Comparison of Preoperative Magnetic Resonance Imaging and Intraoperative Frozen Section Analysis with Final Pathological Outcomes in the Assessment of Myometrial Invasion in Endometrial Cancer Cases
Abstract
1. Introduction
2. Methods
2.1. Study Population and Patient Selection
2.2. MRI Protocol
2.3. Intraoperative Frozen Section Analysis and Definitive Histopathological Evaluation Protocol
2.4. Statistical Analysis
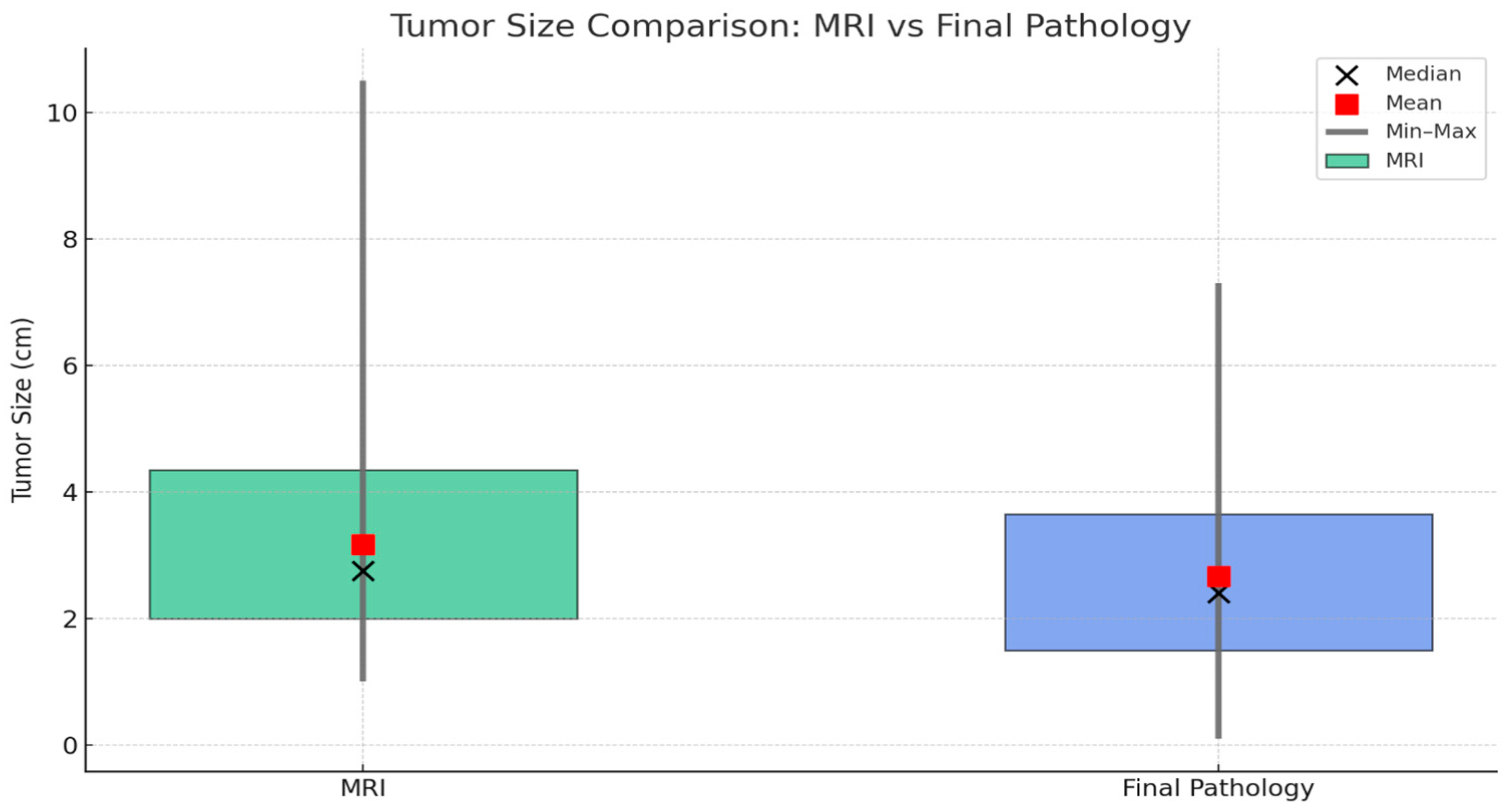
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| EC | Endometrial Cancer |
| ESGO | European Society of Gynecological Oncology |
| ESP | European Society of Pathology |
| ESTRO | European Society for Radiotherapy and Oncology |
| FIGO | International Federation of Gynecology and Obstetrics |
| FS | Frozen Section |
| L/S | Laparoscopy |
| L/T | Laparotomy |
| LVSI | Lymphovascular Space Invasion |
| MRI | Magnetic Resonance Imaging |
| NPV | Negative Predictive Value |
| PPV | Positive Predictive Value |
| SD | Standard Deviation |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Lee, N.K.; Cheung, M.K.; Shin, J.Y.; Husain, A.; Teng, N.N.; Berek, J.S.; Kapp, D.S.; Osann, K.; Chan, J.K. Prognostic factors for uterine cancer in reproductive-aged women. Obs. Gynecol. 2007, 109, 655–662. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.J.; Pannu, H.K. Radiological assessment of gynecologic malignancies. Obs. Gynecol. Clin. N. Am. 2011, 38, 45–68, vii. [Google Scholar] [CrossRef] [PubMed]
- Ocak, B.; Sahin, A.; Oz Atalay, F.; Ozsen, M.; Dakiki, B.; Ture, S.; Sali, S.; Tanriverdi, O.; Bayrak, M.; Ozan, H.; et al. Why do some patients with stage 1A and 1B endometrial endometrioid carcinoma experience recurrence? A retrospective study in search of prognostic factors. Ginekol. Pol. 2022, 93, 112–120. [Google Scholar]
- Espindola, D.; Kennedy, K.A.; Fischer, E.G. Management of abnormal uterine bleeding and the pathology of endometrial hyperplasia. Obs. Gynecol. Clin. N. Am. 2007, 34, 717–737, ix. [Google Scholar] [CrossRef]
