Prevalence of the Sphenoidal Emissary Foramen in a Chilean Osteological Sample: Anatomical and Surgical Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
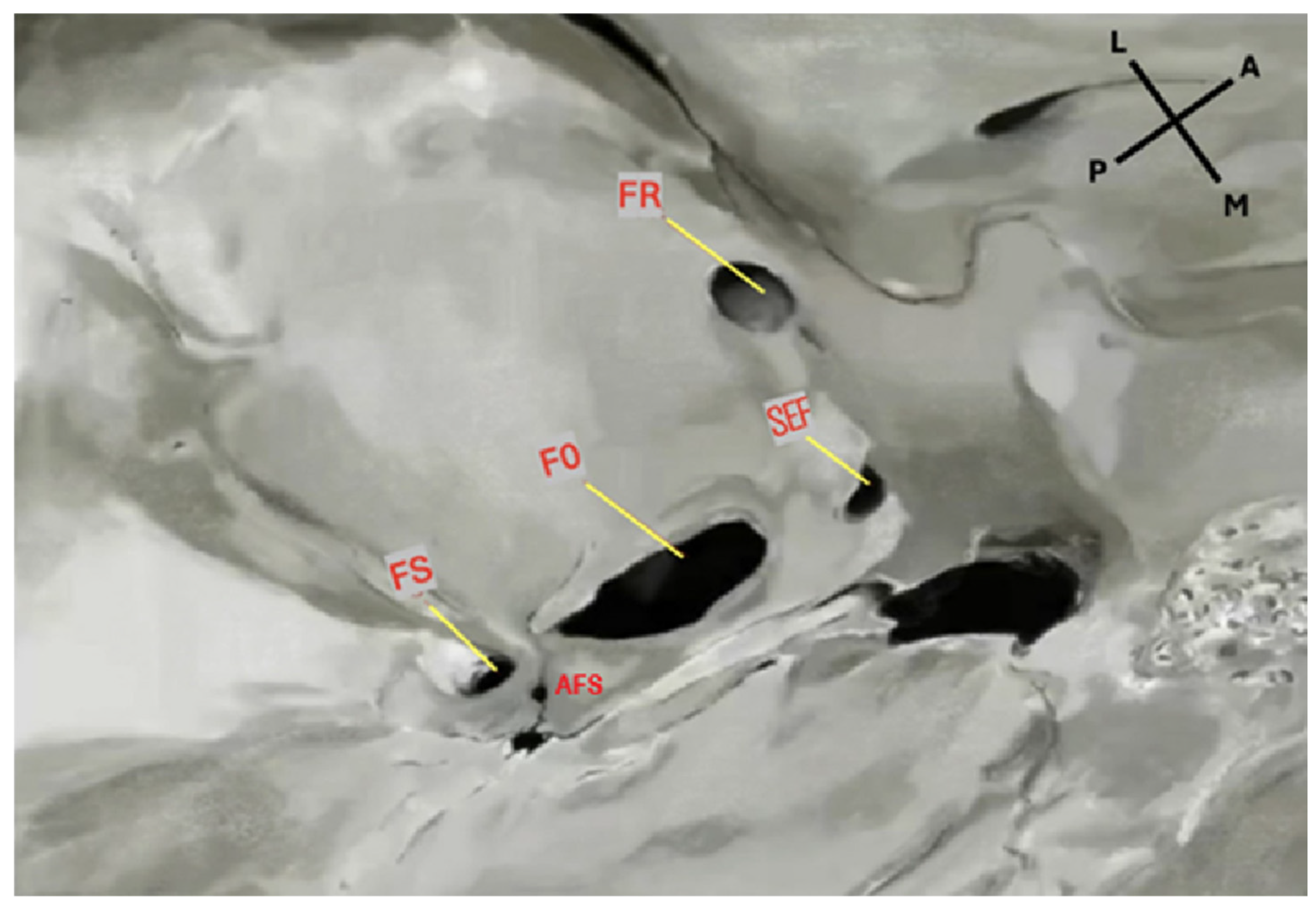
2.3. Assessments
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Comparison with Previous Studies
4.2. Clinical and Anatomical Implications
4.3. Geographic and Methodological Considerations
4.4. Methodological Impact
4.5. Study Strengths
4.6. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, J. Clinical Anatomy of the Head: Neurocranium, Orbit and Craniocervical Region; Springer Nature: Durham, NC, USA, 1991; Volume 34, pp. 1–9. [Google Scholar] [CrossRef]
- Rhoton, A.L., Jr. The foramen ovale. Neurosurgery 2007, 61 (Suppl. S1), 37–43. [Google Scholar] [CrossRef]
- Osunwoke, E.A.; Mbadugha, C.C.; Gwunireama, I.U.; Orish, C.N. Morphometric analysis of foramen ovale and foramen spinosum of the human sphenoid bone in southern Nigerian population. J. Appl. Biosci. 2010, 26, 1631–1635. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Hill, M.; May, W.R.; Middlebrooks, E.; Kominek, S.Z.; Marchase, N.; Shoja, M.M.; Loukas, M.; Oakes, W.J. Does the maxillary division of the trigeminal nerve traverse the cavernous sinus? An anatomical study and review of the literature. Surg. Radiol. Anat. 2008, 30, 37–40. [Google Scholar] [CrossRef]
- O′Connor, K.P.; Pelargos, P.E.; Palejwala, A.H.; Shi, H.; Villeneuve, L.; Glenn, C.A. Resection of Pediatric Trigeminal Schwannoma Using Minimally Invasive Approach: Case Report, Literature Review, and Operative Video. World Neurosurg. 2019, 127, 518–524. [Google Scholar] [CrossRef]
- Cochinski, R.; Agarwal, M.; Albuquerque, J.; de Almeida, C.A.; Stricker, R.P.; Uberti, M.F.; Casqueiro, A.P.K.; Mendonça, G.S.; Nascimento, G.R.S.D.; Miraldi, F.; et al. Anatomy and Diseases of the Greater Wings of the Sphenoid Bone. Radiographics 2022, 42, 1177–1195. [Google Scholar] [CrossRef] [PubMed]
- Palamenghi, A.; Cellina, M.; Cè, M.; Cappella, A.; Sforza, C.; Gibelli, D. Correlation Analysis on Anatomical Variants of Accessory Foramina in the Sphenoid Bone for Oncological Surgery. Cancers 2023, 15, 5341. [Google Scholar] [CrossRef]
- James, T.M.; Presley, R.; Steel, F.L.D. The foramen ovale and sphenoidal angle in man. Anat. Embryol. 1980, 160, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, S.D.; Kurşun-Çakmak, E.Ş.D.; Atakan, C.; Orhan, K.D. Anatomic Study on Sphenoidal Emissary Foramen by Using Cone-Beam Computed Tomography. J. Craniofac. Surg. 2018, 29, e477–e480. [Google Scholar] [CrossRef]
