoratos, C.; Barra, V.; Pistoia, L.; Gargani, L.; et al. Myocardial T1 Values at 1.5 T: Normal Values for General Electric Scanners and Sex-Related Differences. J. Magn. Reson. Imaging 2021, 54, 1486–1500. [Google Scholar] [CrossRef]
- Meloni, A.; Luciani, A.; Positano, V.; De Marchi, D.; Valeri, G.; Restaino, G.; Cracolici, E.; Caruso, V.; Dell’amico, M.C.; Favilli, B.; et al. Single region of interest versus multislice T2* MRI approach for the quantification of hepatic iron overload. J. Magn. Reson. Imaging 2011, 33, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; De Marchi, D.; Positano, V.; Neri, M.G.; Mangione, M.; Keilberg, P.; Lendini, M.; Cirotto, C.; Pepe, A. Accurate estimate of pancreatic T2* values: How to deal with fat infiltration. Abdom. Imaging 2015, 40, 3129–3136. [Google Scholar] [CrossRef]
- Meloni, A.; Restaino, G.; Borsellino, Z.; Caruso, V.; Spasiano, A.; Zuccarelli, A.; Valeri, G.; Toia, P.; Salvatori, C.; Positano, V.; et al. Different patterns of myocardial iron distribution by whole-heart T2* magnetic resonance as risk markers for heart complications in thalassemia major. Int. J. Cardiol. 2014, 177, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Enriquez, C.; Ghugre, N.; Tyzka, J.M.; Carson, S.; Nelson, M.D.; Coates, T.D. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005, 106, 1460–1465. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [PubMed]
- Meloni, A.; Righi, R.; Missere, M.; Renne, S.; Schicchi, N.; Gamberini, M.R.; Cuccia, L.; Lisi, R.; Spasiano, A.; Roberti, M.G.; et al. Biventricular Reference Values by Body Surface Area, Age, and Gender in a Large Cohort of Well-Treated Thalassemia Major Patients Without Heart Damage Using a Multiparametric CMR Approach. J. Magn. Reson. Imaging 2021, 53, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Moss, J.; Thisted, R. Predictors of body surface area. J. Clin. Anesth. 1992, 4, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V.; et al. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: A T2* magnetic resonance imaging study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.T.; Elsedfy, H.; Yaarubi, S.A.; Skordis, N.; Khater, D.; El Kholy, M.; Stoeva, I.; Fiscina, B.; Angastiniotis, M.; et al. The ICET-A Recommendations for the Diagnosis and Management of Disturbances of Glucose Homeostasis in Thalassemia Major Patients. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016058. