Lung Ultrasound in Pediatrics: A Review with Core Principles That Every User Should Know
Abstract
1. Introduction
2. Relevant Sections
- A-lines;
- B-lines;
- Pleural line;
- Sea-shore sign;
- Sinusoid sign;
- Lung point sign;
- Double-lung-point sign;
- White lung.
3. Discussion
- LUS is an operator-dependent method.
- LUS has a significantly limited ability to image paravertebral areas, particularly those below the angle of the scapula.
- The quality and accuracy of LUS imaging can be negatively affected by obesity and/or the presence of subcutaneous emphysema.
3.1. Key Artifacts and Diagnostic Signs in Lung Ultrasound Imaging: A Comparative Overview of Normal and Pathological Findings
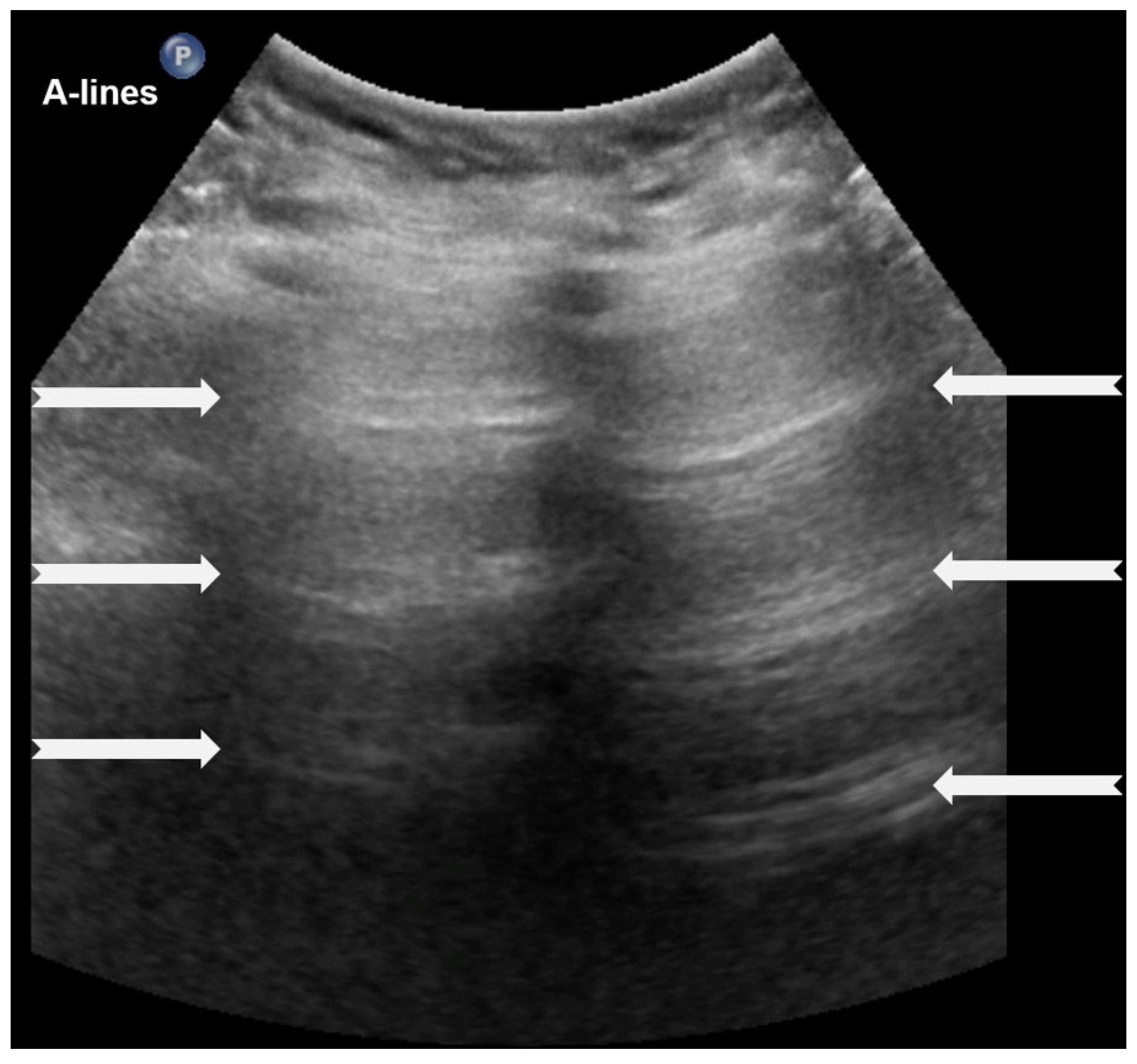
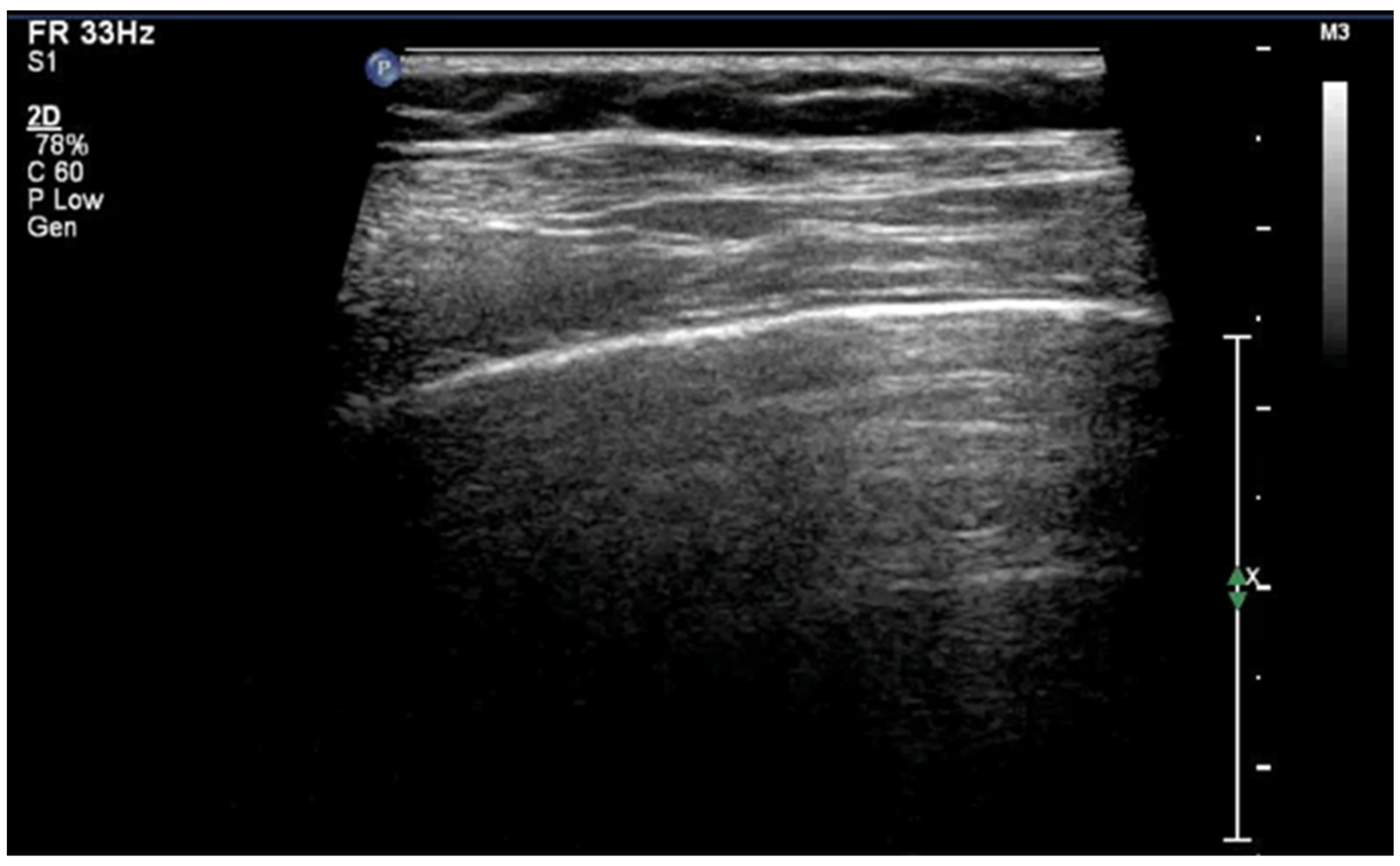
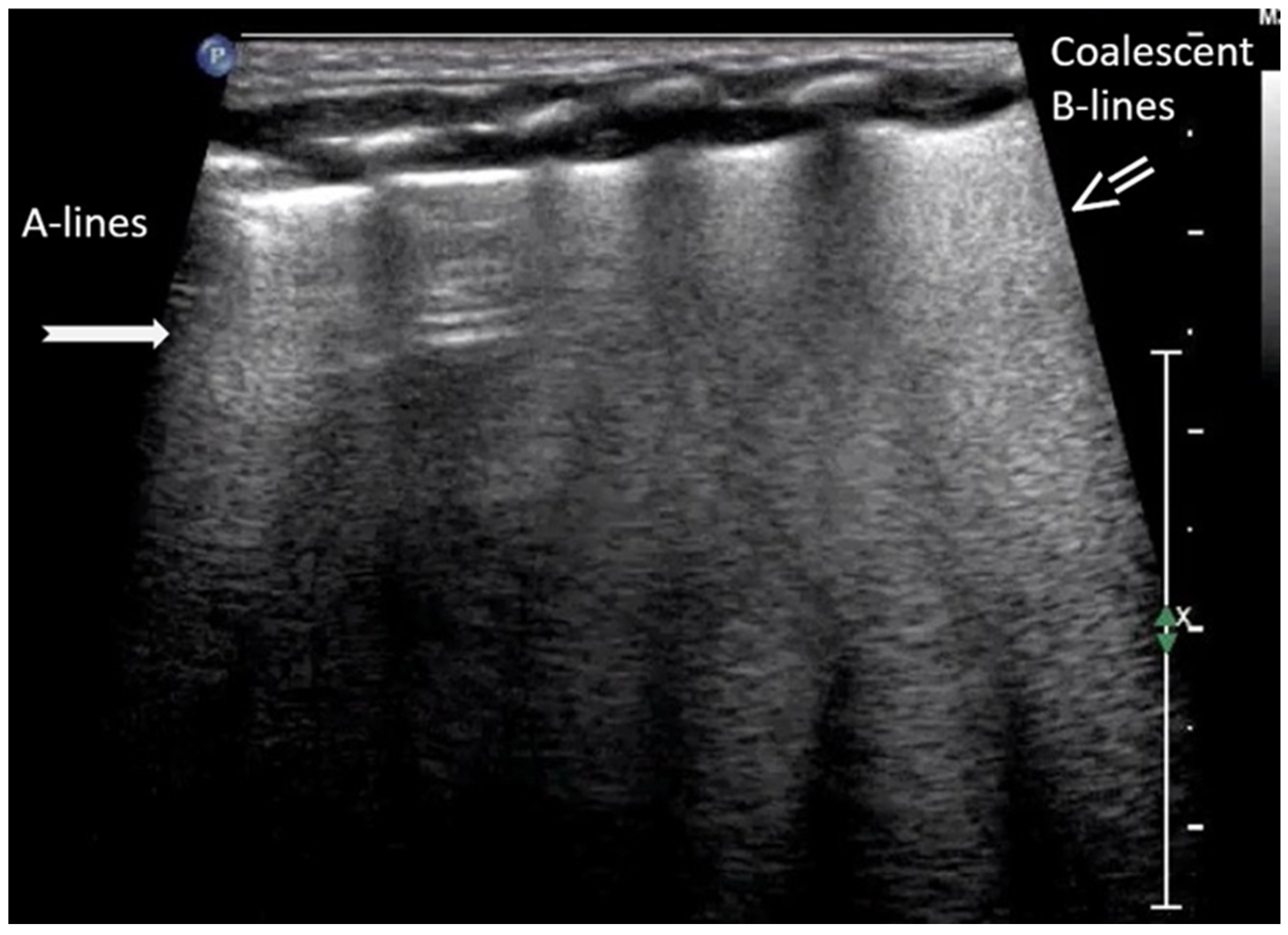
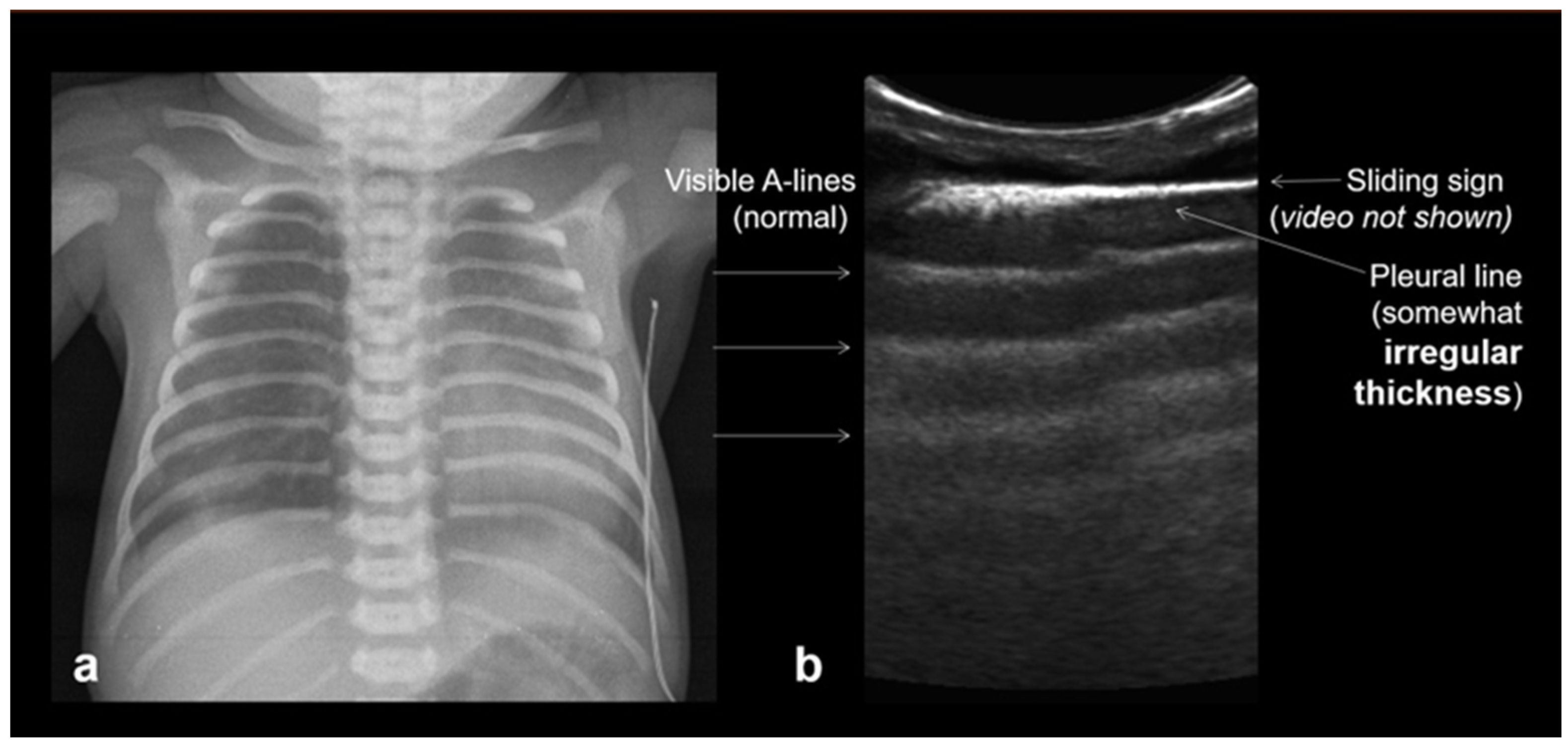
3.1.1. A-Lines
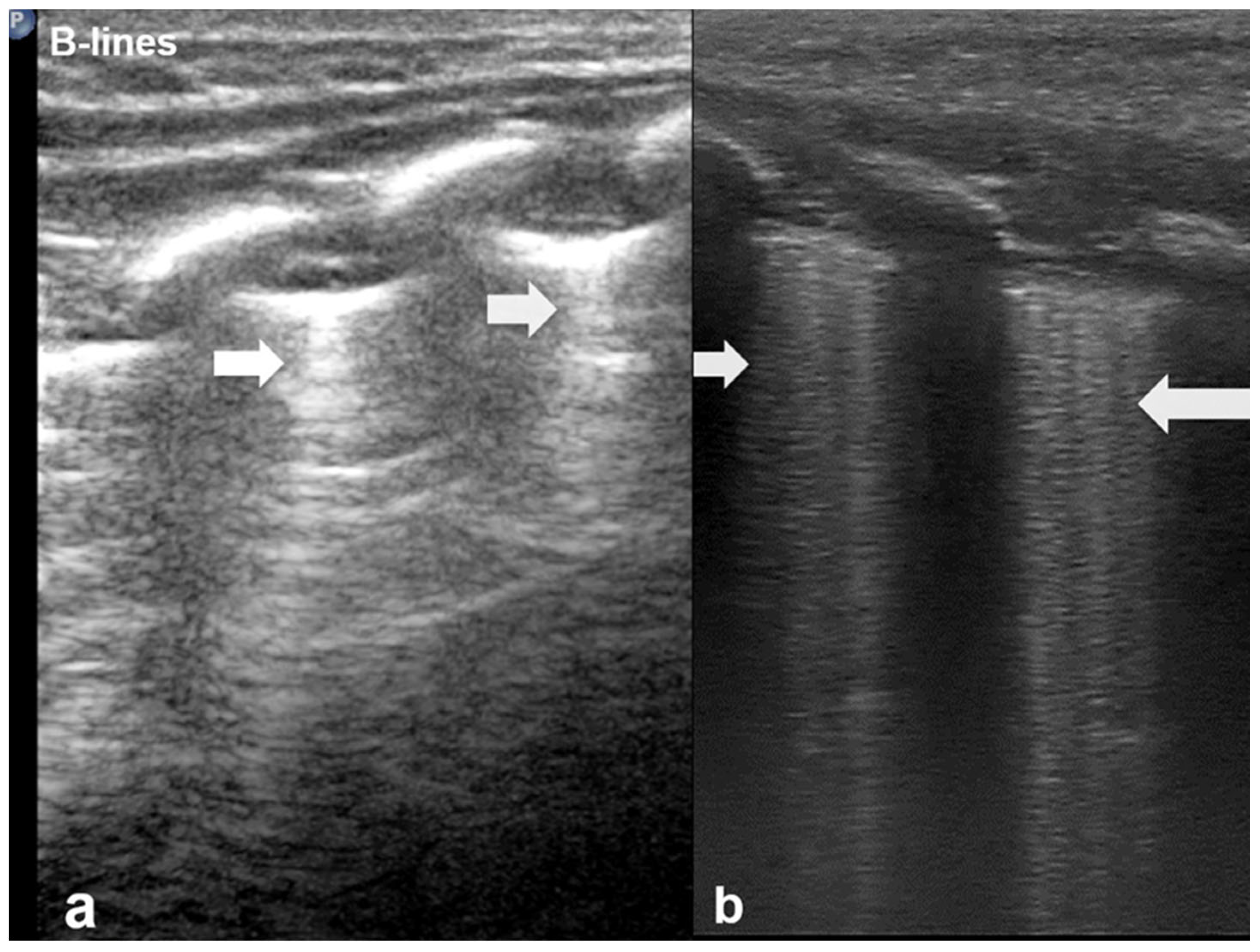
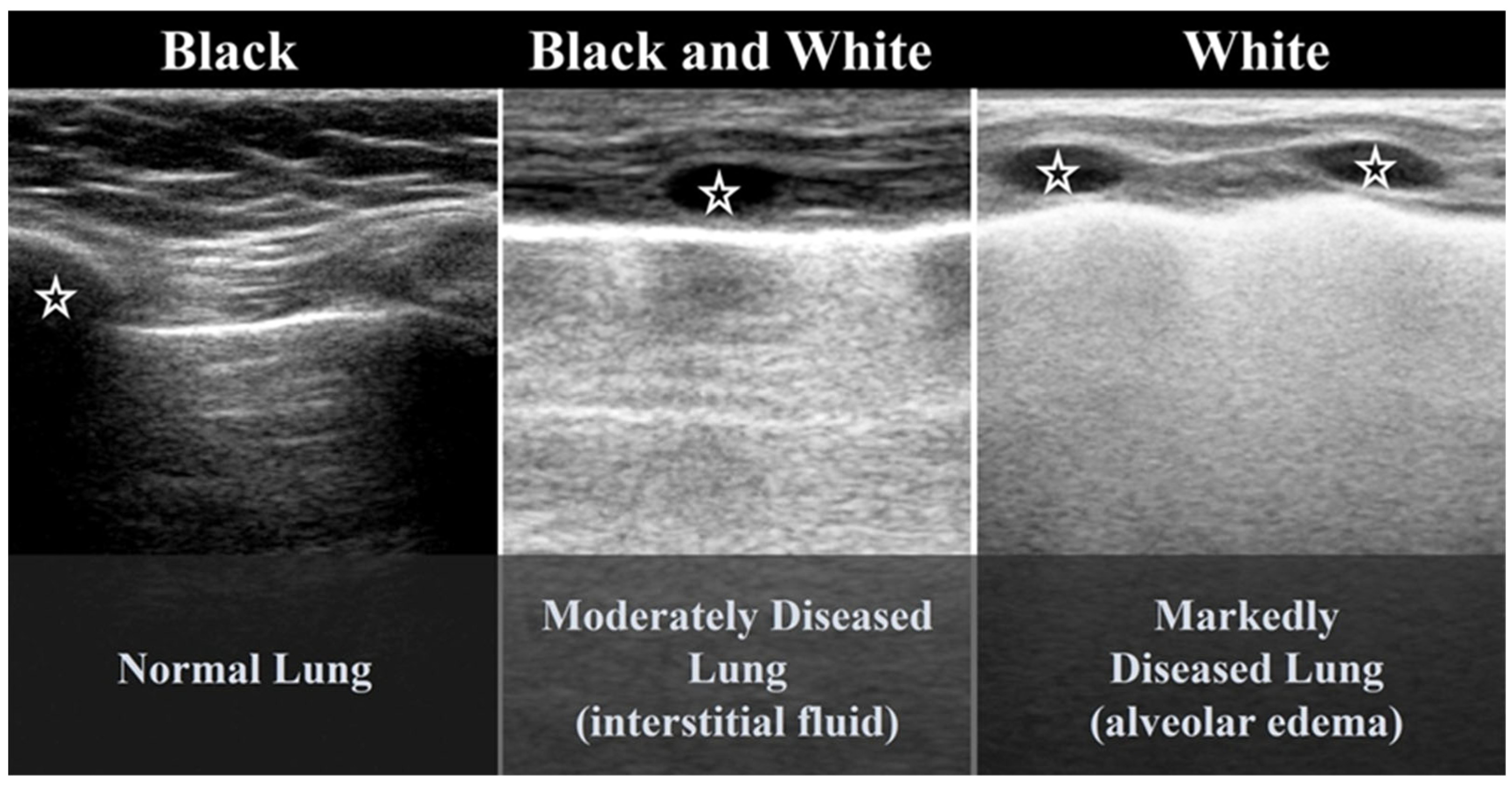
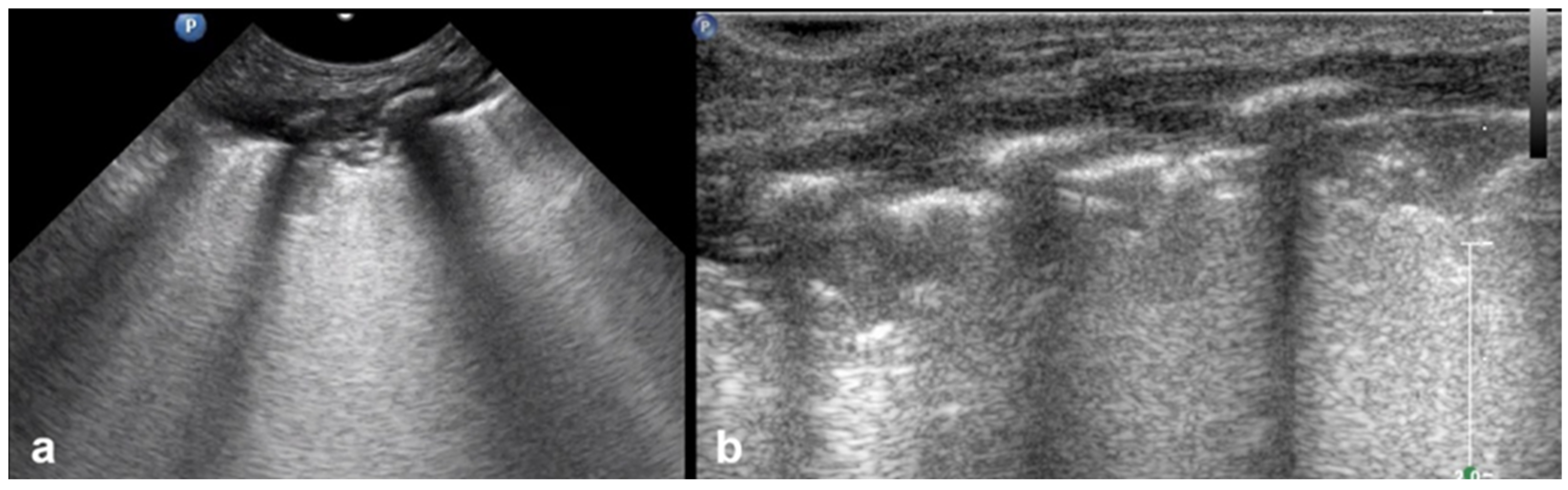
3.1.2. B-Lines
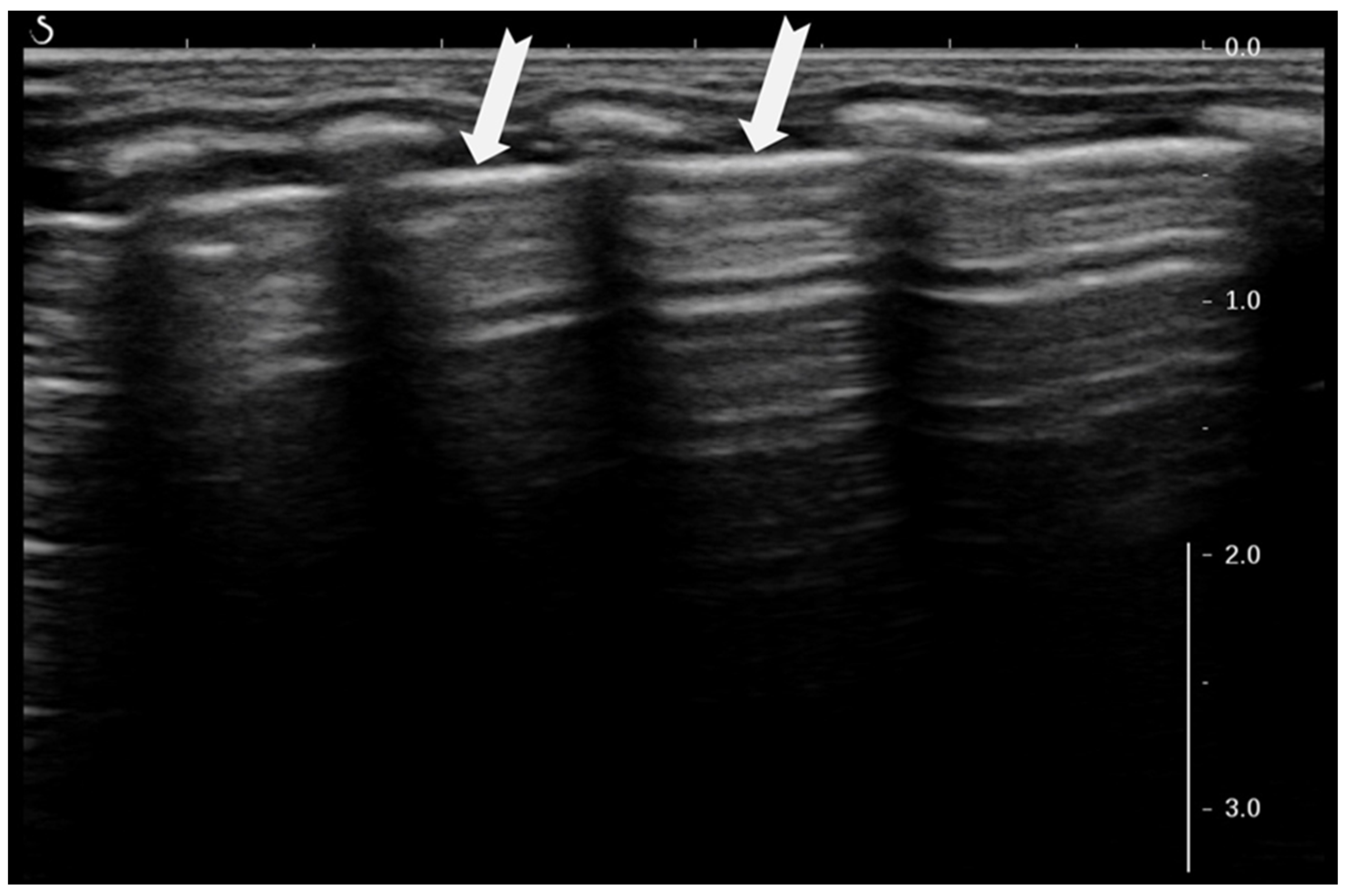
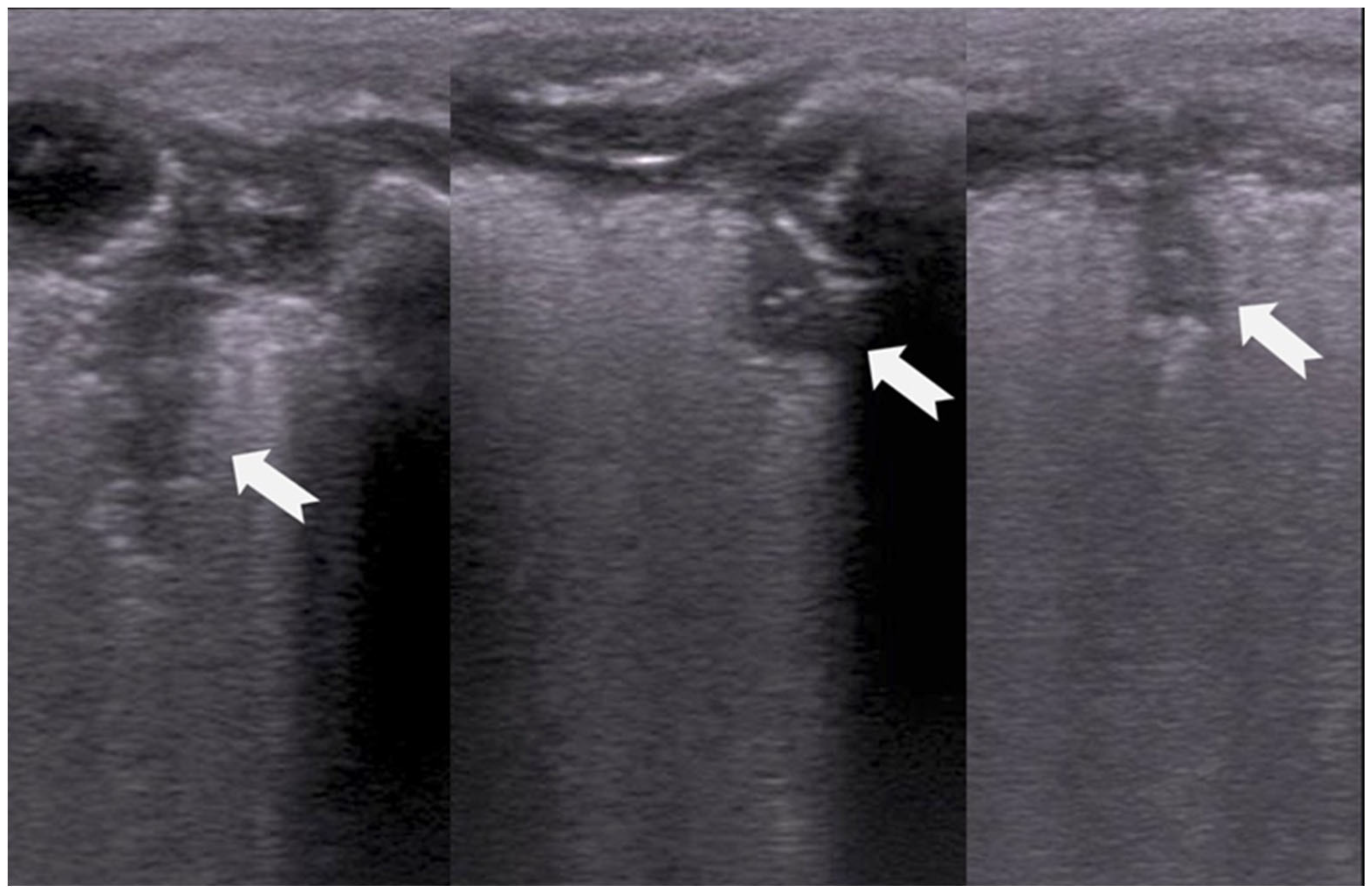
3.1.3. Pleural Line
3.1.4. Sliding Lung Sign
3.1.5. Sea-Shore Sign
3.1.6. Sinusoid Sign
3.1.7. Lung Point Sign
3.1.8. Double-Lung-Point Sign
3.1.9. White Lung
3.2. Main LUS Findings Associated with Neonatal Lung Diseases
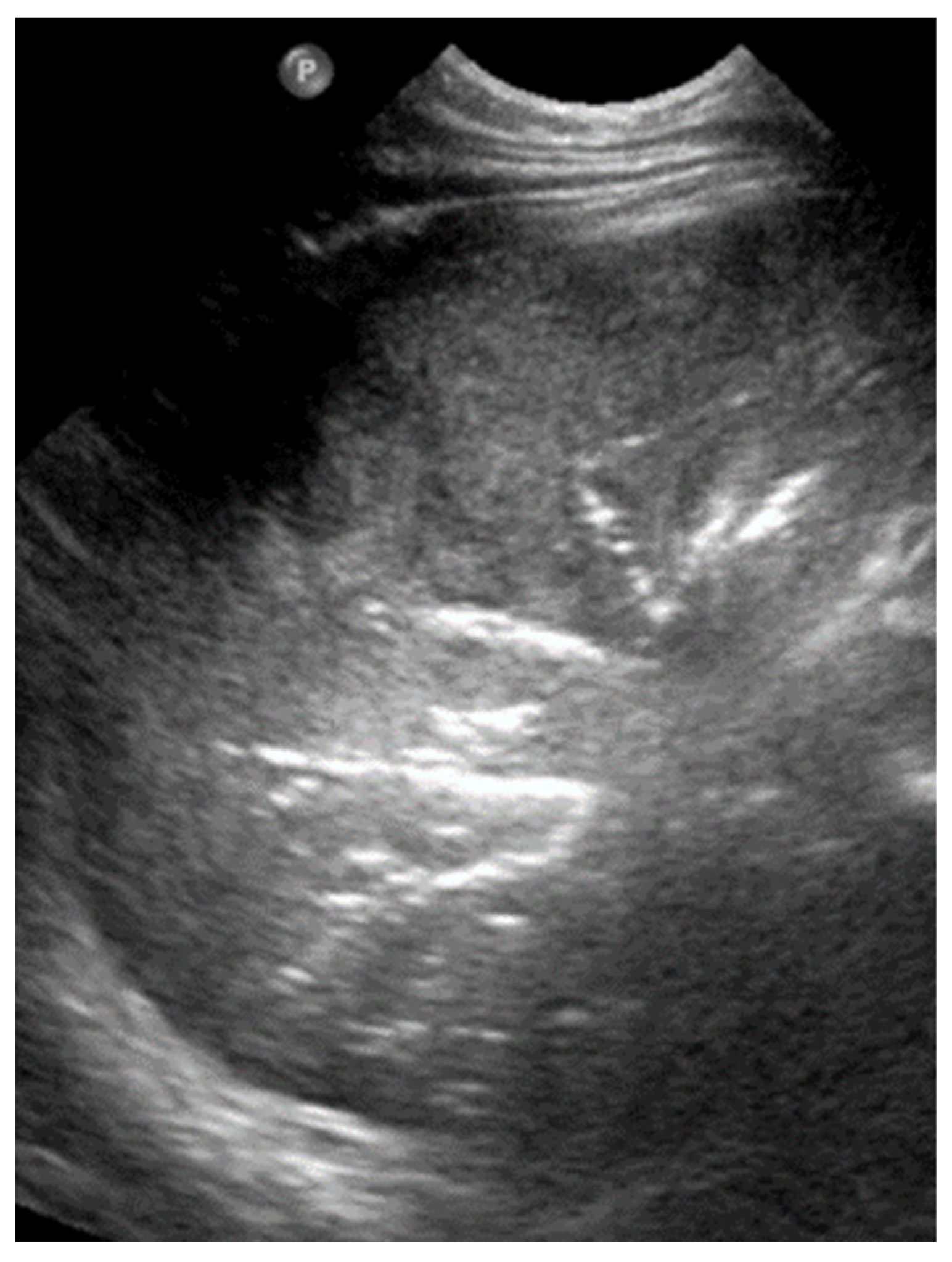
3.2.1. Alveolar–Interstitial Syndrome
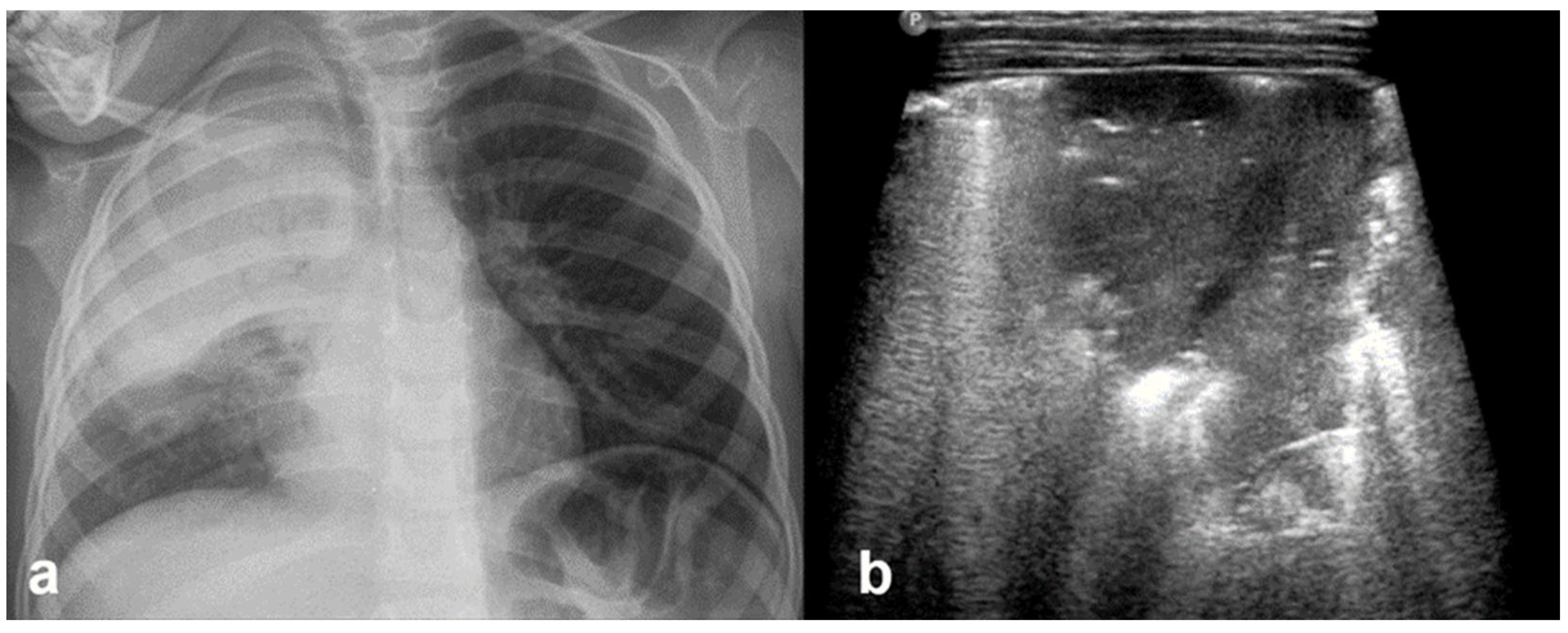
3.2.2. Transient Tachypnoea of the Newborn—Respiratory Distress Syndrome
3.2.3. Transient Tachypnoea of the Newborn (TTN)
3.2.4. Respiratory Distress Syndrome (RDS)
3.2.5. Meconium Aspiration Syndrome (MAS)
3.2.6. Bronchopulmonary Dysplasia (BPD)
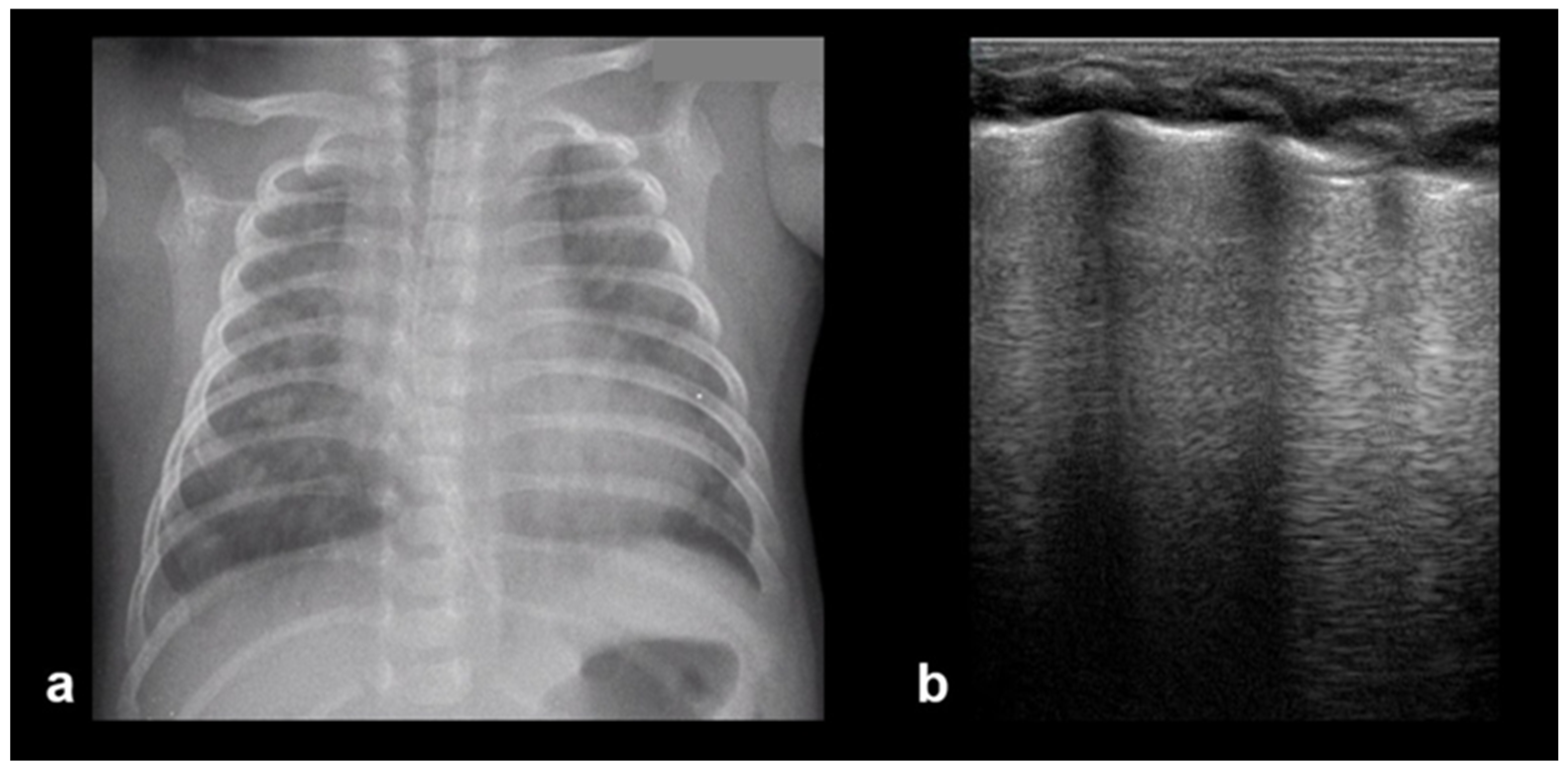
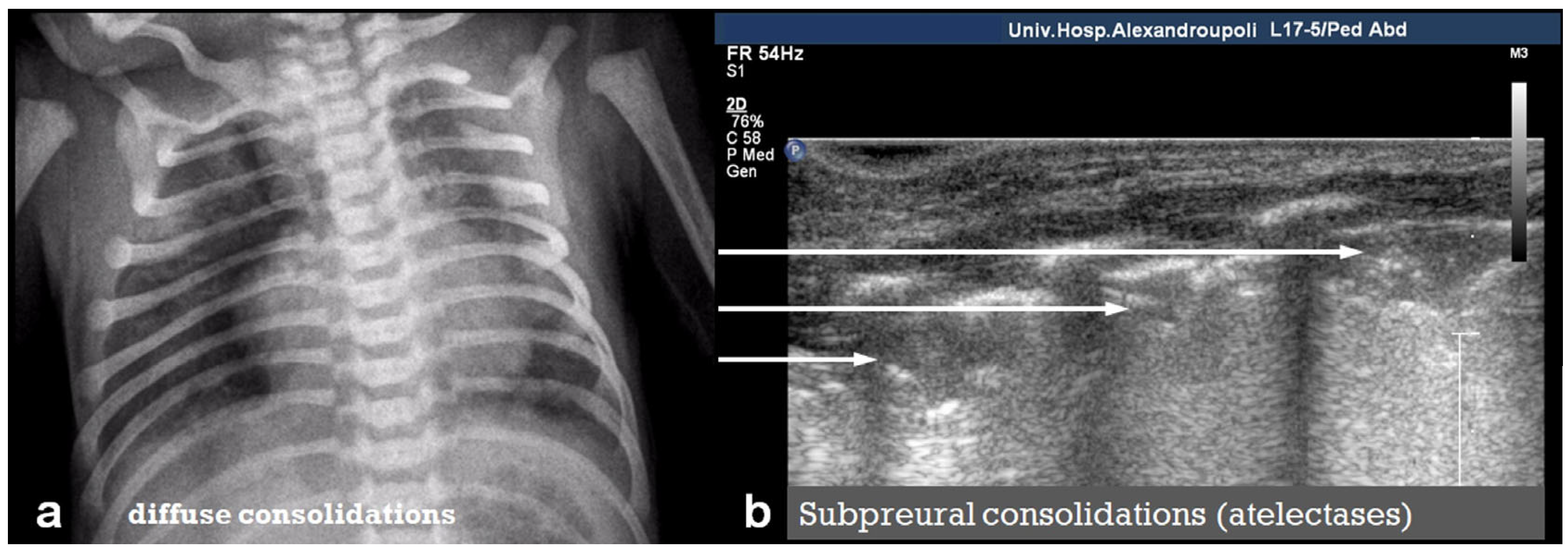
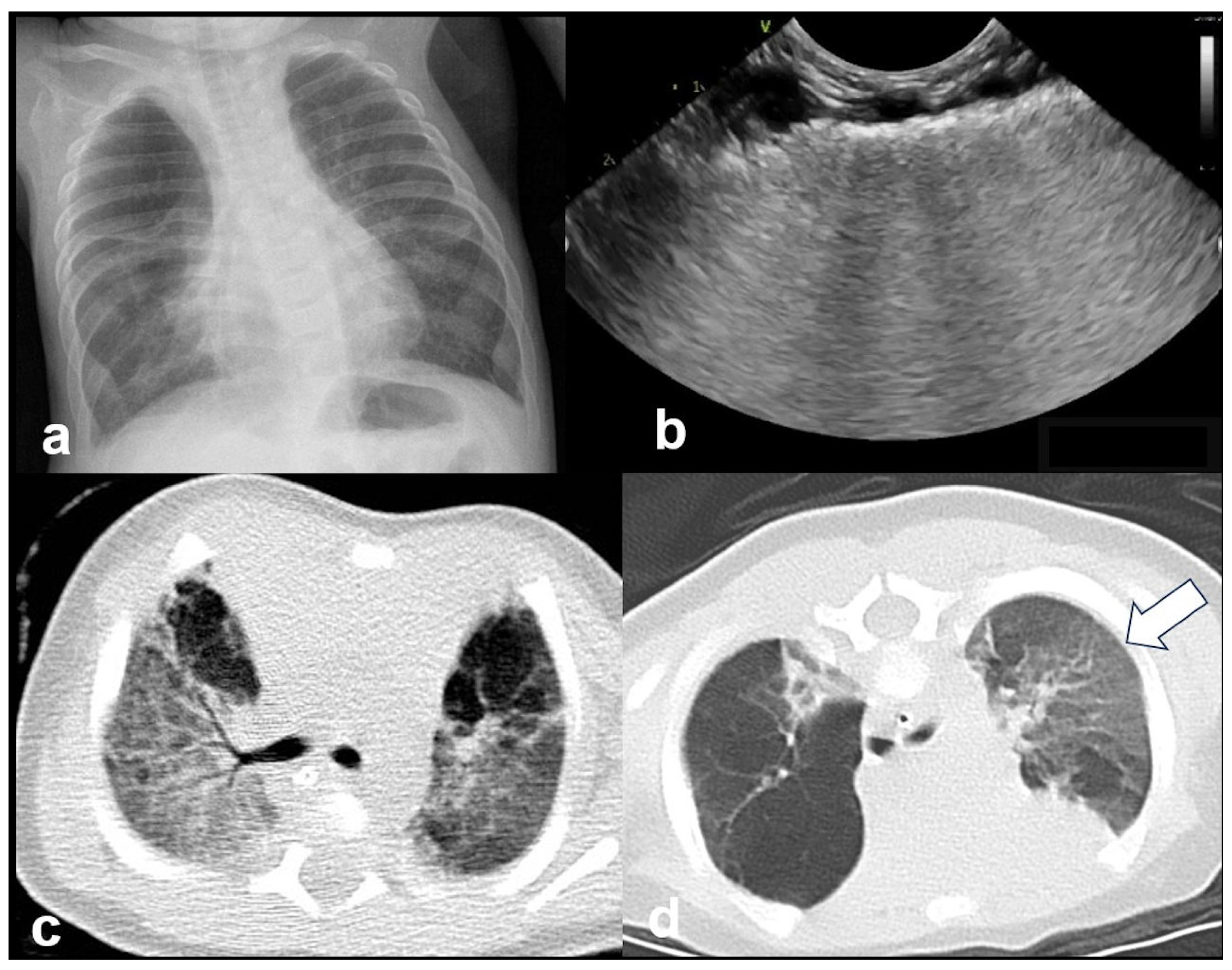
3.2.7. Lung Consolidation
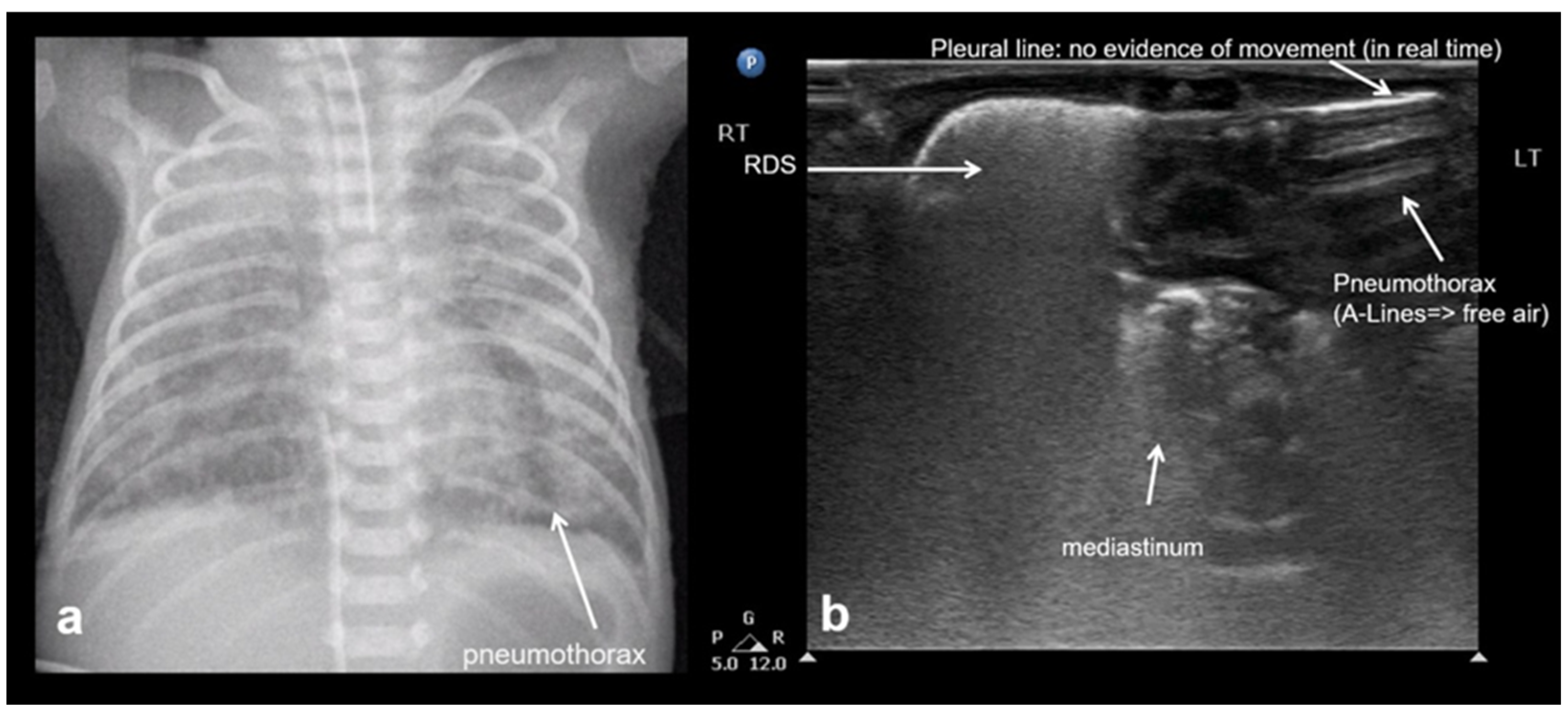
3.2.8. Pneumothorax
3.2.9. Pneumomediastinum