- Görürgöz, C.; Paksoy, C.S. Morphology and morphometry of the foramen venosum: A radiographic study of CBCT images and literature review. Surg. Radiol. Anat. 2020, 42, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Chaisuksunt, V.; Kwathai, L.; Namonta, K.; Rungruang, T.; Apinhasmit, W.; Chompoopong, S. Occurrence of the foramen of Vesalius and its morphometry relevant to clinical consideration. Sci. World J. 2012, 2012, 817454. [Google Scholar] [CrossRef]
- Alves, N. Anatomical study of the Foramen Venosum and its clinical implications. J. Anat. Soc. India 2017, 3, 101–112. [Google Scholar] [CrossRef]
- Ozer, M.A.; Govsa, F. Measurement accuracy of foramen of vesalius for safe percutaneous techniques using computer-assisted three-dimensional landmarks. Surg. Radiol. Anat. 2014, 36, 147–154. [Google Scholar] [CrossRef]
- Aviles-Solis, J.C.; Olvera-Barrios, A.; De la Garza Castro, O.; Elizondo-Omaña, R.E.; Guzmán-López, S. Prevalencia y Características Morfométricas del Foramen Venoso en Cráneos del Noreste de México. Int. J. Morphol. 2011, 29, 158–163. [Google Scholar] [CrossRef]
- Lazarus, L.; Naidoo, N.; Satyapal, K.S. An Osteometric Evaluation of the Foramen Spinosum and Venosum. Int. J. Morphol. 2015, 33, 452–458. [Google Scholar] [CrossRef]
- Shinohara, A.L.; de Souza Melo, C.G.; Silveira, E.M.V.; Lauris, J.R.P.; Andreo, J.C.; de Castro Rodrigues, A. Incidence, morphology and morphometry of the foramen of Vesalius: Complementary study for a safer planning and execution of the trigeminal rhizotomy technique. Surg. Radiol. Anat. 2010, 32, 159–164. [Google Scholar] [CrossRef]
- Leonel, L.C.P.C.; Peris-Celda, M.; de Sousa, S.D.G.; Haetinger, R.G.; Liberti, E.A. The sphenoidal emissary foramen and the emissary vein: Anatomy and clinical relevance. Clin. Anat. 2020, 33, 767–781. [Google Scholar] [CrossRef]
- Unver, D.N.; Fazliogullari, Z.; Uysal, I.I.; Seker, M.; Karabulut, A.K. Anatomical Examination of the Foramens of the Middle Cranial Fossa. Int. J. Morphol. 2014, 32, 43–48. [Google Scholar] [CrossRef]
- Raval, B.B.; Singh, P.R.; Rajguru, J. A morphologic and morphometric study of foramen vesalius in dry adult human skulls of gujarat region. J. Clin. Diagn. Res. 2015, 9, AC04–AC07. [Google Scholar] [CrossRef]
- Toledo, J.; Silva de Lima, M.; Moreira, C.; Rodrigues, C.; Magalhães, D. Foramen Venosum: Prevalence, Patency and Correlation with Cephalic Index. Int. J. Morphol. 2016, 34, 1328–1332. [Google Scholar] [CrossRef]