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Vogel, M.; Anderson, L.J.; Holden, S.; Deanfield, J.E.; Pennell, D.J.; Walker, J.M. Tissue Doppler echocardiography in patients with thalassaemia detects early myocardial dysfunction related to myocardial iron overload. Eur. Heart J. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Liguori, C.; Pitocco, F.; Di Giampietro, I.; de Vivo, A.E.; Schena, E.; Cianciulli, P.; Zobel, B.B. Relationship between myocardial T2 values and cardiac volumetric and functional parameters in β-thalassemia patients evaluated by cardiac magnetic resonance in association with serum ferritin levels. Eur. J. Radiol. 2013, 82, e441–e447. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Frazer, D.M.; Bowden, D.K.; Anderson, G.J. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with β-thalassemia major: A longitudinal study. Blood 2013, 122, 124–133. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Tower, J.; Pomatto, L.C.D.; Davies, K.J.A. Sex differences in the response to oxidative and proteolytic stress. Redox Biol. 2020, 31, 101488. [Google Scholar] [CrossRef] [PubMed]
- Pennell, D.J.; Porter, J.B.; Cappellini, M.D.; Chan, L.L.; El-Beshlawy, A.; Aydinok, Y.; Ibrahim, H.; Li, C.K.; Viprakasit, V.; Elalfy, M.S.; et al. Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with beta-thalassemia major. Haematologica 2012, 97, 842–848. [Google Scholar] [CrossRef]
- Berdoukas, V.; Chouliaras, G.; Moraitis, P.; Zannikos, K.; Berdoussi, E.; Ladis, V. The efficacy of iron chelator regimes in reducing cardiac and hepatic iron in patients with thalassaemia major: A clinical observational study. J. Cardiovasc. Magn. Reson. 2009, 11, 20. [Google Scholar] [CrossRef]
- Pennell, D.J.; Porter, J.B.; Piga, A.; Lai, Y.R.; El-Beshlawy, A.; Elalfy, M.; Yesilipek, A.; Kilinc, Y.; Habr, D.; Musallam, K.M.; et al. Sustained improvements in myocardial T2* over 2 years in severely iron-overloaded patients with beta thalassemia major treated with deferasirox or deferoxamine. Am. J. Hematol. 2015, 90, 91–96. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Pistoia, L.; Cuccia, L.; Gamberini, M.R.; Lisi, R.; D’Ascola, D.G.; Rosso, R.; Allo, M.; Spasiano, A.; et al. MRI multicentre prospective survey in thalassaemia major patients treated with deferasirox versus deferiprone and desferrioxamine. Br. J. Haematol. 2018, 183, 783–795. [Google Scholar] [CrossRef]
- Entezari, S.; Haghi, S.M.; Norouzkhani, N.; Sahebnazar, B.; Vosoughian, F.; Akbarzadeh, D.; Islampanah, M.; Naghsh, N.; Abbasalizadeh, M.; Deravi, N. Iron Chelators in Treatment of Iron Overload. J. Toxicol. 2022, 2022, 4911205. [Google Scholar] [CrossRef] [PubMed]