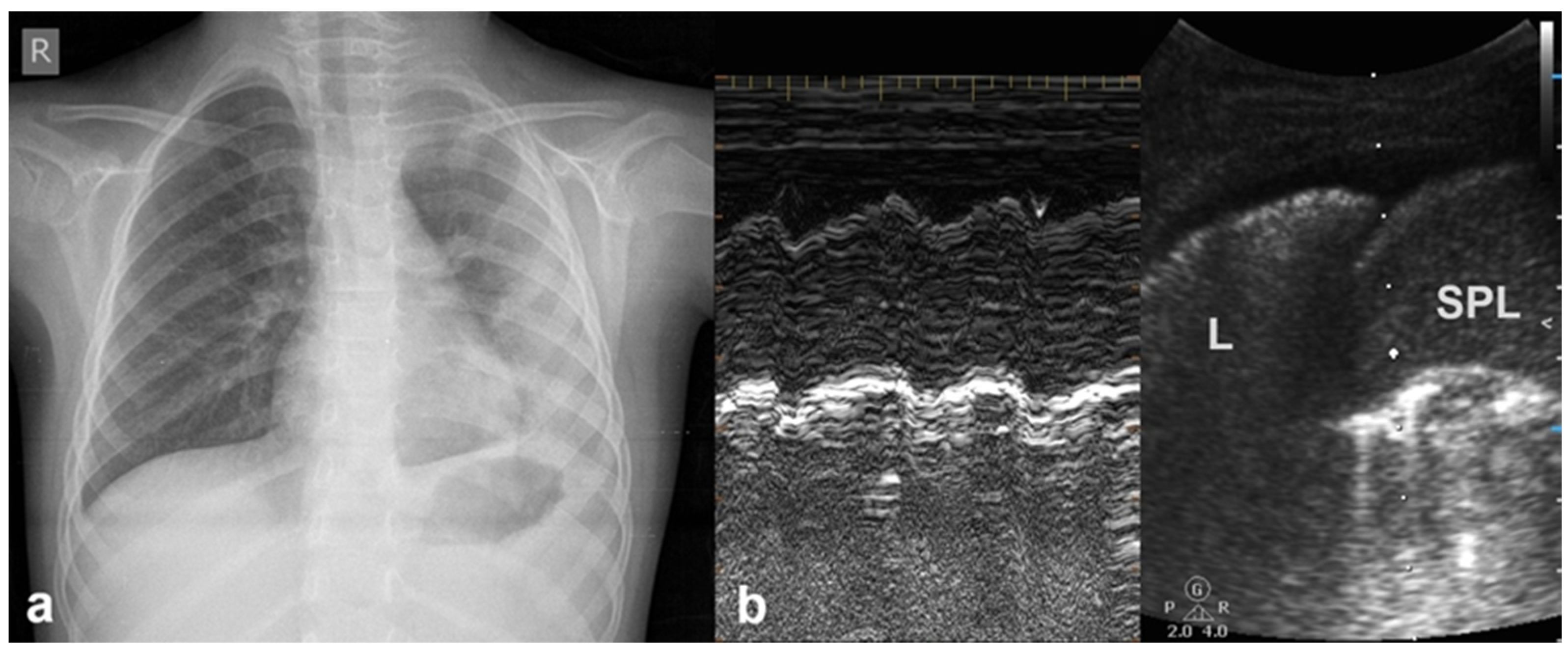
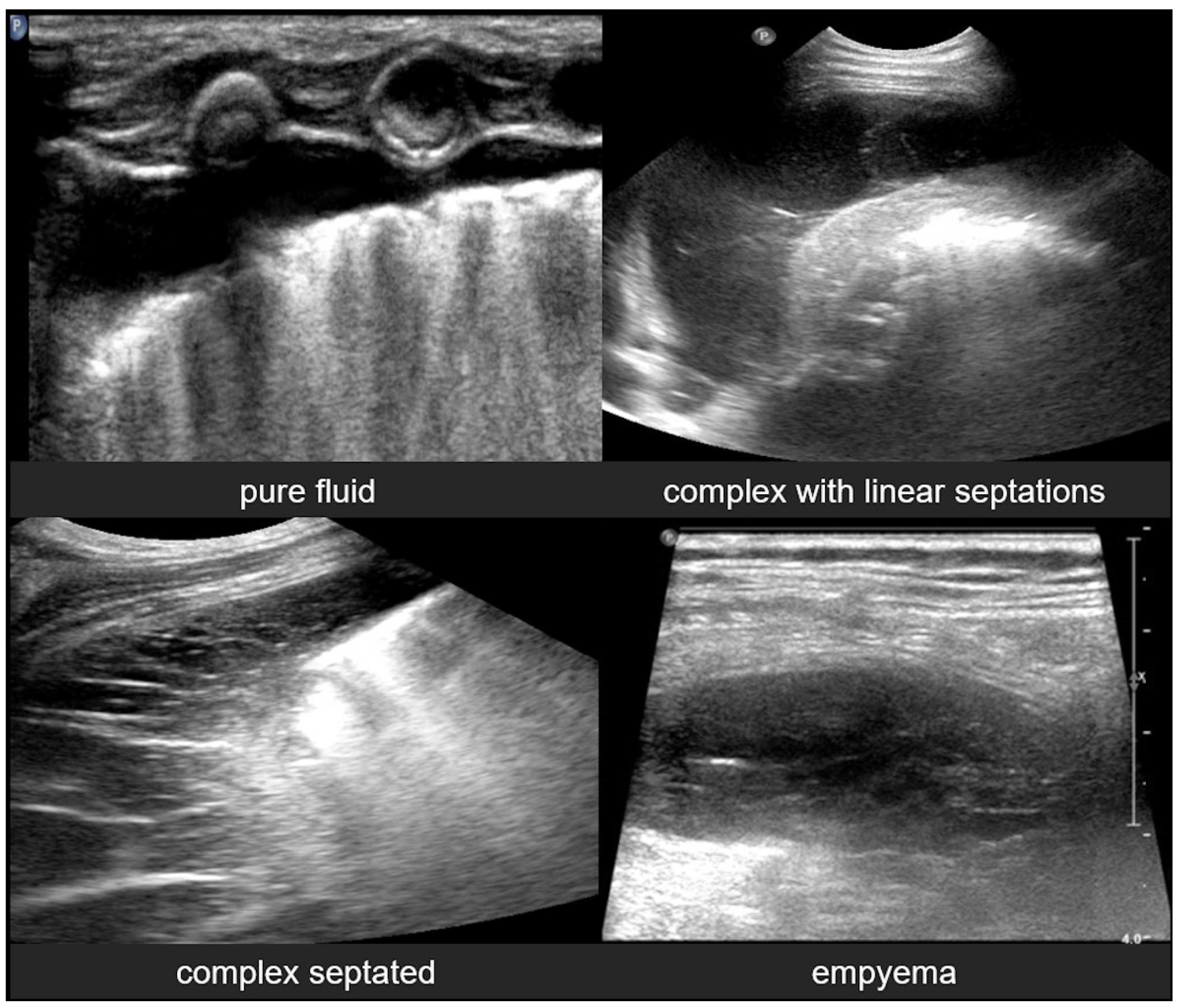
3.2.10. Pleural Effusion
3.2.11. Empyema
4. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LUS | Lung Ultrasound |
| TTN | Transient Tachypnoea of the Newborn |
| RDS | Respiratory Distress Syndrome |
| AIS | Alveolar–Interstitial Syndrome |
| BPD | Bronchopulmonary Dysplasia |
| MAS | Meconium Aspiration Syndrome |
References
- Hall, E.J. Lessons we have learned from our children: Cancer risks from diagnostic radiology. Pediatr. Radiol. 2002, 32, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, H.Y.; Wang, X.L.; Xiao, L.J. The significance and the necessity of routinely performing lung ultrasound in the neonatal intensive care units. J. Matern.-Fetal Neonatal Med. 2016, 29, 4025–4030. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Fu, W.; Liu, J.; Wang, Y. Routine application of lung ultrasonography in the neonatal intensive care unit. Medicine 2017, 96, e5826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surak, A.; Shaireen, H.; Elsayed, Y. Applications of lung ultrasound as an emerging tool in neonates. J. Neonatal-Perinat. Med. 2025, 18, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, H.; Chen, H.; Wan, K.; Mao, R.; Xiao, P.; Chang, X. Application of ultrasonography in neonatal lung disease: An updated review. Front. Pediatr. 2022, 10, 1020437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Musolino, A.M.; Tomà, P.; De Rose, C.; Pitaro, E.; Boccuzzi, E.; De Santis, R.; Morello, R.; Supino, M.C.; Villani, A.; Valentini, P.; et al. Ten years of pediatric lung ultrasound: A narrative review. Front. Physiol. 2022, 12, 721951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escourrou, G.; De Luca, D. Lung ultrasound decreased radiation exposure in preterm infants in a neonatal intensive care unit. Acta Paediatr. 2016, 105, e237–e239. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Attanasi, M.; Di Filippo, P.; Sferrazza Papa, G.F.; Di Pillo, S.; Chiarelli, F. Usefulness of lung ultrasound in paediatric respiratory diseases. Diagnostics 2021, 11, 1783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riccabona, M.; Laffan, E. Chest and lung ultrasound in childhood: Applications, role, value and limitations. J. Ultrason. 2018, 18, 281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pereda, M.A.; Chavez, M.A.; Hooper-Miele, C.C.; Gilman, R.H.; Steinhoff, M.C.; Ellington, L.E.; Gross, M.M.; Price, C.; Tielsch, J.M.; Checkley, W. Lung ultrasound for the diagnosis of pneumonia in children: A meta-analysis. Pediatrics 2015, 135, 714–722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.Q.; Qiu, R.X.; Liu, J.; Zhang, L.; Ren, X.L.; Qin, S.J. Lung ultrasound completely replaced chest X-ray for diagnosing neonatal lung diseases: A 3-year clinical practice report from a neonatal intensive care unit in China. J. Matern.-Fetal Neonatal Med. 2022, 35, 3565–3572. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.R.; Hernández, R.G.; Guerediaga, I.S.; Gato, J.M.; Fanjul, J.R.; Bilbao, V.A.; Quintela, P.A.; Ojembarrena, A.A. Usefulness of lung ultrasound in the diagnosis and follow-up of respiratory diseases in neonates. An. Pediatría (Engl. Ed.) 2022, 96, 252.e1–252.e13. [Google Scholar] [CrossRef] [PubMed]
- Marini, T.J.; Rubens, D.J.; Zhao, Y.T.; Weis, J.; O’connor, T.P.; Novak, W.H.; Kaproth-Joslin, K.A. Lung ultrasound: The essentials. Radiol. Cardiothorac. Imaging 2021, 3, e200564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safai Zadeh, E.; Görg, C.; Prosch, H.; Kifjak, D.; Dietrich, C.F.; Laursen, C.B.; Findeisen, H. Lung Ultrasound and Pleural Artifacts: A Pictorial Review. Diagnostics 2024, 14, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lichtenstein, D.; Mauriat, P. Lung ultrasound in the critically ill neonate. Curr. Pediatr. Rev. 2012, 8, 217–223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurepa, D.; Zaghloul, N.; Watkins, L.; Liu, J. Neonatal lung ultrasound exam guidelines. J. Perinatol. 