- Kaplan, M.; Erol, F.S.; Ozveren, M.F.; Topsakal, C.; Sam, B.; Tekdemir, I. Review of complications due to foramen ovale puncture. J. Clin. Neurosci. 2007, 14, 563–568. [Google Scholar] [CrossRef]
- Yeo, G.S.; Kim, H.Y.; Kwak, E.J.; Jung, Y.S.; Park, H.S.; Jung, H.D. Cavernous sinus thrombosis caused by a dental infection: A case report. J. Korean Assoc. Oral. Maxillofac. Surg. 2014, 40, 195–198, Erratum in J. Korean Assoc. Oral. Maxillofac. Surg. 2014, 40, 258. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.; Standring, S.; Ellis, H. Gray’s Anatomy, 41st ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 27; p. 422. [Google Scholar]
- Lanzieri, C.F.; Duchesneau, P.M.; Rosenbloom, S.A.; Smith, A.S.; Rosenbaum, A.E. The significance of asymmetry of the foramen of Vesalius. AJNR Am. J. Neuroradiol. 1988, 9, 1201–1204. [Google Scholar]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S. An abnormal foramen connecting the middle cranial fossa with sphenoidal air sinus: A case report. Internet J. Biol. Anthropol. 2008, 2. [Google Scholar][Green Version]
- Parveen, N.; Singh, S.; Mishra, S. Foramen venosum: A clinicoanatomic insight into its occurrence and morphometry. Surg. Radiol. Anat. 2023, 45, 409–415. [Google Scholar] [CrossRef]
- Peper, C.; Iwanaga, J.; Dumont, A.S.; Tubbs, R.S. A giant foramen of Vesalius: Case report. Anat. Cell Biol. 2022, 55, 373–375. [Google Scholar] [CrossRef]
- Triantafyllou, G.; Papadopoulos-Manolarakis, P.; Tsakotos, G.; Piagkou, M. Concomitant Sphenopterygoid Canal and Duplicated Foramen Rotundum Detected on Computed Tomography Scan. J Craniofac. Surg. 2025, 36, 1381–1382. [Google Scholar] [CrossRef]
- Kodialbail, A.; Mudaliar, R.P.; Chandrachari, J.K.; Shetty, S. Morphometry of the Foramen Ovale in Adult Human Skulls from a Clinical Perspective. Kurume Med. J. 2024, 70, 91–95. [Google Scholar] [CrossRef]
- Ginsberg, L.E.; Pruett, S.W.; Chen, M.Y.; Elster, A.D. Skull-base foramina of the middle cranial fossa: Reassessment of normal variation with high-resolution CT. AJNR Am. J. Neuroradiol. 1994, 15, 283–291. [Google Scholar]
- Abdelghani, N.; Barut, C.; Ogut, E. The investigation of cranial fossae in the intracranial cavity of fixed cadaveric skull bases: Associations with sex, laterality, and clinical significance. Surg. Radiol. Anat. 2024, 46, 1305–1329. [Google Scholar] [CrossRef] [PubMed]