- Sala, E.; Crawford, R.; Senior, E.; Shaw, A.; Simcock, B.; Vrotsou, K.; Palmer, C.; Rajan, P.; Joubert, I.; Lomas, D. Added value of dynamic contrast-enhanced magnetic resonance imaging in predicting advanced stage disease in patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2009, 19, 141–146. [Google Scholar] [CrossRef]
- Sanjuán, A.; Escaramís, G.; Ayuso, J.R.; Román, S.M.; Torné, A.; Ordi, J.; Lejárcegui, J.A.; Pahisa, J. Role of magnetic resonance imaging and cause of pitfalls in detecting myometrial invasion and cervical involvement in endometrial cancer. Arch. Gynecol. Obs. 2008, 278, 535–539. [Google Scholar] [CrossRef]
- Wang, J.; Yu, T.; Bai, R.; Sun, H.; Zhao, X.; Li, Y. The value of the apparent diffusion coefficient in differentiating stage IA endometrial carcinoma from normal endometrium and benign diseases of the endometrium: Initial study at 3-T magnetic resonance scanner. J. Comput. Assist. Tomogr. 2010, 34, 332–337. [Google Scholar] [CrossRef]
- Bedner, R.; Rzepka-Górska, I. Hysteroscopy with directed biopsy versus dilatation and curettage for the diagnosis of endometrial hyperplasia and cancer in perimenopausal women. Eur. J. Gynaecol. Oncol. 2007, 28, 400–402. [Google Scholar]
- Shen, S.H.; Chiou, Y.Y.; Wang, J.H.; Yen, M.S.; Lee, R.C.; Lai, C.R.; Chang, C.Y. Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. AJR Am. J. Roentgenol. 2008, 190, 481–488. [Google Scholar] [CrossRef]
- Lee, Y.J.; Moon, M.H.; Sung, C.K.; Chun, Y.K.; Lee, Y.H. MR assessment of myometrial invasion in women with endometrial cancer: Discrepancy between T2-weighted imaging and contrast-enhanced T1-weighted imaging. Abdom. Radiol. 2016, 41, 127–135. [Google Scholar] [CrossRef]
- Kido, A.; Himoto, Y.; Kurata, Y.; Minamiguchi, S.; Nakamoto, Y. Preoperative Imaging Evaluation of Endometrial Cancer in FIGO 2023. J. Magn. Reson. Imaging 2024, 60, 1225–1242. [Google Scholar] [CrossRef]