2018, 38, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bhoil, R.; Ahluwalia, A.; Chopra, R.; Surya, M.; Bhoil, S. Signs and lines in lung ultrasound. J. Ultrason. 2021, 21, e225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, A. Practical approach to lung ultrasound. BJA Educ. 2016, 16, 39–45. [Google Scholar] [CrossRef]
- Caiulo, V.A.; Gargani, L.; Caiulo, S.; Fisicaro, A.; Moramarco, F.; Latini, G.; Picano, E.; Mele, G. Lung ultrasound characteristics of community-acquired pneumonia in hospitalized children. Pediatr. Pulmonol. 2013, 48, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Jonusas, S.F.; Cribioli, C.M.; De Gregorio, A.S. Basic notions of lung ultrasound in neonatology. Arch. Argent. Pediatr. 2022, 120, e246–e254. [Google Scholar] [CrossRef] [PubMed]
- Iovine, E.; Nenna, R.; Bloise, S.; La Regina, D.P.; Pepino, D.; Petrarca, L.; Frassanito, A.; Lubrano, R.; Midulla, F. Lung Ultrasound: Its Findings and New Applications in Neonatology and Pediatric Diseases. Diagnostics 2021, 11, 652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Potter, S.K.; Griksaitis, M.J. The role of point-of-care ultrasound in pediatric acute respiratory distress syndrome: Emerging evidence for its use. Ann. Transl. Med. 2019, 7, 507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ammirabile, A.; Buonsenso, D.; Di Mauro, A. Lung Ultrasound in Pediatrics and Neonatology: An Update. Healthcare 2021, 9, 1015. [Google Scholar] [CrossRef]