- Al-Shuaili, A.; Al-Ajmi, E.; Mogali, S.R.; Al-Qasmi, S.; Al-Mufargi, Y.; Kariyattil, R.; Sirasanagandla, S.R. Computed-tomography evaluation of parietal foramen topography in adults: A retrospective analysis. Surg. Radiol. Anat. 2024, 46, 263–270. [Google Scholar] [CrossRef]
- Park, C.K.; Park, B.J. Surgical Treatment for Trigeminal Neuralgia. J. Korean Neurosurg. Soc. 2022, 65, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, S.; Cao, D.; Luo, C.; Liang, Z.; Liang, S.; Zhang, G.; Zhao, Q.; Ruan, G.; Liu, L.; et al. Prognostic Value of Skull Base Foramen Invasion Subclassification in T Category Modification and Induction Chemotherapy Management for Nasopharyngeal Carcinoma: Post-Hoc Analysis of a Dual-Center Retrospective Cohort Study. Adv. Sci. 2025, 12, e2408182. [Google Scholar] [CrossRef] [PubMed]
- Piagkou, M.; Kostares, M.; Duparc, F.; Papanagiotou, P.; Politis, C.; Tsakotos, G.; Pantazis, N.; Natsis, K. The sphenoidal emissary foramina prevalence: A meta-analysis of 6369 subjects. Surg. Radiol. Anat. 2023, 45, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wysiadecki, G.; Varga, I.; Klejbor, I.; Balawender, K.; Ghosh, S.K.; Clarke, E.; Koziej, M.; Bonczar, M.; Ostrowski, P.; Żytkowski, A. Reporting anatomical variations: Should unified standards and protocol (checklist) for anatomical studies and case reports be established? Transl. Res. Anat. 2024, 35, 100284. [Google Scholar] [CrossRef]


| Author | Year | Country | Sample/Study Type | Frequency % | ||||
|---|---|---|---|---|---|---|---|---|
| Total (%) | Bilateral | Unilateral | ||||||
| T | L | R | ||||||
| Shinohara et al. [16] | 2010 | Brazil | 400 MS | 33.75 | 15.5 | 18.25 | 10.55 | 7.75 |
| Aviles et al. [14] | 2011 | Mexico | 25 DS | 20.0 | 4.0 | 16.0 | 8.0 | 8.0 |
| Chaisuksunt et al. [11] | 2012 | Thailand | 377 DS | 16.1 | 4.2 | 11.9 | 8.2 | 3.7 |
| Ozer et al. [13] | 2014 | Turkey | 172 DS | 34.8 | 9.3 | 25.5 | 15.1 | 10.4 |
| Lazarus et al. [15] | 2015 | South Africa | 200 DS | 5.0 | - | - | - | - |
| Alves et al. [12] | 2017 | Brazil | 178 MS | 32.0 | 23.06 | 8.46 | 4.21 | 4.21 |
| Bayrak et al. [9] | 2018 | Turkey | 317 CBCTs | 28.1 | 6.9 | 21.1 | 9.8 | 11.3 |
| Görürgöz et al. [10] | 2020 | Turkey | 260 CBCTs | 73.1 | 42.3 | 30.8 | 16.2 | 14.65 |
| Leonel et al. [17] | 2020 | Brazil | 1000 CTs | 46.8 | 25.4 | 21.4 | 9.6 | 11.8 |
| 170 DS | 45.2 | 18.8 | 26.4 | 14.7 | 11.7 | |||
| 50 cadavers | 14.0 | 6.0 | 8.0 | 4.0 | 4.0 | |||
| Palamenghi et al. [7] | 2023 | Italy | 300 CTs | 67.7 | - | - | - | - |
| Unver et al. [18] | 2014 | Turkey | 44 DS & 18 cadavers | 32.3 (31.8 & 33.3) | - | - | - | - |