- Reinhold, C.; Ueno, Y.; Akin, E.A.; Bhosale, P.R.; Dudiak, K.M.; Jhingran, A.; Kang, S.K.; Kilcoyne, A.; Lakhman, Y.; Nicola, R.; et al. ACR Appropriateness Criteria® Pretreatment Evaluation and Follow-Up of Endometrial Cancer. J. Am. Coll. Radiol. 2020, 17, S472–S486. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef]
- Sany, O.; Singh, K.; Jha, S. Correlation between preoperative endometrial sampling and final endometrial cancer histology. Eur. J. Gynaecol. Oncol. 2012, 33, 142–144. [Google Scholar]
- Hemida, R.A.; Zayed, A.E.; Shalaby, A.; Goda, H.; Fawzy, M.; El Refaeey, A.A. Agreement of histopathological findings of preoperative uterine curettage and hysterectomy specimens: Impact of time factor and hormonal therapy. J. Exp. Ther. Oncol. 2013, 10, 165–168. [Google Scholar]
- Rizescu, R.A.; Sălcianu, I.A.; Șerbănoiu, A.; Ion, R.T.; Florescu, L.M.; Gheonea, I.A.; Iana, G.; Bratu, A.M. Can MRI Accurately Diagnose and Stage Endometrial Adenocarcinoma? Medicina 2024, 60, 512. [Google Scholar] [CrossRef]
- Tanaka, T.; Terai, Y.; Fujiwara, S.; Tanaka, Y.; Sasaki, H.; Tsunetoh, S.; Yamamoto, K.; Yamada, T.; Narumi, Y.; Ohmichi, M. Preoperative diffusion-weighted magnetic resonance imaging and intraoperative frozen sections for predicting the tumor grade in endometrioid endometrial cancer. Oncotarget 2018, 9, 36575–36584. [Google Scholar] [CrossRef]
- Kopatsaris, S.; Apostolopoulou, A.; Tsakiridis, I.; Tranidou, A.; Zachomitros, F.; Papanikolaou, E.; Daponte, A.; Kalogiannidis, I.; Dagklis, T. Accuracy of Frozen Section Biopsy in the Diagnosis of Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 1200. [Google Scholar] [CrossRef]
- Ugaki, H.; Kimura, T.; Miyatake, T.; Ueda, Y.; Yoshino, K.; Matsuzaki, S.; Fujita, M.; Kimura, T.; Morii, E.; Enomoto, T. Intraoperative Frozen Section Assessment of Myometrial Invasion and Histology of Endometrial Cancer Using the Revised FIGO Staging System. Int. J. Gynecol. Cancer 2011, 21, 1180–1184. [Google Scholar] [CrossRef]