- Origa, R.; Cinus, M.; Pilia, M.P.; Gianesin, B.; Zappu, A.; Orecchia, V.; Clemente, M.G.; Pitturru, C.; Denotti, A.R.; Corongiu, F.; et al. Safety and Efficacy of the New Combination Iron Chelation Regimens in Patients with Transfusion-Dependent Thalassemia and Severe Iron Overload. J. Clin. Med. 2022, 11, 2010. [Google Scholar] [CrossRef]
- Antohi, E.-L.; Chioncel, O. Understanding cardiac systolic performance beyond left ventricular ejection fraction. Explor. Med. 2020, 1, 75–84. [Google Scholar] [CrossRef]
- Batouty, N.M.; Tawfik, A.M.; Sobh, D.M.; Gadelhak, B.N.; El-Ashwah, S.; Hussein, M.A.; Gad, M.; Aziz, A.; El-Shahed, M.A.; Karam, R. Global and regional cardiac magnetic resonance feature tracking left ventricular strain analysis in assessing early myocardial disease in β thalassemia major patients. J. Cardiovasc. Imaging 2024, 32, 18. [Google Scholar] [CrossRef]
- Meloni, A.; Saba, L.; Positano, V.; Taccori, M.; Pistoia, L.; De Marco, E.; Sanna, P.M.G.; Longo, F.; Giovangrossi, P.; Gerardi, C.; et al. Left ventricular diastolic and systolic functions by cardiac magnetic resonance in beta-thalassemia major: Correlation with clinical findings and cardiac complications. Int. J. Cardiovasc. Imaging 2025, 41, 847–857. [Google Scholar] [CrossRef]
- Meloni, A.; Martini, N.; Positano, V.; De Luca, A.; Pistoia, L.; Sbragi, S.; Spasiano, A.; Casini, T.; Bitti, P.P.; Allò, M.; et al. Myocardial iron overload by cardiovascular magnetic resonance native segmental T1 mapping: A sensitive approach that correlates with cardiac complications. J. Cardiovasc. Magn. Reson. 2021, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [PubMed]
- Ferreira, V.M.; Piechnik, S.K. CMR Parametric Mapping as a Tool for Myocardial Tissue Characterization. Korean Circ. J. 2020, 50, 658–676. [Google Scholar] [CrossRef] [PubMed]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.R.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 63, 447–456. [Google Scholar] [CrossRef]
- Xu, J.; Yang, W.; Zhao, S.; Lu, M. State-of-the-art myocardial strain by CMR feature tracking: Clinical applications and future perspectives. Eur. Radiol. 2022, 32, 5424–5435. [Google Scholar] [CrossRef]
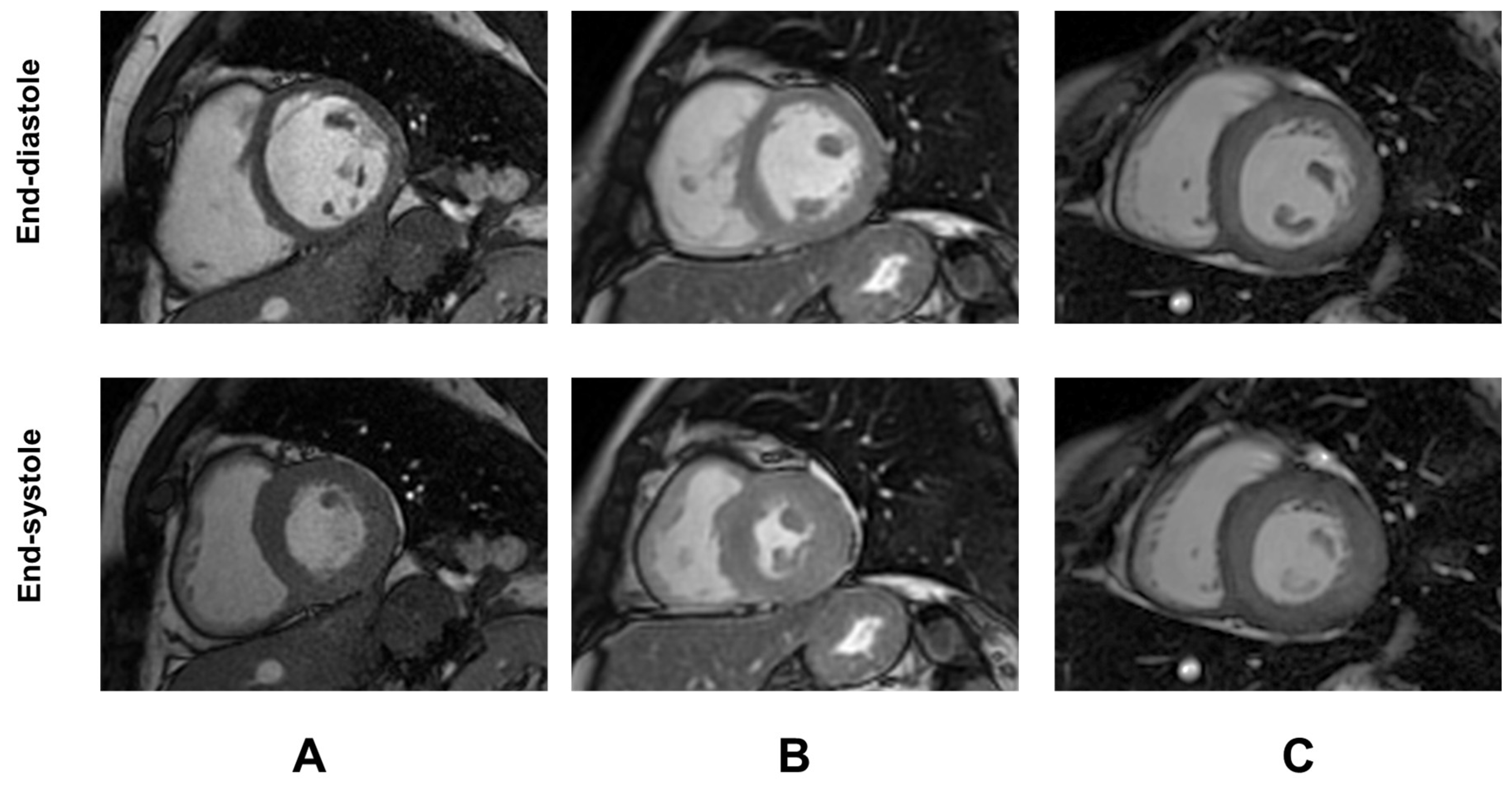
| Variable | TDT Patients (N = 1154) |
|---|---|
| Females, N (%) | 611 (52.9) |
| Age (years) | 37.46 ± 10.67 |
| Transfusion starting age (months) | 14.98 ± 15.37 |
| Chelation starting age (years) | 5.23 ± 6.05 |
| Splenectomy, N (%) | 650 (56.3) |
| Pre-transfusion hemoglobin (g/dL) | 9.66 ± 0.53 |
| Serum ferritin (ngm/L) | 1062.43 ± 1325.07 |