| Raval et al. [19] | 2015 | Western India | 150 DS | 60.0 | 32.23 | 35.56 | 20.0 | 12.0 |
| Toledo et al. [20] | 2016 | Brazil | 84 DS | 41.6 | 16.6 | - | - | - |
| Present Study | 2025 | Chile | 133 DS | 40.17 | 26.79 | 13.38 | 9.12 | 4.26 |
| SEF Occurrence Type | Frequency (n) | Percentage (%) | p-Value | Effect Size |
|---|---|---|---|---|
| (Cramer’s V, 95% CI) | ||||
| Bilateral SEF | 36 | 26.79 | 0.012 | 0.21 (0.05–0.36) |
| Unilateral SEF (Left) | 12 | 9.12 | 0.03 | 0.19 (0.02–0.33) |
| Unilateral SEF (Right) | 6 | 4.26 | - | - |
| SEF Absent | 79 | 59.83 | - | - |
| Total | 133 | 100 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela Fuenzalida, J.J.; Alcaíno Adasme, C.; Soublette Tocornal, T.; Alvial-Ahumada, F.; Perez Gutierrez, M.; Bruna-Mejias, A.; Orellana-Donoso, M.; Nova-Baeza, P.; Suazo-Santibañez, A.; Gutierrez-Espinoza, H.; et al. Prevalence of the Sphenoidal Emissary Foramen in a Chilean Osteological Sample: Anatomical and Surgical Implications. Diagnostics 2025, 15, 2800. https://doi.org/10.3390/diagnostics15212800
Valenzuela Fuenzalida JJ, Alcaíno Adasme C, Soublette Tocornal T, Alvial-Ahumada F, Perez Gutierrez M, Bruna-Mejias A, Orellana-Donoso M, Nova-Baeza P, Suazo-Santibañez A, Gutierrez-Espinoza H, et al. Prevalence of the Sphenoidal Emissary Foramen in a Chilean Osteological Sample: Anatomical and Surgical Implications. Diagnostics. 2025; 15(21):2800. https://doi.org/10.3390/diagnostics15212800
Chicago/Turabian StyleValenzuela Fuenzalida, Juan José, Catalina Alcaíno Adasme, Trinidad Soublette Tocornal, Felipe Alvial-Ahumada, Macarena Perez Gutierrez, Alejandro Bruna-Mejias, Mathias Orellana-Donoso, Pablo Nova-Baeza, Alejandra Suazo-Santibañez, Hector Gutierrez-Espinoza, and et al. 2025. "Prevalence of the Sphenoidal Emissary Foramen in a Chilean Osteological Sample: Anatomical and Surgical Implications" Diagnostics 15, no. 21: 2800. https://doi.org/10.3390/diagnostics15212800
APA StyleValenzuela Fuenzalida, J. J., Alcaíno Adasme, C., Soublette Tocornal, T., Alvial-Ahumada, F., Perez Gutierrez, M., Bruna-Mejias, A., Orellana-Donoso, M., Nova-Baeza, P., Suazo-Santibañez, A., Gutierrez-Espinoza, H., Sanchis-Gimeno, J., Piagkou, M., Triantafyllou, G., Samolis, A., & León-Rojas, J. E. (2025). Prevalence of the Sphenoidal Emissary Foramen in a Chilean Osteological Sample: Anatomical and Surgical Implications. Diagnostics, 15(21), 2800. https://doi.org/10.3390/diagnostics15212800