- Papadia, A.; Azioni, G.; Brusacà, B.; Fulcheri, E.; Nishida, K.; Menoni, S.; Simpkins, F.; Lucci, J.A., 3rd; Ragni, N. Frozen section underestimates the need for surgical staging in endometrial cancer patients. Int. J. Gynecol. Cancer 2009, 19, 1570–1573. [Google Scholar] [CrossRef]
- Mavromatis, I.D.; Antonopoulos, C.N.; Matsoukis, I.L.; Frangos, C.C.; Skalkidou, A.; Creatsas, G.; Petridou, E.T. Validity of intraoperative gross examination of myometrial invasion in patients with endometrial cancer: A meta-analysis. Acta Obs. Gynecol. Scand. 2012, 91, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, R.; Khalil, A.; Ghunaim, S.; El Housheimi, A.; Khalife, D.; Sassine, D.; Khoury, K.; Mailhac, A.; Nassour, F.; Saliba, M.; et al. The accuracy and clinical impact of intraoperative frozen section in determining the extent of surgical intervention in patients with early stage endometrial cancer. J. Obs. Gynaecol. 2022, 42, 1474–1481. [Google Scholar] [CrossRef]
- Kisu, I.; Banno, K.; Lin, L.Y.; Ueno, A.; Abe, T.; Kouyama, K.; Okuda, S.; Masugi, Y.; Umene, K.; Nogami, Y.; et al. Preoperative and intraoperative assessment of myometrial invasion in endometrial cancer: Comparison of magnetic resonance imaging and frozen sections. Acta Obs. Gynecol. Scand. 2013, 92, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Jónsdóttir, B.; Marcickiewicz, J.; Borgfeldt, C.; Bjurberg, M.; Dahm-Kähler, P.; Flöter-Rådestad, A.; Hellman, K.; Holmberg, E.; Kjølhede, P.; Rosenberg, P.; et al. Preoperative and intraoperative assessment of myometrial invasion in endometrial cancer-A Swedish Gynecologic Cancer Group (SweGCG) study. Acta Obs. Gynecol. Scand. 2021, 100, 1526–1533. [Google Scholar] [CrossRef]
- Iitsuka, C.; Asami, Y.; Hirose, Y.; Nagashima, M.; Mimura, T.; Miyamoto, S.; Onuki, M.; Ohgiya, Y.; Kushima, M.; Sekizawa, A.; et al. Preoperative Magnetic Resonance Imaging versus Intraoperative Frozen Section Diagnosis for Predicting the Deep Myometrial Invasion in Endometrial Cancer: Our Experience and Literature Review. J. Obs. Gynaecol. Res. 2021, 47, 3331–3338. [Google Scholar] [CrossRef] [PubMed]