- Bouhemad, B.; Zhang, M.; Lu, Q.; Rouby, J.J. Clinical review: Bedside lung ultrasound in critical care practice. Crit. Care 2007, 11, 205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cattarossi, L.; Copetti, R.; Brusa, G.; Pintaldi, S. Lung ultrasound diagnostic accuracy in neonatal pneumothorax. Can. Respir. J. 2016, 2016, 6515069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.W.; Zhang, M.Y.; Liu, J. Application of lung ultrasonography in the diagnosis of childhood lung diseases. Chin. Med. J. 2015, 128, 2672–2678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J. Lung ultrasonography for the diagnosis of neonatal lung disease. J. Matern.-Fetal Neonatal Med. 2014, 27, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Tomà, P.; Owens, C.M. Chest ultrasound in children: Critical appraisal. Pediatr. Radiol. 2013, 43, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Farahbakhsh, N. Role of chest ultrasound in neonatal lung disease: A review of current evidences. J. Matern.-Fetal Neonatal Med. 2019, 32, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Copetti, R.; Cattarossi, L.; Macagno, F.; Violino, M.; Furlan, R. Lung ultrasound in respiratory distress syndrome: A useful tool for early diagnosis. Neonatology 2008, 94, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Mezière, G.; Seitz, J. The dynamic air bronchogram: A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009, 135, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Nobile, S.; Sette, L.; Esposito, C.; Riitano, F.; Di Sipio Morgia, C.; Sbordone, A.; Vento, G.; Perri, A. Diagnostic Accuracy of Lung Ultrasound in Neonatal Diseases: A Systematized Review. J. Clin. Med. 2024, 13, 3107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa, F.; Titolo, A.; Ferrocino, M.; Biagi, E.; Dell’Orto, V.; Perrone, S.; Esposito, S. Lung Ultrasound in Neonatal Respiratory Distress Syndrome: A Narrative Review of the Last 10 Years. Diagnostics 2024, 14, 2793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lovrenski, J. Pediatric lung ultrasound—Pros and potentials. Pediatr. Radiol. 2020, 50, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Stoller, J.Z.; Fraga, M.V. Point-of-care ultrasound in the neonatal ICU. Curr. Opin. Pediatr. 2020, 32, 216–227. [Google Scholar] [CrossRef] [PubMed]