| Diabetes, N (%) | 174/1105 (15.7) |
| MRI LIC (mg/g dw) | 6.38 ± 9.73 |
| Global pancreas T2* (ms) | 12.76 ± 10.35 |
| Global heart T2* (ms) | 37.08 ± 9.61 |
| MIO, N (%) | |
| no | 1055 (91.4) |
| moderate-to-mild | 64 (5.6) |
| severe | 35 (3.0) |
| Number of segments with T2* < 20 ms | 1.58 ± 4.19 |
| LV end-diastolic volume index (mL/m2) | 82.48 ± 16.96 |
| LV end-systolic volume index (mL/m2) | 31.57 ± 10.81 |
| LV stroke volume index (mL/m2) | 51.39 ± 10.58 |
| LV mass index (g/m2) | 54.91 ± 13.69 |
| LV ejection fraction (%) | 62.32 ± 6.99 |
| Replacement myocardial fibrosis, N (%) | 95/366 (26.0) |
| GTI (mm/m2) | 3.99 ± 0.73 |
| Univariable Regression | Multivariable Regression | |||
|---|---|---|---|---|
| B (95% CIs) | p-Value | B (95% CIs) | p-Value | |
| Male sex | 0.111 (0.027 to 0.195) | 0.009 | 0.119 (0.036 to 0.203) | 0.005 |
| Age > 75th percentile | 0.021 (−0.076 to 0.118) | 0.671 | ||
| Splenectomy | −0.017 (−0.102 to 0.067) | 0.687 | ||
| Diabetes | 0.106 (−0.010 to 0.222) | 0.072 | ||
| Severe MIO | 0.344 (0.100 to 0.588) | 0.006 | 0.367 (0.123 to 0.611) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Pistoia, L.; Peritore, G.; Zerbini, M.; Renne, S.; Fina, P.; Vallone, A.; Longo, F.; Spasiano, A.; Borsellino, Z.; et al. Global Myocardial Wall Thickness in Transfusion-Dependent Thalassemia: A Cross-Sectional MRI Analysis. Diagnostics 2025, 15, 2805. https://doi.org/10.3390/diagnostics15212805
Meloni A, Pistoia L, Peritore G, Zerbini M, Renne S, Fina P, Vallone A, Longo F, Spasiano A, Borsellino Z, et al. Global Myocardial Wall Thickness in Transfusion-Dependent Thalassemia: A Cross-Sectional MRI Analysis. Diagnostics. 2025; 15(21):2805. https://doi.org/10.3390/diagnostics15212805
Chicago/Turabian StyleMeloni, Antonella, Laura Pistoia, Giuseppe Peritore, Michela Zerbini, Stefania Renne, Priscilla Fina, Antonino Vallone, Filomena Longo, Anna Spasiano, Zelia Borsellino, and et al. 2025. "Global Myocardial Wall Thickness in Transfusion-Dependent Thalassemia: A Cross-Sectional MRI Analysis" Diagnostics 15, no. 21: 2805. https://doi.org/10.3390/diagnostics15212805
APA StyleMeloni, A., Pistoia, L., Peritore, G., Zerbini, M., Renne, S., Fina, P., Vallone, A., Longo, F., Spasiano, A., Borsellino, Z., Cecinati, V., Messina, G., Corigliano, E., Positano, V., Barison, A., & Clemente, A. (2025). Global Myocardial Wall Thickness in Transfusion-Dependent Thalassemia: A Cross-Sectional MRI Analysis. Diagnostics, 15(21), 2805. https://doi.org/10.3390/diagnostics15212805