- Boronow, R.C.; Morrow, C.P.; Creasman, W.T.; Disaia, P.J.; Silverberg, S.G.; Miller, A.; Blessing, J.A. Surgical staging in endometrial cancer: Clinical-pathologic findings of a prospective study. Obs. Gynecol. 1984, 63, 825–832. [Google Scholar]
- Abeler, V.M.; Kjørstad, K.E. Endometrial adenocarcinoma in Norway. A study of a total population. Cancer 1991, 67, 3093–3103. [Google Scholar] [CrossRef] [PubMed]
- Berretta, R.; Patrelli, T.S.; Migliavacca, C.; Rolla, M.; Franchi, L.; Monica, M.; Modena, A.B.; Gizzo, S. Assessment of tumor size as a useful marker for the surgical staging of endometrial cancer. Oncol. Rep. 2014, 31, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Schink, J.C.; Lurain, J.R.; Wallemark, C.B.; Chmiel, J.S. Tumor size in endometrial cancer: A prognostic factor for lymph node metastasis. Obs. Gynecol. 1987, 70, 216–219. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Haddock, M.G.; Calori, G.; Podratz, K.C. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am. J. Obs. Gynecol. 2000, 182, 1506–1519. [Google Scholar] [CrossRef]
- Beddy, P.; Moyle, P.; Kataoka, M.; Yamamoto, A.K.; Joubert, I.; Lomas, D.; Crawford, R.; Sala, E. Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: Comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 2012, 262, 530–537. [Google Scholar] [CrossRef] [PubMed]

| Variable | Value |
|---|---|
| Age, mean ± SD, (years) | 57.57 ± 9.65 |
| BMI, mean ± SD, (kg/m2) | 32.33 ± 6.74 |
| Comorbidities n (%) Absent Present | 46 (52.3) 42 (47.7) |
| Menopausal Status n (%) Premenopausal Postmenopausal | 24 (27.3) 64 (72.7) |
| Preoperative Complaints n (%) None Abnormal Uterine Bleeding Pelvic Pain Postmenopausal Bleeding Increased Endometrial Thickness Vaginal Discharge | 2 (2.3) 23 (26.1) 1 (1.1) 59 (67.0) 2 (2.3) 1 (1.1) |