- El-Fattah, N.M.A.; El-Mahdy, H.S.; Hamisa, M.F.; Ibrahim, A.M. Lung Ultrasound Findings in the Diagnosis of Transient Tachypnea of Newborn in Late Preterm and Term Neonates. Asian J. Pediatr. Res. 2023, 13, 205–212. [Google Scholar] [CrossRef]
- Cattarossi, L. Lung ultrasound: Its role in neonatology and pediatrics. Early Hum. Dev. 2013, 89, S17–S19. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ojembarrena, A.; Gregorio-Hernández, R.; Raimondi, F. Neonatal point-of-care lung ultrasound: What should be known and done out of the NICU? Eur. J. Pediatr. 2024, 183, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Aggarwal, N.; Makhaik, S.; Jhobta, A.; Kapila, S.; Bhoil, R. Role of lung ultrasound in diagnosing and differentiating transient tachypnea of the newborn and respiratory distress syndrome in preterm neonates. J. Ultrason. 2022, 22, e1–e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Cao, H.Y.; Wang, H.W.; Kong, X.Y. The role of lung ultrasound in diagnosis of respiratory distress syndrome in newborn infants. Iran. J. Pediatr. 2015, 25, e323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xin, H.; Wang, L.; Hao, W.; Hu, H.; Li, H.; Liu, B. Lung Ultrasound in the Evaluation of Neonatal Respiratory Distress Syndrome. J. Ultrasound Med. 2023, 42, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Hiles, M.; Culpan, A.M.; Watts, C.; Munyombwe, T.; Wolstenhulme, S. Neonatal respiratory distress syndrome: Chest X-ray or lung ultrasound? A systematic review. Ultrasound 2017, 25, 80–91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Malah, H.E.-D.G.M.; Hany, S.; Mahmoud, M.K.; Ali, A.M. Lung ultrasonography in evaluation of neonatal respiratory distress syndrome. Egypt. J. Radiol. Nucl. Med. 2015, 46, 469–474. [Google Scholar] [CrossRef]