| Ultrasound Findings n (%) Normal Increased Endometrial Thickness Intrauterine Mass Intracavitary Fluid Irregular Endometrium | 11 (12.5) 33 (37.5) 29 (33.0) 2 (2.3) 13 (14.8) |
| Surgical Approach n (%) Laparoscopy (L/S) Laparotomy (L/T) Conversion from L/S to L/T | 46 (52.3) 34 (38.6) 8 (9.1) |
| Assessment Parameter | Category | n | % |
|---|---|---|---|
| MRI Myometrial Invasion Depth | Superficial invasion | 51 | 58.0 |
| Deep invasion | 37 | 42.0 | |
| Frozen Section Myometrial Invasion Depth | Superficial invasion | 59 | 67.0 |
| Deep invasion | 29 | 33.0 | |
| Frozen Section Cervical Stromal Involvement | Absent | 85 | 96.6 |
| Present | 3 | 3.4 | |
| Final Pathology—Myometrial Invasion | Superficial invasion | 50 | 56.8 |
| Deep invasion | 38 | 43.2 | |
| Final Pathology—Cervical Stromal Involvement | Absent | 73 | 83.0 |
| Present | 15 | 17.0 |
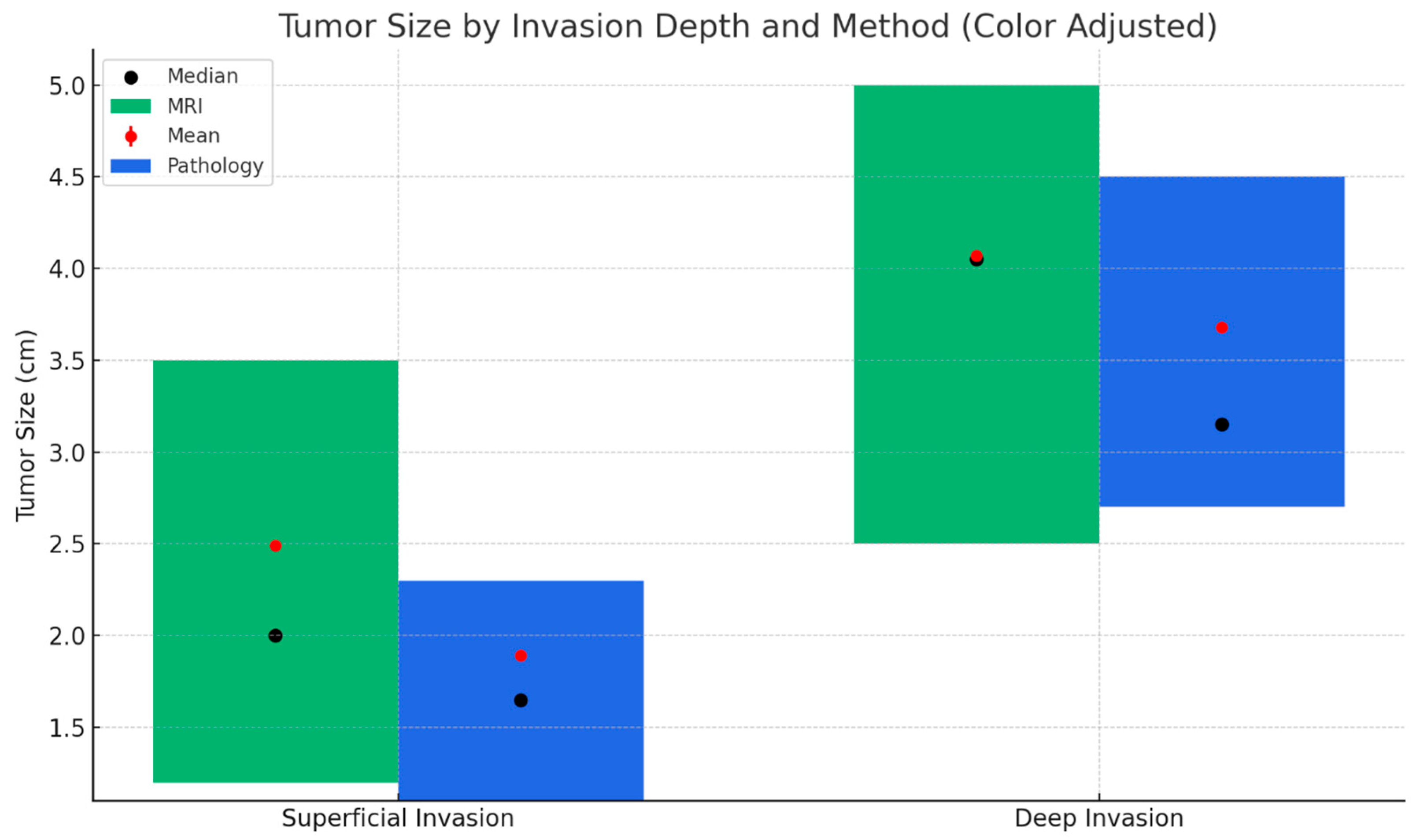
| Final Pathology Myometrial Invasion Depth | Superficial Invasion | Deep Invasion | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | p-Value | ||
| MRI Myometrial Invasion Depth | Superficial invasion | 44 | 88.0 | 7 | 18.4 | <0.001 b |
| Deep invasion | 6 | 12.0 | 31 | 81.6 | ||
| Frozen Section Myometrial Invasion Depth | Superficial invasion | 49 | 98.0 | 10 | 26.3 | <0.001 b |
| Deep invasion | 1 | 2.0 | 28 | 73.7 | ||
| Frozen Section Cervical Stromal Involvement | Absent | 49 | 98.0 | 36 | 94.7 | 0.576 a |
| Present | 1 | 2.0 | 2 | 5.3 | ||
| Final Pathology—Cervical Stromal Involvement | Absent | 48 | 96.0 | 25 | 65.8 | <0.001 b |
| Present | 2 | 4.0 | 13 | 34.2 | ||
| Final Tumor Grade | Grade 1 | 42 | 84.0 x | 18 | 47.4 y | 0.001 a |
| Grade 2 | 7 | 14.0 x | 16 | 42.1 y | ||
| Grade 3 | 1 | 2.0 x | 4 | 10.5 x | ||
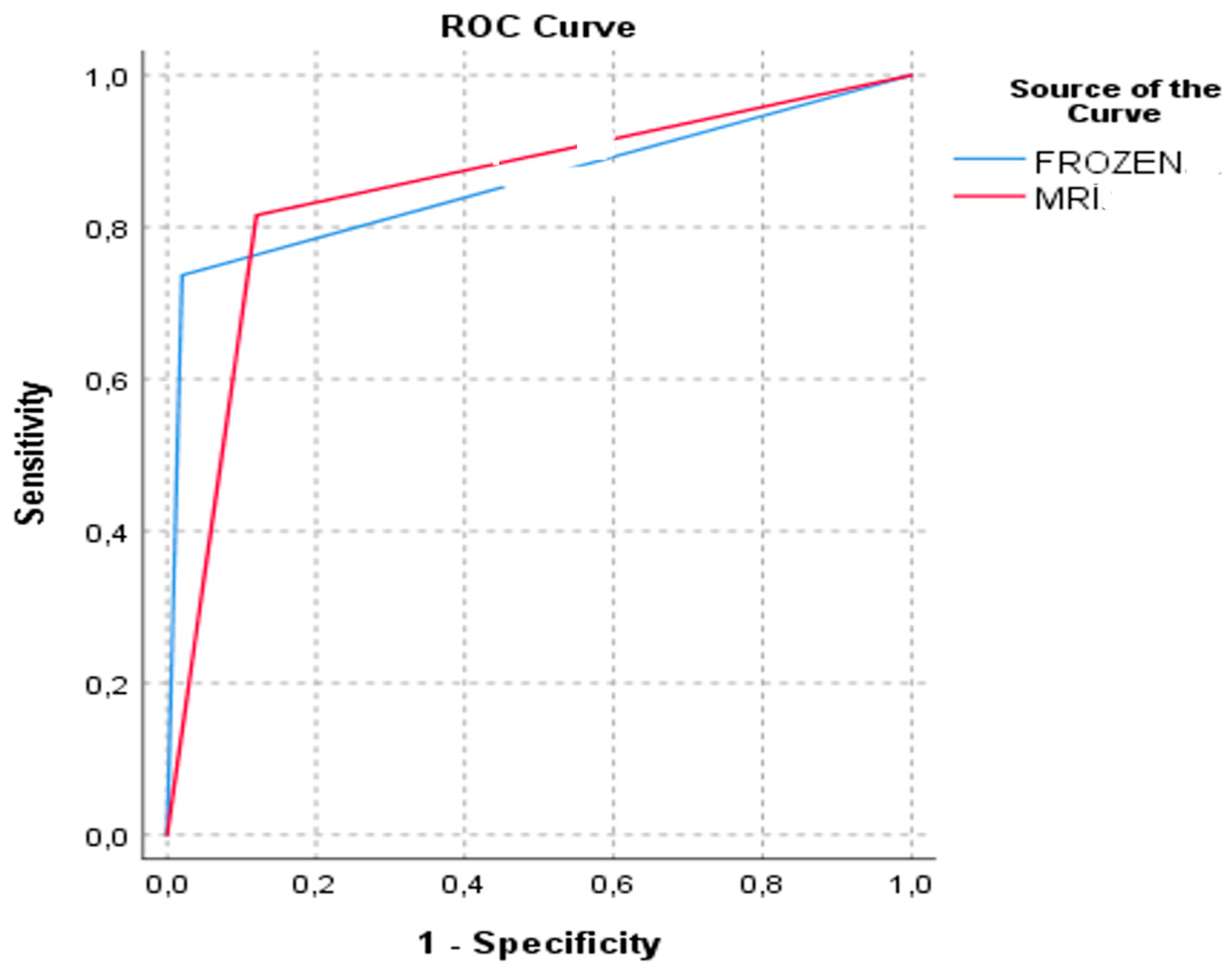
| Final Pathology Myometrial Invasion Depth | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Superficial Invasion | Deep Invasion | AUC | AUC Difference | p-Value | ||||||
| n | NPV (%) | Specificity (%) | n | PPV (%) | Sensitivity (%) | |||||
| MRI Myometrial Invasion Depth | Superficial invasion | 44 | 86.3 | 88.0 | 7 | 13.7 | 18.4 | 0.848 | 0.01 (−0.103–0.082) | 0.823 |
| Deep invasion | 6 | 16.2 | 12.0 | 31 | 83.8 | 81.6 | ||||
| Frozen Section Myometrial Invasion Depth | Superficial invasion | 49 | 83.1 | 98.0 | 10 | 16.9 | 26.3 | 0.858 | ||
| Deep invasion | 1 | 3.4 | 2.0 | 28 | 96.6 | 73.7 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metin Çakır, T.; Güner, F.C.; Iltar, E.; Dinç, C.; Öz, Ö.F.; Şimşek, T. Comparison of Preoperative Magnetic Resonance Imaging and Intraoperative Frozen Section Analysis with Final Pathological Outcomes in the Assessment of Myometrial Invasion in Endometrial Cancer Cases. Diagnostics 2025, 15, 2799. https://doi.org/10.3390/diagnostics15212799
Metin Çakır T, Güner FC, Iltar E, Dinç C, Öz ÖF, Şimşek T. Comparison of Preoperative Magnetic Resonance Imaging and Intraoperative Frozen Section Analysis with Final Pathological Outcomes in the Assessment of Myometrial Invasion in Endometrial Cancer Cases. Diagnostics. 2025; 15(21):2799. https://doi.org/10.3390/diagnostics15212799
Chicago/Turabian StyleMetin Çakır, Tuba, Fatma Ceren Güner, Elif Iltar, Can Dinç, Ömer Faruk Öz, and Tayup Şimşek. 2025. "Comparison of Preoperative Magnetic Resonance Imaging and Intraoperative Frozen Section Analysis with Final Pathological Outcomes in the Assessment of Myometrial Invasion in Endometrial Cancer Cases" Diagnostics 15, no. 21: 2799. https://doi.org/10.3390/diagnostics15212799
APA StyleMetin Çakır, T., Güner, F. C., Iltar, E., Dinç, C., Öz, Ö. F., & Şimşek, T. (2025). Comparison of Preoperative Magnetic Resonance Imaging and Intraoperative Frozen Section Analysis with Final Pathological Outcomes in the Assessment of Myometrial Invasion in Endometrial Cancer Cases. Diagnostics, 15(21), 2799. https://doi.org/10.3390/diagnostics15212799