- Bao, L.Y.; Dao, X.Y.; Du, K. Progress in the Application of Lung Ultrasound for the Evaluation of Neonates with Respiratory Distress Syndrome. J. Multidiscip. Healthc. 2024, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ismail, R.; El Raggal, N.M.; Hegazy, L.A.; Sakr, H.M.; Eldafrawy, O.A.; Farid, Y.A. Lung Ultrasound Role in Diagnosis of Neonatal Respiratory Disorders: A Prospective Cross-Sectional Study. Children 2023, 10, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raimondi, F.; Migliaro, F.; Sodano, A.; Vallone, G.; Ferrara, T.; Maddaluno, S.; Coppola, C.; Capasso, L. Point-of-care chest ultrasound in the Neonatal Intensive Care Unit. J. Pediatr. Neonatal Individ. Med. (JPNIM) 2013, 2, e020214. [Google Scholar] [CrossRef]
- Liu, J.; Inchingolo, R.; Suryawanshi, P.; Guo, B.-B.; Kurepa, D.; Cortés, R.G.; Yan, W.; Chi, J.-H.; Acosta, C.M.; Jagła, M.; et al. Guidelines for the use of lung ultrasound to optimise the management of neonatal respiratory distress: International expert consensus. BMC Med. 2025, 23, 114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J. The Lung Ultrasound Score Cannot Accurately Evaluate the Severity of Neonatal Lung Disease. J. Ultrasound Med. 2020, 39, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, H.Y.; Fu, W. Lung ultrasonography to diagnose meconium aspiration syndrome of the newborn. J. Int. Med. Res. 2016, 44, 1534–1542. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carnazzo, S.M.; Nasikas, S.; Comisi, F.F. Lung Ultrasound in Neonates: A Narrative Review Along With Diagnostic Insights and Early Postnatal Applications. Cureus 2024, 16, e70487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elabbas, A.; Choudhary, R.; Gullapalli, D.; Mistry, S.; Farzana, M.H.; Mallick, A.H.; Kevu, E.P.; Asif, J.; Mostafa, J.A. Lung Ultrasonography Beyond the Diagnosis of Pediatrics Pneumonia. Cureus 2022, 14, e22460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mong, A.; Epelman, M.; Darge, K. Ultrasound of the pediatric chest. Pediatr. Radiol. 2012, 42, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, P. The Use of Lung Ultrasound Rather than Chest X-Ray to Diagnose Neonatal Lung Disease: Time for Action. Diagnostics 2025, 15, 1583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rambhia, S.H.; D’Agostino, C.A.; Noor, A.; Villani, R.; Naidich, J.J.; Pellerito, J.S. Thoracic ultrasound: Technique, applications, and interpretation. Curr. Probl. Diagn. Radiol. 2017, 46, 305–316. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foutzitzi, S.; Prassopoulos, P.; Chatzimichail, A.; Kambouri, K.; Moschouris, H.; Psatha, E.A.; Oikonomou, P.; Deftereos, S.P. Lung Ultrasound in Pediatrics: A Review with Core Principles That Every User Should Know. Diagnostics 2025, 15, 2782. https://doi.org/10.3390/diagnostics15212782
Foutzitzi S, Prassopoulos P, Chatzimichail A, Kambouri K, Moschouris H, Psatha EA, Oikonomou P, Deftereos SP. Lung Ultrasound in Pediatrics: A Review with Core Principles That Every User Should Know. Diagnostics. 2025; 15(21):2782. https://doi.org/10.3390/diagnostics15212782
Chicago/Turabian StyleFoutzitzi, Soultana, Panos Prassopoulos, Athanasios Chatzimichail, Katerina Kambouri, Hippocrates Moschouris, Evlampia A. Psatha, Panagoula Oikonomou, and Savas P. Deftereos. 2025. "Lung Ultrasound in Pediatrics: A Review with Core Principles That Every User Should Know" Diagnostics 15, no. 21: 2782. https://doi.org/10.3390/diagnostics15212782
APA StyleFoutzitzi, S., Prassopoulos, P., Chatzimichail, A., Kambouri, K., Moschouris, H., Psatha, E. A., Oikonomou, P., & Deftereos, S. P. (2025). Lung Ultrasound in Pediatrics: A Review with Core Principles That Every User Should Know. Diagnostics, 15(21), 2782. https://doi.org/10.3390/diagnostics15212782




